Successful treatment of hepatic hydrothorax: A case report
Mariam Jmal, Maroi Dammak, Olfa Chakroun-Walha, Noureddine Rekik
Emergency Department, University Hospital Habib Bourguiba Sfax, Tunisia
ABSTRACT
KEYWORDS: Hepatic hydrothorax; Management; Chest tube; Medical treatment
1. Introduction
Cirrhosis is a serious liver disease with potentially life-threatening complications. These complications include gastrointestinal bleeding related to portal hypertension, encephalopathy, ascites fluid infection, and hydrothorax. We reported a case of a less known complication, hepatic hydrothorax that was successfully treated without surgery. Hepatic hydrothorax is a pleural effusion with no associated cardiac or renal explanation. Its mechanisms are still debated. Surgery is the common management.
2. Case report
Informed consent was obtained from the patient, and the ethics approval was authorized by the Institutional Ethics Committee prior to reporting this case. A 51-year-old patient was presented to the emergency room for increasing breathlessness. This patient had hepatic cirrhosis secondary to hepatitis B, prolonged consumption of alcohol, and metabolic syndrome. His diagnosis was retained six months earlier; since then, he had been treated by diuretics and beta-blockers. When he voluntarily stopped his treatments for 40 d, the symptoms relapsed.
His medical history began a month prior to his admission to the emergency room. He had developed edema in the lower limbs with dyspnea. He was first hospitalized in a polyclinic, where an abdominal ultrasound was performed. It showed signs of chronic liver disease with portal hypertension and abundant ascites. The chest X-ray revealed a massive right pleural effusion with a chest tube. The gastroscopy showed grade 2 esophageal varices of the lower third of esophagus without any bleeding. Therefore, ascites fluid punctation was performed, which showed sensitivity Escherichia coli. The blood analysis showed a decrease of the prothrombin ratio (38%; normal range: >70%), an elevation of the INR (2.04; normal range: >1), thrombocytopenia (7 000 elemts/mm3; normal range: 150 000-500 000 elemts/mm3), hepatic cholestasis, hepatic cytolysis, hyperammonemia (117 mmol/L; normal range: <25 mmol/L), hyponatremia (128 mmol/L; normal range: 135-145 mmol/L), and hypoproteinemia (51 g/L; normal range: 60-70 g/L).
A chest tube was placed at the clinic, which drained 4 L of pleural fluid (Figure 1), and then, the patient was transferred to the emergent room. On admission, the patient was confused (GCS=14). His hemodynamic state was stable (blood pressure=110/90 mmHg; pulse rate=80 b/min). He had edema in the lower limbs and a bloated abdomen.
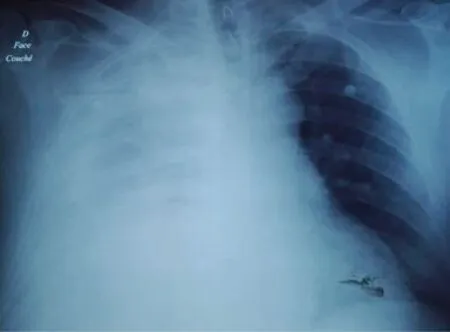
Figure 1. Chest X-ray showing a massive right pleural effusion with a chest tube on admission to the emergency room.
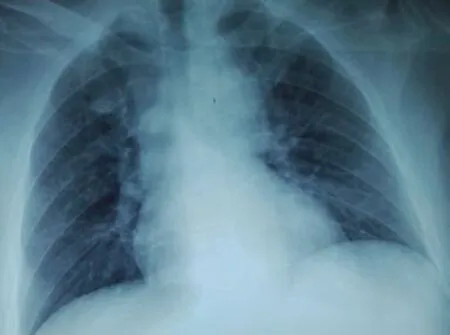
Figure 3. A dried-up pleural fluid 3 d after the increase of the doses of diuretics with a strict salt-free diet.
A chest and abdominal CT scan showed a massive right pleural effusion, cirrhosis, ascites, and splenomegaly. The two causes of decompensation were non-adherence to medication and ascites fluid infection. The patient was given diuretics (furosemide, spironolactone) and antibiotic treatments. He was also given human albumin.
Despite the improvement of the patient’s general state, at that time the medical staff was still unable to remove the chest tube due to the persistence of the effusion (Figure 2). The patient was treated with increased doses of diuretics and a strict salt-free diet. After 3 d, the pleural fluid dried up. An attempt was made to clamp the chest tube and its ablation was successfully performed. A chest control radio was very satisfactory (Figure 3). The patient was discharged 2 weeks later with high doses of diuretics and a strict salt-free diet. A clinical and radiological check after one week was satisfactory (Figure 4). The patient is currently well.
3. Discussion
Hepatic hydrothorax is defined as a pleural effusion of more than 500 mL secondary to portal hypertension in cirrhotic patients without any significant underlying cardiac, pulmonary or pleural disease[1]. The localization of such an effusion is predominantly right-sided (85%), yet, followed by left-sided (13%) and bilateral (2%)[2].

Figure 2. A persisting pleural effusion after the chest tube ablation.
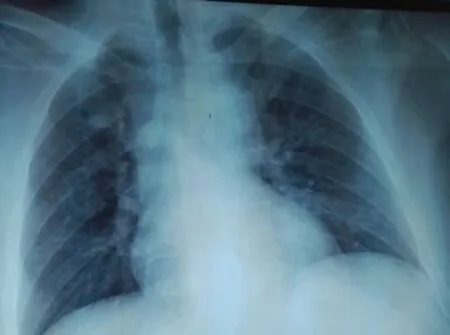
Figure 4. A satisfactory radiological check one week after discharge from the emergency room.
Several hypotheses have been cited to explain the mechanism of hydrothorax in cirrhotic patients[3-6]. The reason can be a hypoalbuminemia causing a decrease in oncotic pressure; plasma leakage from large anastomoses between the portal system and the azygous venous system; leakage from the thoracic duct; passage of ascites in the pleural cavity through trans-diaphragmatic lymphatic or passage of ascites from the peritoneal cavity into the pleural space through diaphragmatic breaches, which was the pathogenesis of our case.
The size of the membranes’ gaps varies from 0.03 to 5 or 6 mm in diameter, which makes their detection difficult. They are located more on the right diaphragmatic cupola because of its weak resistance compared to the left cupola[3-6]. These gaps are covered on one side by the peritoneal membrane and on the other by the pleura[4]. In our case, an abdominal hyper pressure secondary to the formation of ascites, and these membranes’ gaps generate peritoneal-pleural communication[3]. Therefore, the passage of the liquid of ascites towards the pleural cavity is made under the effect of a hydrostatic pressure gradient[3].
Furthermore, the negative intrathoracic pressure relative to the peritoneal space perpetuates the sequestration of ascitic fluid preferentially into the thoracic cavity[4]. The semiology of hydrothorax varies from the absence of respiratory symptoms to respiratory distress in case of abundant effusion or rapid constitution which requires prompt evacuation[3,6]. Therapeutic modalities for these patients are based on the following approaches. The administration of diuretics is the first step of the management to keep a negative sodium balance. A restricted sodium diet (less than 90 mmol/24 h) combined with a diuretic therapy should be prescribed[3,4,6]. However, pleural fluid will only be mobilized once ascites formation has been controlled[3]. Several studies have recommended the combination of spironolactone and furosemide[4,6]. In most cases, these measures would be sufficient. In the rare cases of failure of these alternatives, other measures must be proposed[3,4,6].
Thoracocentesis is recommended if urgent drainage of hydrothorax is required[4,6]. A chest X-ray is sufficient to assess the volume of effusion and the effectiveness of pleural punctures. Thoracocentesis can be performed on an outpatient basis[6]. The main risk of pleural punctures is pneumothorax[7]. Other complications are rare, including vagal discomfort, empyema, hepatic or splenic injury, hemoptysis, air embolism, and/or subcutaneous emphysema[4]. In the cases of therapeutic pleural puncture failure, other treatments should be considered.
Several studies have stated that chest tube insertion should be avoided as it may lead to prolonged drainage and increase the risk of empyema, massive fluid, and protein loss. This was reported by Runyon et al. in two cases[8]. In the study, the placement of the pleural drain resulted in a massive loss of proteins and electrolytes and consequently, the death of the patients[8]. In addition, it is very difficult to remove the chest tube due to the ascites leakage along the drain path after removal[6,8]. In our case, the patient was admitted to the emergency room with a chest tube already installed at a private clinic. It was so difficult to remove, which made us reinforce the diuretics treatment (increased doses of spironolactone from 75 mg to 225 mg/d, and furosemide from 40 mg to 120 mg/d). This pharmacological treatment was associated with a strict saltfree diet and close monitoring of the patient’s hemodynamic state. Eventually, when the pleural fluid dried up, the chest tube was removed successfully.
Trans-jugular intrahepatic portosystemic shunt (TIPS) involves the creation of a communication between a hepatic vein and a branch of the portal vein[9]. This communication is kept open by laying an expansive metal prosthesis. By decreasing the portal pressure, this device will cause a decrease in splanchnic arterial vasodilation and an improvement of arterial vascular filling[9]. Consequently, it decreases extravasation, ascites formation and, thus, hydrothorax.
The complications of TIPS are numerous, including immediate complications such as perforation of the Glisson capsule, intraperitoneal hemorrhage, hemobilia, infection, hemolysis, and late complications such as thrombosis, occlusion of the shunt, etc. Installation of polytetrafluoroethylene prosthesis could reduce this risk[10].
The development of hepatic encephalopathy occurs in about 30% of the patients after the insertion of TIPS[9]. The risk is higher before shunt insertion and in patients over 60 years or with a history of hepatic encephalopathy[9].
In conclusion, hepatic hydrothorax is a serious complication of cirrhosis related to portal hypertension and hepatocellular insufficiency. The medical treatment includes diuretics and a strict salt-free diet. Thoracocentesis is recommended if urgent drainage is required. Chest tube should be avoided because of its numerous complications. TIPS is effective in about 70% of the cases, but it needs to be better evaluated. However, liver transplantation remains the definitive treatment for refractory hydrothorax.
Conflict of interest statement
The authors report no conflict of interest.
Authors’ contribution
O.C.W. is responsible for the concept, designing and intellectual content. M.J. and M.D. are responsible for the manuscript preparation. O.C.W. and N.R. are responsible for manuscript editing and review.
 Journal of Acute Disease2020年3期
Journal of Acute Disease2020年3期
- Journal of Acute Disease的其它文章
- Knowledge, attitude, and practice towards coronavirus disease 2019 (COVID-19) among medical students: A cross-sectional study
- Factors affecting outcomes of surgically treated patients with cranial extradural hematoma: A cross-sectional study
- Clinical characteristics and treatment of acute epiglottitis: A retrospective study of 28 cases
- Aberration detection of pertussis from the Mazandaran province, Iran, from 2012 to 2018: Application of discrete wavelet transform
- Identification of Panton-Valentine leukocidin virulence gene in methicillinresistant Staphylococcus aureus isolated from clinical specimens of burn patients in Zare Hospitals of Sari, Iran
- Suicide attempt of an overt hypothyroid patient with levothyroxine: A case report
