Assessment of the individual and combined effects of Rht8 and Ppd-D1a on plant height,time to heading and yield traits in common wheat
Kunpu Zhng*,Junjun Wng,Hunju Qin,Zhiying Wei,Libo Hng,Pengwei Zhng,Mtthew Reynols,Dowen Wng*
aState Key Laboratory of Plant Cell and Chromosome Engineering, Institute of Genetics and Developmental Biology, Chinese Academy of Sciences,Beijing 100101,China
bCollege of Agronomy and State Key Laboratory of Wheat and Maize Crop Science,Henan Agricultural University,Zhengzhou 450002,Henan,China
cZhaoxian Institute of Agricultural Sciences,Zhaoxian 051530,Hebei,China
dCIMMYT,Int. Apdo.Postal 6-641,06600 México,DF, Mexico
Keywords:Association analysis QTL mapping Grain number Grain yield Triticum aestivum
ABSTRACT Grain yield in cereal crops is a complex trait controlled by multiple genes and influenced by developmental processes and environment. Here we report the effects of alleles Rht8 and Ppd-D1a on plant height, time to heading, and grain yield and its component traits. Association analysis and quantitative trait locus mapping using phenotypic data from 15 environments led to the following conclusions.First,both Rht8 and Ppd-D1a reduce plant height.However,Ppd-D1a but not Rht8 causes earlier heading.Second, both Rht8 and Ppd-D1a promote grain yield and affect component traits. Their combined effects are substantially larger than those conferred by either allele alone.Third,promotion of grain yield by Rht8 and Ppd-D1a is through increasing fertile spikelet number. We speculate that Rht8 and Ppd-D1a act independently and additively in control of plant height,grain yield and yield component.Combination of the two alleles is desirable for adjusting plant height and enhancing grain yield and abiotic stress tolerance.
1. Introduction
Increased cereal yield is a major goal of agricultural research.However, efforts to reach such a goal have met considerable difficulties in recent years,because the rate of genetic gain in grain yield (GY) is slowing down [1]. Furthermore, crop productivity is increasingly limited by reduced availability of natural resources(such as water for irrigation)and ecological concerns (e.g., water pollution by excessive use of chemical fertilizers) [2]. It is well known that GY and its related component traits are generally controlled by polygenes whose expression is affected by plant development and environmental factors [3,4]. It is essential, therefore, to study the genes controlling plant development,GY and related traits and their interactions with environmental factors in order to increase the efficiency of enhancing GY by breeding.
Common wheat (Triticum aestivum L, AABBDD, 2n = 6× =42) is the most widely cultivated staple crop. Considerable effort has been devoted to investigating the genes and quantitative trait loci (QTL) involved in controlling wheat development and GY [5-8]. The most frequently studied wheat development genes include those controlling vernalization response(Vrn1,Vrn2 and Vrn3),photoperiod sensitivity(Ppd1), and GA-insensitive plant height (Rht-B1b and Rht-D1b)[9-11]. The function of these genes tends to be allele-specific and environmentally sensitive. Moreover, they often interact in affecting plant development and GY.
The GA-sensitive Rht8 located on wheat chromosome arm 2DS has long been known for its involvement in regulating plant height (PH) in common wheat [12,13]. The Rht8 allele that originated from the Japanese variety Akakomugi confers a semi-dwarf phenotype [14]; the contrasting allele rht8 is associated with a taller phenotype [14]. Compared to Rht-B1b and Rht-D1b (located on chromosomes 4B and 4D, respectively)that decrease PH by 15%-30%[15-18],the effect of Rht8 on height is less (8%-15%) [13,14,17,19,20]. Although Rht8 has not yet been characterized at the molecular level, it has been tagged by specific genetic markers. The 192 bp amplicon of the microsatellite marker Xgwm261 has been widely used for identifying Rht8 in global wheat germplasm [13,20-23]. A recent study revealed that the semi-dwarf phenotype conferred by Rht8 is associated with reduced sensitivity to the brassinosteroid hormone[14].That study also developed new gene-based markers more closely linked to Rht8 than Xgwm261. Rht8 does not significantly affect GY in European environments although it slightly improves spike fertility under some conditions [19,24-26]. Rebetzke et al. [17]found that Rht8 improves GY and grain number per spike (GNS) in Australian environments.Several quantitative genetic studies in China have reported QTL controlling GY and related traits around Xgwm261,indicating that Rht8 might promote GY and its component traits[27-29].
The molecularly characterized Ppd-D1 on chromosome arm 2DS plays an important role in regulation of photoperiod sensitivity[30,31].Two main alleles,Ppd-D1a and Ppd-D1b,are distinguished in most wheat varieties[30,32].Ppd-D1a confers photoperiod insensitivity and promotes early heading and flowering [30,31,33]. It decreases PH possibly due to a shortened vegetative growth period and accelerated ear emergence [14,25,33-35]and tends to increase grain number and final grain yield, particularly in the environments with post-anthesis high temperature and drought stress[19,33,36].
The combination of Rht8 and Ppd-D1a likely leads to plants with reduced height and early flowering [14]. These traits are important for adaptation to the environments with risk of summer heat and drought. This is consistent with the observation that both alleles are present in a high proportion of the wheat lines from Southern European countries, where summer heat and drought stress commonly occur during the late stages of growth and grain development [19]. The close association between Rht8 and Ppd-D1a (i.e., both located on 2DS and frequently found together)has caused researchers to question whether it is possible to separate the effects of the two genes. Rebetzke et al. [17]suggested that the positive effects of Rht8 on GY and GNS might be associated with its linkage with Ppd-D1a. Wilhelm et al. [18]speculated that Rht8 might be the main cause of height reduction associated with Ppd-D1a in their analysis of 372 worldwide common wheat accessions. Hence, there was a need for a systematic investigation of the effects of Rht8 and Ppd-D1a on key developmental and yield traits under diverse cultivation conditions, as the outcomes could aid the development of the wheat varieties with improved water and nutrient use efficiencies.
The main objective of this study was to assess the individual and combined effects of Rht8 and Ppd-D1a on PH,TH, GY, and GY-related traits using data from two types of genetic analysis. The first was an association analysis of 94 elite common wheat varieties grown in 12 environments differing in location and water and fertilizer applications[37].The second was a QTL analysis involving 168 double haploid lines (DHLs) grown in three fertilized and irrigated environments [38]. The genotypes included in both analyses came mainly from the Yellow and Huai river valley (YHRV), and encompassed the major wheat growing regions in Henan and Shandong provinces and some parts of Hebei, Shaanxi,Shanxi, Jiangsu and Anhui, with a total annual wheat cultivation area of about 12 Mha [39]. Wheat productivity in the YHRV is adversely affected by drought and heat stress particularly during flowering and grain filling stages[39].Rht8 and Ppd-D1a have each been detected in >50%of the varieties planted in YHRV [21,22,33], suggesting that both alleles were selected by breeders in coping with drought and heat stresses.Considering that both Rht8 and Ppd-D1a have been reported to exert beneficial effects on spike fertility and grain number[17,19,24,33], the effects of the individual alleles and their combination on GNS, spikelet number per spike (SNS), fertile spikelet number per spike (FSNS) and spike length (SL) were investigated.
2. Materials and methods
2.1. Genetic populations and field trials
The population for association analysis contained 94 winter wheat varieties,89 of which came from the YHRV region.The other five lines were from the neighboring Northern winter wheat zone. They were grown at Hengshui (HS) in Hebei province in 2008/2009 and 2009/2010, and at Jiyuan (JY) in Henan province during 2009/2010 where they were subjected to four treatments,namely irrigated and fertilized(IF),rainfed(RF), reduced nitrogen (RN), and reduced phosphorus (RP) as detailed previously [37].
The QTL mapping population consisted of 168 DHLs derived from a cross between Huapei 3 and Yumai 57 [38].Huapei 3 has higher grain weight and relatively high yield potential [40], whereas Yumai 57 has broad environmental adaptation and comparatively high yield potential at multiple locations in the YHRV [41]. This DH population had been grown in three IF environments, two at Tai'an (TA) in Shandong province (during 2005/2006 and 2006/2007) and one at Suzhou(SZ)in Anhui province (during 2006/2007) [38].
2.2. Trait evaluation
The PH of each genotype was measured as the average height(cm) of 10 stems from the soil surface to the tip of the spike(excluding awns). TH was recorded as the number of days from sowing to 50% spike emergence. Ten main-stem spikes from the middle of each replication were used for investigating GNS, SNS, FSNS and SL (excluding awns). GY was measured as the weight of all grains harvested from the entire plot.
2.3. Molecular markers and chromosomal linkage map
Alleles Rht8 and rht8 were detected using microsatellite marker Xgwm261 [42]and the gene-based marker DG273 [14].Allelic variants at Ppd-D1 were amplified using a multiplex PCR assay[30].The primer set Ppd-D1_F(5′-ACGCCTCCCACTACACTG-3′) and Ppd-D1_R1 (5′-TGTTGGTTCAAACAGAGAGC-3′) yielded a 414 bp fragment diagnostic for Ppd-D1b,whereas primer pair Ppd-D1_F and Ppd-D1_R2 (5′-CACTGGTGGTAG CTGAGATT-3′) gave a 288 bp fragment specific for Ppd-D1a.The results of genotyping of the 94 cultivars with the three markers are provided in Table S1.Polymorphisms of the three markers among the 168 DHLs enabled estimation of genetic distances among Xgwm261,DG273, and Ppd-D1. The genotypic data were analyzed by MAPMAKER/Exp version 3.0b [43]. Map positions of the three markers were integrated with those of previously published markers on 2D[44]using Mapchart version 2.1[45].
2.4. Association analysis and QTL mapping
The mixed linear model (MLM) model with population structure (Q) and kinship (K) in the TASSEL 2.1 software package (http://www2.maize genetics.net/) was used for genome wide association analysis of PH, TH, GY, and GY component traits (GNS, SNS, FSNS, and SL) for each of the 12 environments as described by Zhang et al. [37]. Q and K parameters were estimated previously [37]. The R2(percentage of phenotypic variance explained) values and their significance levels were computed for the examined markers(loci).
QTL mapping of PH,TH,GY,and GY component traits was performed for each of the three IF environments in which the DH population was cultivated using IciMapping software(http://www.isbreeding.net/). The walk speed was 1.0 cM for all QTL. QTL declaration was based on a LOD score threshold of ≥2.5. Marker intervals and peak positions were computed for the Rht8 and Ppd-D1 QTL.
2.5. Statistical analysis
All statistical analyses were carried out using IBM SPSS for Windows 17.0 (SPSS, www.spss.com). Environmental and genetic variations of the traits were examined by analysis of variance (ANOVA) and the least significant difference rank test (α = 0.05) followed by a Behrens-Fish multiple comparison test. Genotyping and comparison of genotypic means were performed to examine the allelic effects of detected loci as described previously [37].
3. Results
3.1. Assessment of phenotypic data
Phenotypic data for seven traits (PH, TH, GY, GNS, SNS,FSNS, and SL) were obtained from all 12 environments in the association analysis experiment (Table S2). All seven traits showed variation.In general,trait means were highest under IF conditions, followed by those under RP or RN conditions,with that in RF environments being the lowest. There was substantial genotypic variance (δ2G) for all seven traits and broad sense heritabilities(H2) were relatively high(Table S2).
To validate the findings from the association analysis, we used the phenotypic data for PH,TH,GY,GNS,SNS,FSNS and SL from the 168 DHLs and their parents grown in three IF environments [38]. The ranges in trait variation and broad sense heritabilities (Table S3) were generally comparable to those determined for the association mapping population(Table S2).
3.2.Association of Rht8 and Ppd-D1 with PH,TH and GY and related traits
We previously conducted an association analysis of chromosome loci controlling grain weight in the 94 common wheat varieties using 1171 microsatellite and DArT markers [37].Here, we incorporated the new Rht8-co-segregating marker and the Ppd-D1 locus into the linkage map of chromosome 2D.Among the five markers DG273 was clearly amplified in all 94 varieties with alleles of 391 and 421 bp.The positions of DG273 and Ppd-D1 on chromosome 2D were then verified by genotyping the 168 Huapei 3 × Yumai 57 DHLs. Huapei 3 carried the DG273391bpand Ppd-D1b alleles,whereas Yumai 57 had DG273421bpand Ppd-D1a. The genetic distance between Xgwm261 and DG273 in the 2D genetic map (Fig. S1) was 0.5 cM, whereas that between DG273 and Ppd-D1 was 11 cM.With the incorporation of DG273 and Ppd-D1,the total number of markers used for association analysis changed from 1171 in the previous study[37]to 1173 in the present work.
Using the MLM program and considering both Q and K, a larger number of loci were associated with each of the seven traits (i.e., PH, TH, GY, GNS, SNS, FSNS, and SL). Xgwm261,DG273 and Ppd-D1,all located on 2DS,were selected for further analysis because they were stably associated with PH, GY,GNS, SNS and FSNS across all 12 environments (P ≤0.01)(Table 1).The phenotypic variance explained(R2)by DG273 for all five traits tended to be larger than that explained by Xgwm261, consistent with the fact that DG273 was more closely linked to Rht8 than Xgwm261[14](Fig.S1).The average effects of DG273 on the five traits were generally larger than those conferred by Ppd-D1 (Table 1). In contrast to the results described above, Ppd-D1, but not Xgwm261 and DG273, was consistently associated with TH (Table 1). Neither Rht8 nor Ppd-D1 was significantly associated with SL.

Table 1-Phenotypic effects(R2,%)of Xgwm261,DG273,and Ppd-D1 loci on PH,TH,GY,GNS,SNS,FSNS,and SL as detected by association analysis.
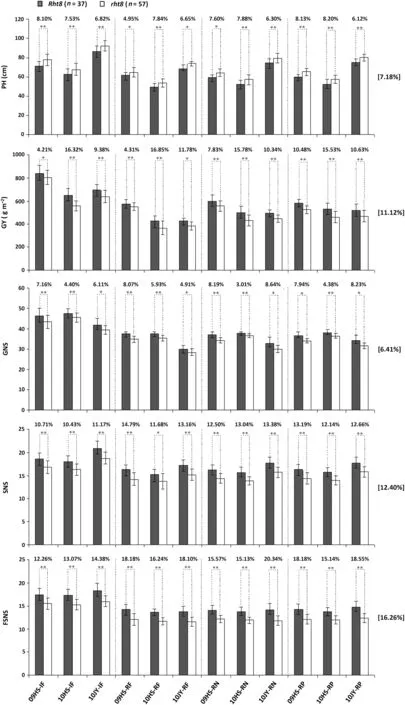
Fig.1-Assessing the allelic effects of Rht8 on plant height(PH),grain yield(GY),grain number per spike(GNS),spikelet number per spike(SNS),and fertile spikelet number per spike(FSNS)using the phenotypic data from 94 common wheat varieties cultivated in 12 environments.These environments were created by growing the varieties in different cropping cycles(08/09 and 09/10)and locations(HS and JY)under four conditions(IF,RF,RN,and RP).The 94 varieties were divided into Rht8 and rht8 groups.Means(±SD)of PH,GY,GNS,SNS,and FSNS were calculated for each group and each environment,and statistically compared(*P ≤0.05,**P ≤0.01).Percentage differences between means are given at the top of each histogram.The percentages at the right indicate averaged effects of Rht8 relative to rht8 across all 12 environments.IF,irrigated and fertilized;HS,Hengshui;JY,Jiyuan;RF,rainfed;RN,reduced nitrogen;RP,reduced phosphorus.

Table 2-Effects of Rht8 and Ppd-D1 loci on PH,TH,GY, GNS,SNS,FSNS.and SL revealed by QTL mapping.
DG273421bp(Rht8), the superior allele, was found in 37 of the 94 cultivars (39.4%, Table S4). Ppd-D1a, the elite allele of Ppd-D1,was present in 86 of the 94 cultivars(91.5%,Table S4).Among the 94 varieties examined, 34 (36.2%) possessed both Rht8 and Ppd-D1a (Table S4). The relatively high numbers of varieties carrying Rht8 (37) or rht8 (57) facilitated a reliable statistical comparison of their effects on PH, GY, GNS, SNS,and FSNS (Fig. 1). Rht8 was significantly more effective than rht8 in decreasing PH, and in promoting GY, GNS, SNS, and FSNS. Rht8 decreased PH by an average 7.18%, and promoted GY by 11.12%, GNS by 6.41%, SNS by 12.40% and FSNS by 16.26%(Fig. 1).
The number of varieties with Ppd-D1b was small (n = 8),making it difficult to statistically compare the allelic effects of Ppd-D1a and Ppd-D1b. Nevertheless, a provisional assessment revealed that Ppd-D1a tended to be more effective than Ppd-D1b in reducing PH, shortening TH, and promoting GY, GNS,SNS,and FSNS in all 12 environments(Table S5).
Stringent statistical comparisons among the means of the four groups of genotypes carrying different allelic combinations of Rht8 and Ppd-D1 (i.e., Rht8 + Ppd-D1a, rht8 + Ppd-D1a,
Rht8 + Ppd-D1b and rht8 + Ppd-D1b)were also difficult because the numbers of varieties with Rht8 + Ppd-D1b (n = 3) or rht8 +Ppd-D1b (n = 5) were low. A tentative examination showed that, on average, genotypes with Rht8 and Ppd-D1a appeared to have the lowest PH and the highest values for GY,GNS,SNS,and FSNS, whereas those carrying rht8 and Ppd-D1a or Rht8 and Ppd-D1b were comparatively less effective in reducing PH and promoting GY,GNS,SNS,and FSNS(Table S6).Genotypes with rht8 and Ppd-D1b had the highest PH and the lowest GY,GNS,SNS,and FSNS values(Table S6).
3.3. Validation of association analysis findings by QTL mapping
Quantitative genetic mapping with 168 DHLs showed that both Rht8 and Ppd-D1 represented stably expressed QTL for PH,GY,GNS,SNS and FSNS in all three IF environments,and that Ppd-D1 but not Rht8 was a significant QTL for TH (Table 2).Neither the marker interval nor the peak position of the Rht8 QTL overlapped with those of the Ppd-D1 QTL(Table 2,Fig.S2).According to the percentage of phenotypic variance explained, Rht8 was generally more effective than Ppd-D1 in affecting PH, GY, GNS, SNS and FSNS (Table 2). This analysis also indicated highly significant (P <0.01) additive interactions between Rht8 and Ppd-D1 on PH,GY,GNS,SNS and FSNS(Table 2).
DHL genotypes carrying Rht8(n = 93),rht8(n = 75),Ppd-D1a(n = 90),or Ppd-D1b(n = 78)were sufficiently large for rigorous statistical comparisons of the allelic effects of the two genes.It is clear that Rht8 was superior to rht8 in lowering PH and increasing GY, GNS, SNS and FSNS (Fig. 2). Across the three environments,the mean effects of Rht8 on the five traits were 6.94% (PH), 9.38% (GY), 9.39% (GNS), 10.94% (SNS), and 11.79%(FSNS). Ppd-D1a showed much stronger effects than Ppd-D1b on advancing TH, reducing PH, and increasing GY, GNS, SNS,and FSNS (Fig. 3). When averaged across the three environments,the effects of Ppd-D1a on the six traits were 1.68%(TH),4.32% (PH), 8.83% (GY), 8.86% (GNS), 10.32% (SNS), and 11.16%(FSNS).The combination of Rht8 and Ppd-D1a was significantly more effective than that of rht8 and Ppd-D1b in reducing PH,and increasing GY, GNS, SNS, and FSNS (Fig. 4). The Rht8 +Ppd-D1a combination was significantly more effective than Rht8 or Ppd-D1a alone in promoting PH, GY, GNS,SNS,and FSNS (Table 3). The allelic effects of Rht8 on the five traits tended to be higher than those of Ppd-D1a,Rht8,Ppd-D1a,and Rht8 + Ppd-D1a exhibited the highest effect on FSNS(Table 3).

Fig.2- Verification of the allelic effects of Rht8 on plant height(PH),grain yield(GY),grain number per spike(GNS),spikelet number per spike(SNS),and fertile spikelet number per spike(FSNS)with the phenotypic data from 168 DHLs grown in three irrigated and fertilized environments(06TA,07TA and 07SZ).The 168 lines were divided into two genotype groups based on Rht8 alleles(Rht8 or rht8).The means(±SD)of PH,GY, GNS,SNS and FSNS were calculated for each group and for each environment and were statistically compared(* P ≤0.05,**P ≤0.01).The number of lines(n) for each group is given in the brackets. For each environment,the percentage of difference between the means of the two groups is given at the top of the figure.Values in square brackets show the averaged effect of Rht8(relative to rht8)across all three environments.SZ,Suzhou;TA,Tai'an.
The effect of an allele is the percentage increase (+) or decrease (-) over the inferior allele. The mean values (±SD)being compared are derived from Fig.2 (Rht8), Fig.3 (Ppd-D1a)and Fig. 4 (Rht8 + Ppd-D1a). FSNS, fertile spikelet number per spike;GNS,grain number per spike;GY,grain yield;PH,plant height; SNS, spikelet number per spike. Different letters following SD indicate statistical significance (P <0.05) among the three genotypes.
Consistent with the lack of association of Rht8 and Ppd-D1 with SL among the 94 elite varieties grown in 12 environments(Table 1),allelic variation of Rht8(Rht8 vs.rht8)had no significant effect on SL, and genotypes carrying Rht8 + Ppd-D1a or rht8 +Ppd-D1b did not differ substantially from each other in mean SL(Table S7).For the 168 DHLs grown in three IF environments,SL was not markedly influenced by allelic variation at the Rht8 or Ppd-D1 loci, nor did it vary significantly between genotypes carrying Rht8 + Ppd-D1a or rht8 + Ppd-D1b(Table S8).
4. Discussion
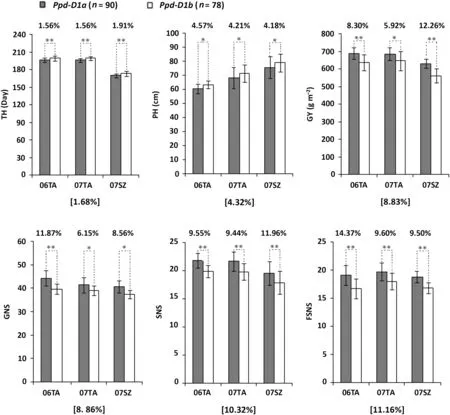
Fig.3- Allelic effects of Ppd-D1 on time to heading(TH),plant height(PH),grain yield(GY), grain number per spike(GNS),spikelet number per spike(SNS),and fertile spikelet number per spike(FSNS)using phenotypic data from 168 DHLs grown in three irrigated and fertilized environments(06TA,07TA,and 07SZ).The lines were divided into two genotype groups based on Ppd-D1 alleles(Ppd-D1a or Ppd-D1b). Means(±SD)of PH,GY, GNS,SNS, FSNS and TH calculated for each group and for each environment were statistically compared(*P ≤0.05,**P ≤0.01).The number of lines(n)for each group is given at the top.The percentage difference between means for the two groups is given above each histogram. Values in square brackets indicate averaged effects of Ppd-D1a(compared to Ppd-D1b)across all three environments. SZ,Suzhou;TA,Tai'an.
In this work we assessed the effects of Rht8 and Ppd-D1a on common wheat traits PH, TH and GY by association analysis and QTL mapping with phenotypic data from 15 environments. The information obtained and implications for wheat improvement are discussed below.
4.1. Distribution of Rht8,Ppd-D1a in common wheat
The frequency of Rht8 in the 94 varieties(39.4%)was lower than reported previously (42.3%-64.0%) [22,46]. This work employed marker DG273 to identify the presence of Rht8,whereas the 192 bp amplicon of Xgwm261 was used in previous studies as an indicator of Rht8. Since DG273 is more closely linked to Rht8[14](Fig.S1),its use should provide more accurate identification of Rht8. The frequency of Ppd-D1a in the 94 cultivars (91.5%) was comparable to previous reports(77.9%-90.6%) for the improved winter wheat varieties in China[33,47].Rht8 and Ppd-D1a were simultaneously detected in 36.2% of the 94 varieties (Table S3), which is quite low considering the beneficial effects of the combination on GY and related traits revealed in this work.
4.2. Rht8 and Ppd-D1a appear to act independently and additively

Fig.4-Comparisons of plant height(PH),grain yield(GY),grain number per spike(GNS),spikelet number per spike(SNS)and fertile spikelet number per spike(FSNS)between lines carrying Rht8 + Ppd-D1a and those with rht8 + Ppd-D1b.The phenotypic data used were from 168 DHLs grown in three IF environments(06TA,07TA and 07SZ).These lines were divided into genotype groups carrying Rht8 + Ppd-D1a or rht8 + Ppd-D1b.The means(±SD)of PH,GY,GNS,SNS,and FSNS calculated for each group and environment were statistically compared(*P ≤0.05,**P ≤0.01).The number of lines(n)for each group is shown at the top.The percentage differences between the means for the two groups are given above the histograms.Values in square brackets illustrate the averaged effects of Rht8 + Ppd-D1a(compared to rht8 + Ppd-D1b)across the three environments.SZ,Suzhou;TA,Tai'an.
Considering the data obtained in this work,we speculate that Rht8 and Ppd-D1a act independently and additively in control of PH,GY,GNS,SNS,and FSNS.The marker interval and peak position of the Rht8 QTL did not overlap with those of the Ppd-D1 QTL (Table 2, Fig. S2). Moreover, Ppd-D1a, but not Rht8,significantly affected TH in all 15 environments(Table 1,Table 2). The fact that the genetic effects of Rht8 on PH, GY, GNS,SNS,and FSNS were generally not identical to those of Ppd-D1a(Table 3)provides additional support for functional difference of the two loci.
The possibility that Rht8 and Ppd-D1a act additively on PH,GY,GNS,SNS,and FSNS is mainly supported by two findings.First,highly significant(P <0.01 or P <0.001)additive interactions on PH, GY, GNS, SNS, and FSNS were detected for Rht8 and Ppd-D1 by QTL mapping (Table 2). Second, the additive effect of Rht8 + Ppd-D1a on each of the five traits was generally close to the sum of their individual effects(Table 3).
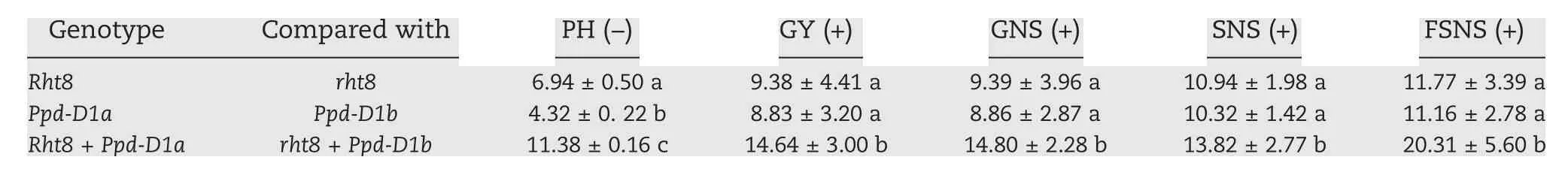
Table 3-Effects of Rht8,Ppd-D1a and their combination(Rht8 + Ppd-D1a)on PH,GY, GNS,SNS,and FSNS.
4.3. Comparison with previous studies
Both Rht8 and Ppd-D1a conferred substantial improvement on GY and related traits relative to their respective inferior counterparts (rht8 and Ppd-D1b). This contrasts with previous findings of limited positive effects of Rht8 on spike fertility[19,24-26]and of Ppd-D1a on GY and grain number in some European environments[9,19].Because post anthesis drought and high temperature stresses are generally severe and common in YHRV environments [39],the beneficial effects of Rht8 and Ppd-D1a on GY and related traits might have been higher under the conditions of our study. Additionally, our analysis used a comprehensive phenotypic data set collected from 94 varieties and 168 DHLs cultivated in 15 different environments, which may be more conducive to uncovering larger allelic effects of Rht8 and Ppd-D1a on GY and related traits.
In agreement with our observations, two genetic studies involving five Chinese wheat populations consistently mapped significant QTL for GY-related traits around Xgwm261 (indicative of Rht8) [28,29]. In another study by Wu et al. [27], wheat yield-related QTL were also detected in the chromosome 2D region of Rht8 and Ppd-D1a in multiple rainfed environments. Furthermore, genetic variation associated with Ppd-D1 has been found to affect GY in a wide range of European conditions [48,49], and a recent study suggested that Ppd-D1a was associated with significant positive effects on GY in both Australian and Mexican environments [8].
4.4. Implications for wheat improvement
From the discussion above,it seems that Rht8 and Ppd-D1a can be used to adjust PH and to enhance GY and tolerance to post-anthesis heat and drought stress of common wheat in the YHRV region and in other areas (e.g., southern European countries) with similar wheat production environments (Fig.5). Because the combined effects of Rht8 and Ppd-D1a are generally larger than the effect of either allele alone,it would be preferable to pyramid them. This can be achieved using DNA markers developed previously [14,30,33]. Since the proportion of cultivars carrying both Rht8 and Ppd-D1a is currently low (36.2%, Table S4), pyramiding the two alleles may enable rapid development of more superior common wheat lines with higher yield potential and better environmental adaptability. In agreement with this view, Rht8 was recently recommended as a useful height reducing gene for breeding common wheat varieties adapted to the environments with recurrent drought problems [50]. The number of common wheat varieties carrying both Rht8 and Ppd-D1a is rising rapidly in southern European regions where postanthesis drought and high temperature stress are frequently encountered[35].
The wide-ranging beneficial effects conferred by Rht8 +Ppd-D1a on wheat developmental and yield traits deserve further study using larger segregating populations. We have now developed a multiparent advanced generation intercross population with >4000 recombinant lines, which should be useful for a more comprehensive elucidation of the individual and combined effects of Rht8 and Ppd-D1a in the growth,development, environmental adaptation and yield performance of wheat in future research.

Fig.5-A diagram depicting the individual and combined effects of Rht8 and Ppd-D1a on plant height(PH),grain yield(GY),grain number per spike(GNS), spikelet number per spike (SNS), fertile spikelet number per spike(FSNS),and time to heading(TH)revealed in this work.Rht8,Ppd-D1a and their combination(Rht8 + Ppd-D1a)cause decrease of PH and promote GY mainly through increasing FSNS.In addition,Ppd-D1a decreases TH.The thicker arrows illustrate the larger effects conferred by Rht8 + Ppd-D1a relative to those by Rht8 or Ppd-D1a alone.Rht8,Ppd-D1a and especially their combination(Rht8 + Ppd-D1a)may be useful for developing the common wheat cultivars adaptable to the environments with post-anthesis heat and drought stresses.
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2019.06.008.
Declaration of competing interest
There is no conflict of interest.
Acknowledgments
This study was supported by the Ministry of Science and Technology of China(2017YFD0101000),Science and Technology Service Network Program (STS Program) of Chinese Academy of Sciences (KFJ-STS-ZDTP-024) and National Natural Science Foundation of China(31371611).The authors thank Professor Jiankang Wang (Institute of Crop Science, Chinese Academy of Agricultural Sciences)for advice on QTL mapping.
- The Crop Journal的其它文章
- Positional cloning of PmCH1357 reveals the origin and allelic variation of the Pm2 gene for powdery mildew resistance in wheat
- The Crop Journal 作物学报(英文版) (Started in 2013, Bimonthly)
- Wheat powdery mildew resistance gene Pm64 derived from wild emmer(Triticum turgidum var.dicoccoides) is tightly linked in repulsion with stripe rust resistance gene Yr5
- Wheat breeding in northern China: Achievements and technical advances
- Breed ing w heat for resistance to Fusarium head blight in the Global North: China,USA,and Canad a
- Pyramiding disease resistance genes in elite winter wheat germplasm for Western Canada

