Combustion Performance of Fe2O3-containing Nanothermites Prepared by Ball Milling Method
JIANG Ai-feng,XIA De-bin,LI Meng-ru,LIN Kai-feng,QIANG Liang-sheng,LI Jia-he,FAN Rui-qing,YANG Yu-lin
(MIIT Key Laboratory of Critical Materials Technology for New Energy Conversion and Storage,School of Chemistry and Chemical Engineering,Harbin Institute of Technology,Harbin 150001,China)
Abstract:Various preparation methods have been widely explored to improve the combustion performance of nanothermites in recent years.In this work,two kinds of Fe2O3-containing nanothermites were successfully prepared by in-situ ball milling method and conventional ultrasonic blending method respectively.The morphologies and performance of as-prepared products have been fully characterized by thermogravimetric analysis(TGA),X-ray diffraction(XRD),contact angle tests,scanning electron microscopy(SEM),high-speed imaging experiments and infrared temperature measurement.The results show that the Fe2O3-doped nanothermites via in-situ ball milling method exhibit better performance than that made by ultrasonic blending method.The optimal nanothermites with 17%Fe2O3doped amount possess the maximum mass gain percentage of 13.1%per 100℃.Compared with the products made by ultrasonic blending method,the heating voltage and initial combustion temperature of in-situ ball milled nanothermites decrease to 12 V and 600℃,respectively.In addition,the combustion flame of in-situ ball milled nanothermites is more stable and homogeneous than the corresponding one.
Key words:nanothermites;in-situ ball milling;ultrasonic blending;contact angle;combustion flame
1 Introduction
Thermites,commonly composed of metal and metal oxide[1-8],is one major kind of energetic materials(EMs)and extremely attracts researcher’s growing attentions due to their high energy densities,rapid reaction rate,ready availability and wide range of tunability[9-13].Especially,nanothermites assembled with nano-scale particles remarkably reduces the mass diffusion distance and promotes the intimate mixing[14-17]of the fuels and oxidizers,and yields an enhanced performance.For example,Aumann et al.[18]presented that Al/MoO3nanocomposites possess improved energy release rate,which was attributed to the nano-scaled particle sizes.Beside the particle size controlling,the well mixture of fuels and oxidizers should be another key point to the high performanceofthermites.Therefore,variousmethods have been employed to prepare the nanothermites,such as ultrasonic blending method[19-21],physical vapor deposition method(PVD)[22-24]and sol-gel method[25-26].However,these methods are still suffered from the low-yield,high-cost and complicated process disadvantages.
Ball milling technique[27-29]is an another typical method for preparing the composite samples,in which particles can be refinedviamechanical forces.The products can achieve the overall-increased surface area and controllable structural defects by ball milling method,which enhance the chemical activity.Therefore,the well-mixed thermites with high density could be obtained cost-effectively and massive productively through ball milling method.However,the particle size controlling together with relatively small output is still a big problem for this method.Very recently,aluminum particles with uniform nano-sized by modified ball milling method were successfully prepared[30].
Based on this experience,a series of Fe2O3-containing nanothermites with different oxide content were successfully prepared byin-situball milling method on a large scale.At the same time,the common ultrasonic blending method has also been utilized to prepare the nanothermites with the same doped amounts for comparison.The mixing uniformity,thermal behavior and hydrophobicity of asprepared products were investigated by a series of measurements and calculations.Additionally,the combustion performances between the composites preparedviatwo methods were also deeply discussed.
2 Experiments
2.1 Reagents and Instruments
The 4A molecular sieve was purchased from Sinopharm Chemical Reagent Co.,Ltd.and treated by calcinating at 400℃for 2 h in a muffle furnace.Dimethyl Sulfoxide(DMSO)was obtained from Aladdin and pre-purifiedviadecompressing distillation technique by refluxing with calcium hydride(CaH2)under a dry nitrogen(N2)atmosphere for 24 h to keep anhydrous and oxygen free.Afterwards,the distilled DMSO solvent mixed with activated 4A molecular sieve under magnetic stirring for 8 h for deeply purified.Micron aluminum powders were received from Anshan Industry Fine Aluminum Powders Company Limited.Ferric oxide(Fe2O3)was purchased from Innochem.CaH2and trimethoxy(vinyl)silane (A171)were obtained from Alfa Aesar.Cyclohexane and ammonium chloride(NH4Cl)were obtained from Aladdin.
2.2 Methods
2.2.1 Sample preparation
The loading,sampling and covering processes were all operated in a glovebox under high purity N2.The ball milling method was carried out by QM-3SP4 planetary ball-mill in the steel vials and the milling mediums were steel balls with a diameter of 5 mm.The mixture of micron aluminum powders,Fe2O3,and NH4Cl,and steel balls with 1∶50 mass ratio,were all added into the steel vials and sealed tightly,then ball milling for 14 h.In addition,the added mass loading of Fe2O3is 1%,5%,9%,13%,17%and 21%of micron aluminum powders,respectively.After ball milling process,all products were washed by purified DMSO for 3 times to remove NH4Cl.
Then,the mixture of 0.5 mL A171 and 100 mL cyclohexane were added into a two-neck flask as primed solution.Afterwards,10 g ball-milled products were added into well-mixed primed solution and stirring for one hour at 80℃.The separation of solids and liquids were executed by vacuum filtration and washed 3 times by using cyclohexane.Finally,the products were dried in the vacuum drying oven for an hour at room temperature.The ultrasonic blending method was also performed as follows:the mixture of 10 g ball-milled aluminum nanoparticles and 100 mL purified cyclohexane were added to a two-neck flask.Then,various Fe2O3(1%~21%)powders and 0.5 mL A171were added in afterwards.Subsequently,the suspension was continued ultrasonic dispersed for 1 h and the solution was removed via vacuum filtration by cyclohexane washing for 3 times.Finally,the samples were dried with the same conditions.
2.2.2 Experimental Measurements
The crystalline of as-prepared composites were performed by X-ray powder diffraction(XRD)analysis(Cu Kα)using a PANalytical Empyrean instru-ment with a range of 10°to 90°.Thermogravimetric analysis(TGA)was detected by SDT Q600 from room temperature to 850oC at the heating rate of 10℃·min-1under air flow.High speed imaging images were measured by Germany Dantec Dynamics high speed camera at a frame rate of 1500 fps and 640×480 resolution.The combustion flames temperature was simultaneously measured by Germany DIAS Infrared Systems under air atmosphere.Samples were treated by ultrasonicating in cyclohexane for 30 min and adhered on a-10 mm long Ni-Cr wire(220 μm).The metal wire was heated by energization and controlled by changing the voltage.Contact angles was conducted via JC2000C from Shanghai Zhongchen.Scanning electron microscope(SEM)were employed by Hitachi SU 8000HSD.
3 Results and discussion
3.1 Particles structure
In order to verify the structures of as-prepared nanothermites by ball milling method,the XRD was conducted and the results shown in Fig.1a.The XRD patterns of samples doped with 1%and 5%Fe2O3mainly shows the aluminum peaks(2θ=38.47°,44.74°,65.13°,78.23°and 82.44°).In addition,the weak diffraction peaks at 2θ=33.12°and 35.61°are presented,which could be attributed to the presence of Fe2O3.When the doped amounts are gradually increased up to 21%,the XRD patterns obviously identified the existence of aluminum and Fe2O3,and the intensity of the Fe2O3diffraction peaks increased by doped amounts.The similar results were also observed in the patterns of nanothermites prepared by ultrasonic blending method (Fig.1b).These resultsexhibitthatFe2O3are successfully doped in aluminum nanoparticles prepared by the two methods.
3.2 Thermal stability
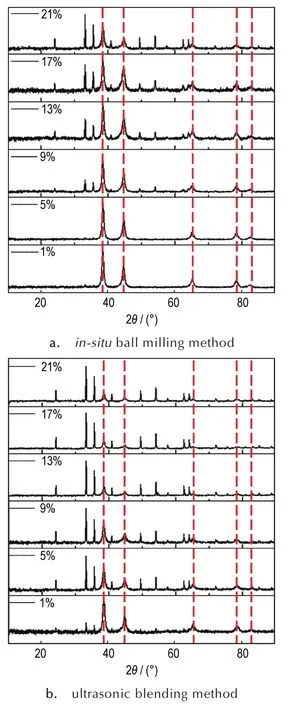
Fig.1 X-ray diffraction(XRD)patterns of nanothermites doped with different amounts of Fe2O3via different methods(Note:the peaks marked by red dotted lines represent Al peaks and the unmarked peaks are all Fe2O3peaks)
In order to compare the oxidation effect of Fe2O3on aluminum nanoparticles prepared byin-situball milling method and ultrasonic blending method,the TGA measurements are conducted(Fig.2).With regard to the nanothermites prepared byin-situball milling method,the initial oxidation temperature of all compisites gradually decreases as the Fe2O3doped amounts increase from 1%to 17%,indicating the addition of Fe2O3is indeed able to accelerate the oxidation rate of aluminum nanoparticles.However,when the doped amount increased to 21%,the initial oxidation temperature is no obvious change and the mass gain slightly decreased.Therefore,the addition of Fe2O3may bring about two ef-fects,decreasing in aluminum content and accelerating the oxidation rate of aluminum nanoparticles.As a consequence,the optimal Fe2O3doped amount of nanothermites prepared byin-situball milling method for accelerating the oxidation rate is 17%.As for the nanothermites prepared by ultrasonic blending method,the optimal product for promoting oxidation of aluminum nanoparticles is the nanothermites doped with 5%Fe2O3(Fig.2b)and the oxidation rate gradually decreases as the doped amounts increase when the Fe2O3doped amounts exceed 9%,indicating that the mixing degree of the nanothermites prepared by the ultrasonic blending method is not so similar as that ofin-situball milling method.
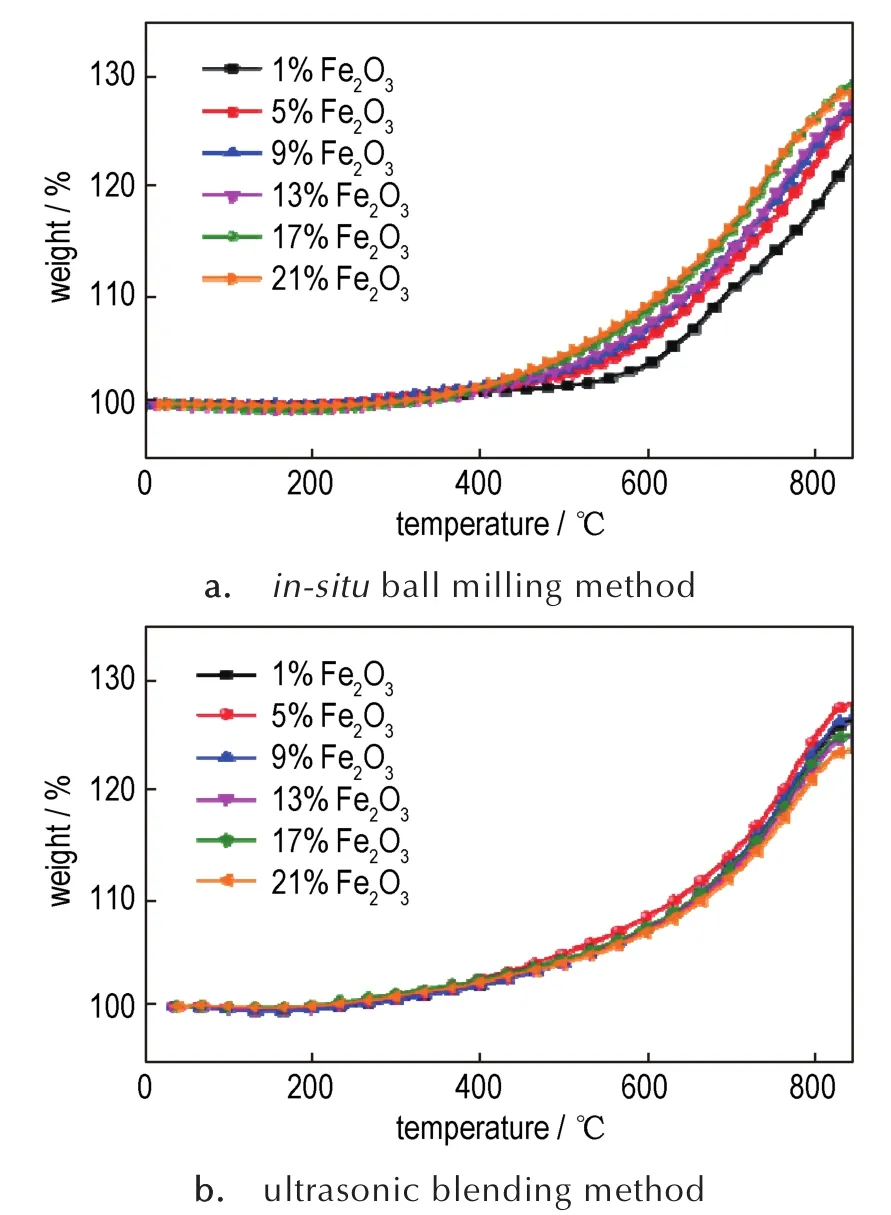
Fig.2 TGA curves of nanothermites doped with different amounts of Fe2O3via two methods
To compare and theoretically analyze the rising rate of the TGA curves of nanothermites obtained from two methods,fitting functions were employed by Origin software[31]and the results are shown in Fig.3,Fig.4 and Table 1.The fitting functions ofinsituball-milled nanothermites and ultrasonic-blended nanothermites were set tof(Bx)andf(Sx),respectively,and the fitting curves derivatives were set torespectively.Given that the curves fitting and calculation processes of different doped amounts of Fe2O3are similar,so the TGA curves of doped 17%Fe2O3ball-milled composite and doped 5%Fe2O3ultrasonic-blended composite were selected and investigated.The functions of the fitting curves are presented as follows:
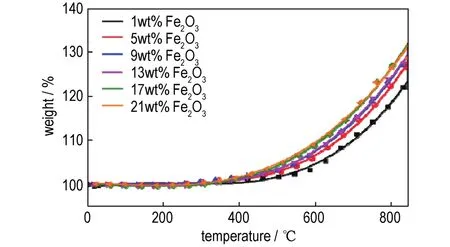
Fig.3 TGA fitting curves of nanothermites doped with different amounts of Fe2O3prepared by in-situ ball milling method.
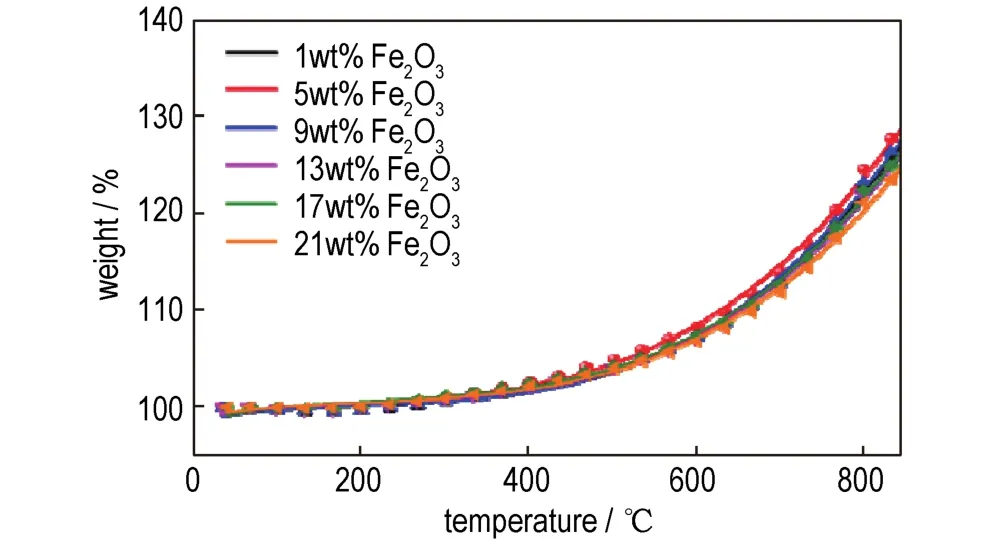
Fig.4 TGA fitting curves of nanothermites doped with different amounts of Fe2O3prepared by ultrasonic blending method.
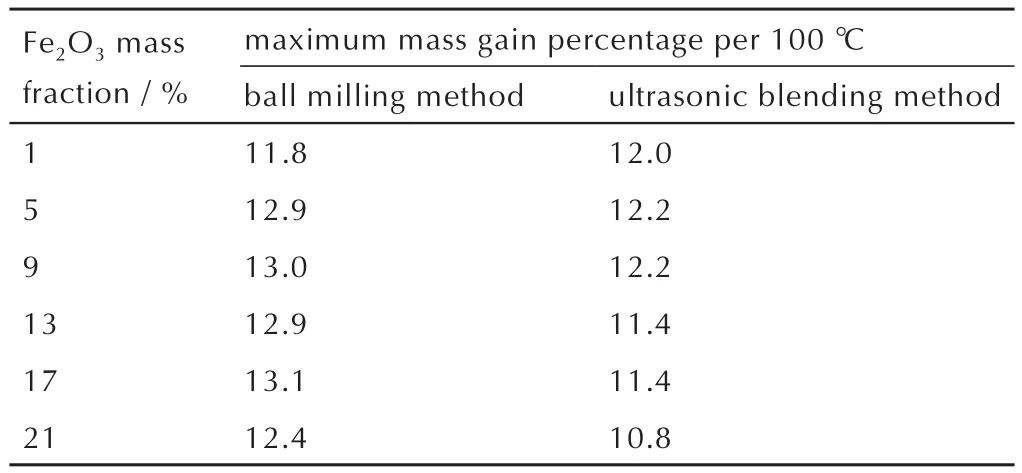
Table 1 Maximum rising rate(850℃)of TGA fitting curves derivatives of nanothermites prepared by in-situ ball milling method and ultrasonic blending method %
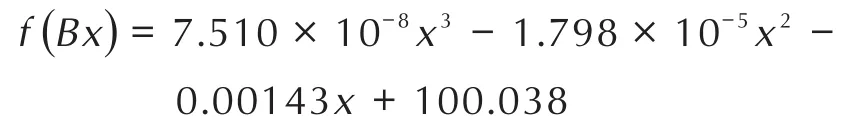
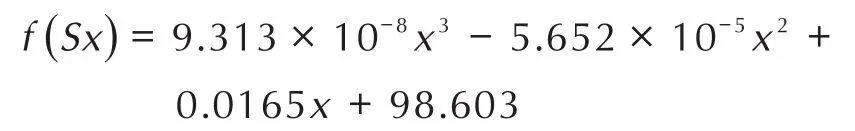
For the purpose of obtaining the rising rates of the fitting curves,the derivative functions of abovementioned functions are calculated as follows:
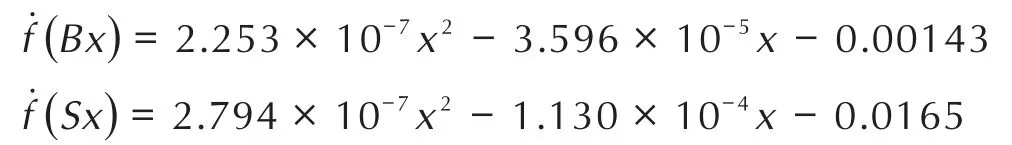
Therefore,the maximum rising rate of TGA fitting curves were calculated at 850℃and the oxidation rates of all samples were reflected by mass gain percentage per 100℃.Obviously,the maximum mass gain percentage per 100℃of optimalin-situball-milled nanothermites was 13.1%.The maximum mass gain percentage per 100℃of ultrasonicblended nanothermites was only 12.2%,indicating the two fitting curves with 5%and 9%doped composites shown similar oxidation rates.However,the mass gain of the 5%doped composite is higher than that of 9%,illustrating the oxidation degree of nanothermites doped with 5%Fe2O3is more complete than that of nanothermites doped with 9%Fe2O3.
As a consequence,the optimal doped amounts of nanothermites prepared by the two methods were 17%and 5%for ball-milled and ultrasonic-blended composites,respectively,and thein-situball-milled nanothermites possess higher oxidation rate in comparison with the ultrasonic-blended nanothermites,which is consistent with the results presented by TGA curves.
3.3 Microscopic morphology
To exhibit the particles size,element distribution and microscopic morphology of nanothermites prepared by the two methods,scanning electron microscope(SEM)and mapping scanning of energy dispersive spectrometer(EDS)analysis were conducted and the results are presented in Fig.5.
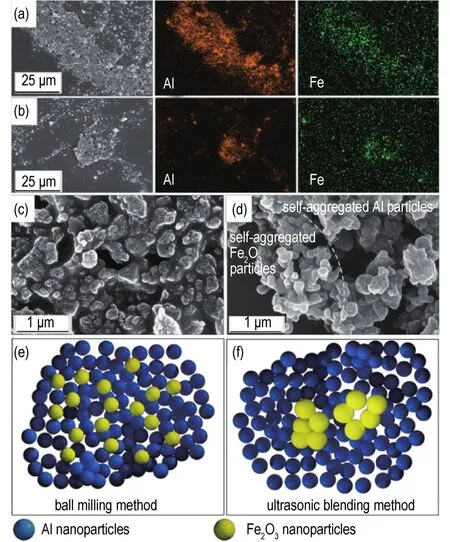
Fig.5 Microscopic morphology and element distribution of in-situ ball-milled and ultrasonic-blended nanothermites:(a)Element distribution of in-situ ball-milled nanothermites doped with 17%Fe2O3from EDS mapping;(b)Element distribution of ultrasonic-blended nanothermites doped with 17%Fe2O3from EDS mapping;(c)corresponding SEM images of in-situ ball-milled nanothermites doped with 17%Fe2O3;(d)corresponding SEM images of ultrasonic-blended nanothermites doped with 5 Fe2O3;(e)Schematic diagram of in-situ ball-milled nanothermites doped with 17%Fe2O3 and(f)Schematic diagram of ultrasonic-blended nanothermites doped with 5%Fe2O3.
Obviously,aluminum and iron elements in the prepared nanothermites are both displayed clearly,(Fig.5a and Fig.5b),which is in agreement with the XRD results.The enlarged SEM image and schematic diagram ofin-situball-milled nanothermites doped with 17%Fe2O3are shown in Fig.5c and Fig.5e,illustrating the ball-milled particles are indeed nanosized particles and spherical.However,the self-agglomeration phenomenon can be observed in the enlarged SEM image and schematic diagram of ultrasonic-blended nanothermites,indicating uneven mixing of Al and Fe2O3particles(Fig.5d and Fig.5f)and greatly reduced mixing intimacy.As a consequence,Al and Fe2O3particles of ball-milled nanothermites dispersed more uniformly than that of ultrasonic-blended nanothermites.
3.4 Hydrophobicity
The hydrophobicity of nanothermites prepared by two methods were investigated and the contact angles were detected and the results presented in Fig.6.
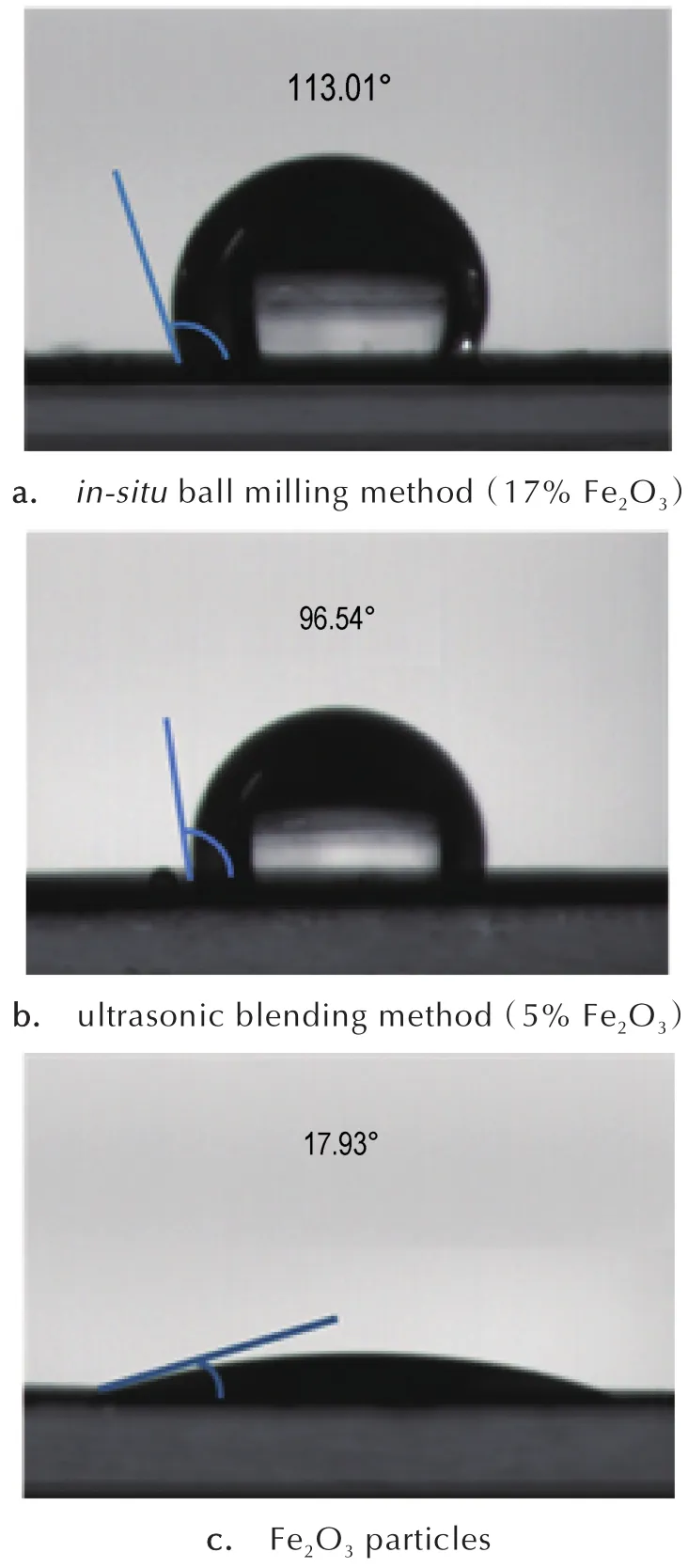
Fig.6 Contact angles of nanothermites doped different amounts of Fe2O3via different methods.
Clearly,the contact angles of nanothermites obtained from ball milling method,ultrasonic blending method and pristine Fe2O3are 113.01°,96.54°and 17.93°,respectively.The result could be ascribed to the exposure of Fe2O3nanoparticles to the surrounding atmosphere and water,stemming from the uneven mixing of Al and Fe2O3particles.In contrast,the contact angle ofin-situball-milled nanothermites doped with 17%Fe2O3(113.01°)is much larger than that of the ultrasonic-blended composite,indicating thatin-situball-milled composites possess better hydrophobicity than that os ultrasonic-blended composites.Therefore,in-situball-milled nanothermites are more conducive to preservation in comparison to ultrasonic blended nanothermites.[32]
3.5 Combustion performance
To obtain the real-time flame temperature and combustion phenomena of nanothermites prepared by the two methods,infrared temperature measurement and high-speed imaging experiments were conducted on two compisites.
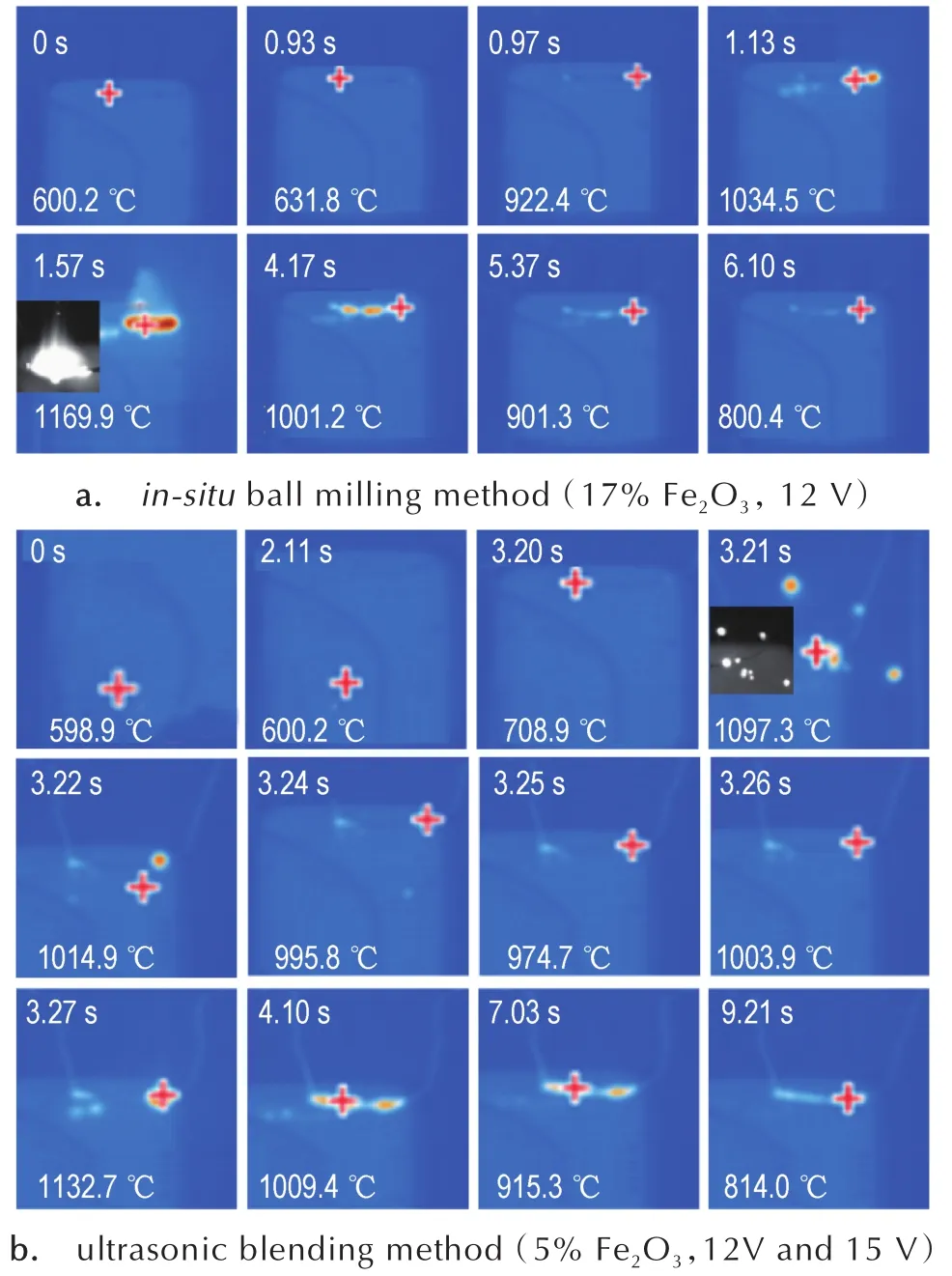
Fig.7 Images of real-time infrared temperature measurementofnanothermitesprepared via differentmethods.(Note:The cross symbols(+)represent the tracked automatically highest temperature points.)
As shown in Fig.7,the initial heating voltage is 12 V and the initial heating temperature is around 600℃.For thein-situball-milled composite,the temperature slowly increases to approximately 630℃,indicating the passivation agent starts to rupture and partial aluminum nanoparticles of nanothermites begin to be oxidized.Then the temperature rapidly increases over 900℃ within 0.4 s,pointing to the oxidation of massive aluminum nanoparticles of nanothermites and heat release.Hereafter,the temperature continued to increase up to about 1170℃and the combustion flame is stable.In con-trast,as for the ultrasonic-blended compoiste,it is difficult to be ignited at 12 V voltage,so higher voltage(15 V)was applied for the ignition.At this time,the composite rapidly reacts and the temperature rises up to nearly 1100℃.With the rapid increase of temperature,the nanothermites prepared by ultrasonic blending method exist the phenomenon of particle sputtering and the temperature dropped rapidly or even fell below 1000℃.This is because the aluminum nanoparticles and the Fe2O3contained in nanothermites prepared by ultrasonic blending method are seriously self-aggregated,resulting in inhomogeneous mixing and unstable combustion.Consequently,the initial combustion temperature of the nanothermites prepared byin-situball milling method is lower than that of the nanothermites preparedviaultrasonic blending method,and the combustion flame is more homogeneous and stable.
4 Conclusions
(1)The Fe2O3powders and aluminum particles of nanothermites preparedviaball milling method are mixed more evenly,nevertheless,the nanothermites prepared by ultrasonic blending method exist clearly distinguished respective agglomerated aluminum particles and Fe2O3particles.
(2)The contact angle of the nanothermites prepared byin-situball milling method is 113.01°,which is significantly larger than that of corresponding ultrasonic blending method(96.54°).With the optimal doped Fe2O3amount(17%)byin-situball milling,the maximum mass gain percentage per 100℃ is 13.1%,which is larger than that for ultrasonic-blended doped 5%Fe2O3product(12.2%).
(3)Thein-situball milled nanothermites could be ignited at lower heating voltage(12 V)and lower initial combustion temperature(~600℃)than those of ultrasonic-blended nanothermites(15 V and~700℃),and the combustion flame is more stable and homogeneous.This investigation is expected to promote the development of nanothermites.

