Precise base editing of non-allelic acetolactate synthase genes confers sulfonylurea herbicide resistance in maize
Ynmin Li,Jinjie Zhu, Ho Wu, Chnglin Liu, Chngling Hung,Jinho Ln,Ynming Zho, Chunxio Xie,*
aInstitute of Crop Sciences,Chinese Academy of Agricultural Sciences,National Key Facility for Crop Gene Resources and Genetic Improvement,Beijing 100081,China
bQingdao Agricultural University, Qingdao 266109,Shandong,China
cAnhui Agricultural University, Hefei 230036,Anhui,China
ABSTRACT
1. Introduction
Maize(Zea mays L.)is one of the most important cereal crops,providing feed,industrial feedstock,and human food. Singlenucleotide polymorphisms (SNP), found in numbers exceeding 10 million in the maize genome,contribute to phenotypic diversity in maize [1,2]. Reverse genetic approaches to recovering targeted point mutations from random mutations(e.g., targeting induced local lesions in genomes,TILLING)are time-consuming and limited by low efficiency [3,4]. Development of more efficient technologies for creating targeted base mutations is a promising approach to resolving SNP variation for both functional genomics and genetic improvement in breeding applications. CRISPR/Cas9 (clustered regularly interspaced short palindromic repeats/CRISPR-associated Cas9),a class 2 bacterial adaptive immune system,is a robust genome editing tool [5] in a variety of organisms including maize[6-9].Programmable base editing of a target cytosine(C)into thymine (T) by fusion of CRISPR/Cas9 with a cytidine deaminase enzyme allows C-to-T (or G-to-A in the antisense strand)substitution[10].Targeted A-to-G base editing has also been recently [11] reported. These targeted conversions of Cto-T [12,13] and A-to-G [14-16] base editing are being rapidly adopted for higher plants, providing precise single-base editing technology for plant mutation breeding. To date, for maize, only one example of single C-to-T editing in the gene ZmCENH3 has been reported in a regenerated plant [13].Further improvement of precise target base-editing efficiency will facilitate precise plant breeding based on SNPs.
Weeds are responsible for over 34% of crop yield loss per year worldwide[17].Cultivation of herbicide-resistant crops is an environmentally friendly and economical approach to weed control in crop production systems. Breeding for herbicide resistance by screening for resistant mutants arising by natural variation [18] is tedious and is limited by both germplasm resources and genetic diversity.
Genetic modification by introduction of exogenous resistance genes into plants has been the main strategy for breeding herbicide-resistant crops in the past few decades[19-21]. However, food safety and environmental and biosafety management procedures for genetically modified organism (GMO) products are cumbersome, time-consuming,and costly, severely limiting the wide application of this approach [22]. Although safety regulation of genome-editing products is still controversial [23], edited plants requiring no introduction of exogenous genes[24]are not regulated,giving them an advantage over plants generated by transgenic approaches.
Acetolactate synthase(ALS)catalyzes the initial step in the biosynthesis of branched-chain amino acids,including valine,leucine and isoleucine [25]. ALS inhibitors are the most commonly used herbicides, and can be classified into four distinct chemical categories: sulfonylureas, imidazolinones,triazolopyrimidine sulfonanilides, and pyrimidinyl oxybenzoates [18]. A one-amino acid substitution in ALS in transgenic tobacco plants left them highly resistant to sulfonylurea herbicide application [25]. The mutation at a corresponding location was tested in several plant species including Arabidopsis[26],soybean[27]and maize[9].Targeted mutation of ALS using homology-directed repair (HDR) strategy has been attempted in both maize[9] and rice [28].There are two genes for acetolactate synthase in the maize genome:ZmALS1 and ZmALS2 [2]. An HDR system yielded a very low efficiency of 0.2%-0.4% for ZmALS2 and failed to recover a ZmALS1 mutant [9]. For maize, a species that is difficult to transform,this reported efficiency is too low for practical use.
We employed C-to-T base editing technology to introduce target amino acid substitution mutations in the two nonallelic ALS genes.By virtue of the property that a thymine base could also pair with a uracil, conversion of cytidine to uracil using cytidine deaminases can eventually result in C-to-T mutation on DNA at a target locus without requiring DSBs[10].Our expectation was to achieve a C-to-T base-editing activity window, resulting in a serine amino acid substitution at proline 165.
2. Materials and methods
2.1. Plasmid construction
To generate a CT-nCas9 vector backbone, a maize ubiquitin promoter, APOBEC1, nCas9 (D10A), and UGI were amplified using a 15-20 bp overlapping primer, then assembled using In-fusion cloning methods with a pEASY-Uni Seamless Cloning and Assembly Kit (TransGen Biotech, Beijing,China). A 20-bp base-editing target in both ALS1 and ALS2 was designed and cloned into an sgRNA backbone driven by our previously reported [29] Pol III promoter. This APOBEC1-XTEN-nCas9(D10A)-UGI fusion sequence was constructed into the CUB vector. The nuclear localization signal sequences of SV40 and nucleoplasmin were embedded in either ends of APOBEC1-XTEN-nCas9(D10A)-UGI.Then it was cloned into CT-nCas9 vector backbone to generate a CT-nCas9 vector. All primer sets used in this study are listed in Table S1
2.2. Maize protoplast preparation and transfection
The maize inbred line B73 was used for protoplast isolation and transfection following a protocol described previously[30]with slight modification. For protoplast isolation, B73 seeds were sterilized with 10% (v/v) H2O2for 30 min, washed with distilled water,sown in soil,and grown under a 16 h-light and 8 h-dark cycle at 28°C in a growth chamber until 1-cm shoots were visible.Seedlings were then moved to a dark room until the second leaf was 10-15 cm in length. The middle parts of the second leaves were cut into 1-mm strips and digested with enzyme solution (1.5% cellulase, 0.4% macerozyme R10,0.4 mol L−1mannitol, 20 mmol L−1KCl, 20 mmol L−1MES at pH 5.7, 10 mmol L−1CaCl2, 0.1% BSA, and 5 mmol L−1 βmercaptoethanol), and then subjected to vacuum infiltration for 30 min. After digestion for 3-4 h at 28 °C in darkness with gentle shaking(40 r min−1),protoplasts were filtered through a 30 μm nylon mesh and collected in a round-bottomed tube by centrifugation for 2 min at 100×g.They were washed with W5 solution, resuspended in W5 solution, held on ice for 30 min,and resuspended in MMG solution to a final concentration of 1× 106protoplasts mL−1.
For PEG-mediated recombinant plasmid transformation,2×105protoplasts(200 μL)were mixed with 20 μL base-editing plasmid (15 μg), and then gently mixed with 220 μL PEG solution(40%(W/V)PEG 4000,100 mmol L−1CaCl2,0.6 mol L−1mannitol) and incubated in the dark at room temperature for 18 min. Transfections were stopped by addition of 800 μL W5 solution and washed once with W5 solution. Finally, protoplasts were incubated in 1 mL W5 solution in the dark at 28°C for 48 h.
2.3. Maize transformation
The CT-nCas9 construct was delivered into the maize inbred line ZC01 using Agrobacterium-mediated immature embryo transformation as described previously [8]. Briefly, the CTnCas9 vector was transformed into A. tumefaciens strain EHA105 and then used to infect 1.8-2.2 mm-long immature maize embryos. Submerged embryos were gently mixed for 15-20 min and then transferred to N6 co-cultivation medium and incubated in the dark at 26°C for 3 days.They were then transferred to subculture medium once every two weeks until the formation of embryonic callus. Resistant embryonic calluses were transferred to regeneration medium under a 16/8 h photoperiod with a light intensity of 2000 lx until both shoot and root developed, and then transplanted to the greenhouse.
2.4. Mutation detection
For mutation detection, genomic DNA was extracted from transformed protoplasts or from transgenic T1 or T2 plants using the CTAB method. PCR amplification was performed with primers designed to amplify the genomic region containing the Cas9 target site. For mutation detection in protoplasts, PCR products were purified with a QiaQuick Spin Column Kit (QIAGEN, Hilden, Germany) following the manufacturer's protocol. They were pooled and quantified with a Qubit 3.0 Fluorometer (Thermo Fisher Scientific, Waltham,USA), and were then used for construction of libraries for next-generation sequencing using the TruePrep DNA library Kit V2 for Illumina (Vazyme, Jiangsu, China). For mutation detection in T1 and T2 plants,PCR products were subjected to Sanger sequencing or Nru I (New England Biolabs International, Beijing, China) digestion following the manufacturer's instructions. Digested products were resolved by 1% agarose gel electrophoresis and visualized by YeaRed Nucleic Acid(Yusheng Biotech.,Shanghai,China)staining.
2.5. Off-target detection
Putative off-target sites, which contained 2-4 nucleotide mismatches relative to the ZmALS target site, were identified by Cas-OFFinder(http://www.rgenome.net/cas-offinder/).Five potential off-target sites of ZmALS (Table S2) were sequenced to screen off-target mutation using Sanger sequencing.
2.6. Herbicide resistance test assay
To detect the effective level of chlorsulfuron (Bio Basic Inc.,Markham, Canada), Seedlings in the greenhouse (28 °C, 16 h light; 23 °C, 8 h dark) of wild type ZC01 at three-week stage after sowing were sprayed with various concentrations of chlorsulfuron. The effective concentration of chlorsulfuron was determined by the obvious death of wild-type seedlings.Base-edited plants with homozygous or heterozygous mutations in ALS alleles were then used in the herbicide-resistance assay. Seedlings in the greenhouse at the three-week stage were treated with a concentration of chlorsulfuron(100 mmol L−1) about 5 times higher than field usage. Wildtype and mutant plants were placed in the same pot to avoid environmental differences.Photos of plants were taken about one month after the herbicide and non-herbicide treatments.At least 3 biological replicates were used for each treatment.
2.7. Herbicide resistance of acetoacetate synthase variants in vitro
Individuals homozygous for each specific ALS mutations and wild type were grown in the greenhouse (28 °C, 16 h light;23 °C, 8 h dark). Acetoacetate synthase was extracted from seedlings at the 4-5-leaf stage. In vitro activity of ALS was assayed following Ray[31].Briefly,an enzyme suspension was prepared by homogenizing 5 g leaf material in 15 mL enzyme extraction buffer (50 mmol L−1K2HPO4-KH2PO4, pH 7.0,0.5 mmol L−1MgCl2, 0. 5 mmol L−1TPP, 1 mmol L−1DTT,1 mmol L−1sodium pyruvate, 10 μmol L−1FAD), followed by(NH4)2SO4precipitated and pellet dissolved in buffer(50 mmol L−1K2HPO4-KH2PO4, pH 7.0, 0.5 mmol L−1MgCl2,20 mmol L−1sodium pyruvate).
The herbicide resistance of acetoacetate synthase variants was measured by addition of the enzyme extracts to reaction buffer (50 mmol L−1K2HPO4-KH2PO4, pH 7.0, 0.5 mmol L−1 MgCl2, 20 mmol L−1sodium pyruvate, 0.5 mmol L−1TPP,10 μmol L−1FAD) in the presence or absence of chlorsulfuron(10 nmol L−1). After incubation at 37 °C for 1 h, H2SO4was added to a final concentration of 0.3 mol L−1to stop the reaction.The reaction was then incubated at 60°C for 15 min to allow the decarboxylate of acetolactate, after which 0.5%creatine and 5%α-naphthol were added to the mixture for the color change reaction. Finally, absorbance was measured at 525 nm. All assays were performed with at least three biological replicates.
3. Results
3.1. Targeted C-to-T base editing vector design and construction
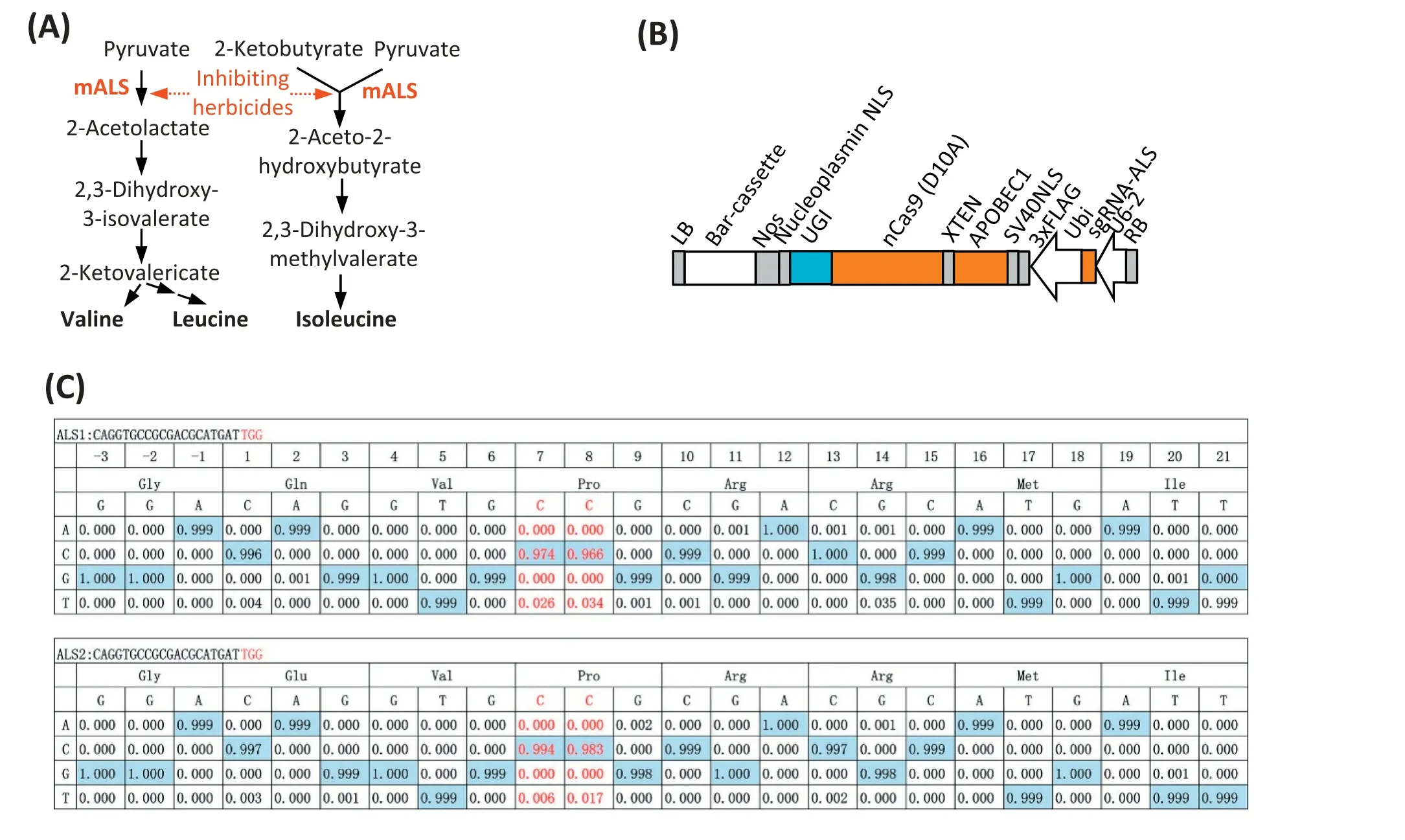
Fig.1-Schematic mechanism of base editing of ALS genes resulting in sulfonylurea herbicide resistance,base-editing vector construction,and its activity validation in maize protoplasts.(A)Schematic mechanism of base editing in ALS genes resulting in sulfonylurea herbicide resistance.mALS,ALS mutant variants were recovered from herbicide inhibition due to targeted mutation.Red arrow with dashed tail:the sulfonylurea herbicide inhibition effect on ALS was released due to the target mutation.(B)Construct of targeted C-to-T base editing vector(CT-nCas9)that targets ZmALS genes.APOBEC1,rat cytidine deaminase;Bar cassette,a CAMV35S promoter-driven BlpR gene with CaMV poly(A)termination signal;LB,T-DNA left boarder;NOS,Nos terminator; RB,T-DNA right boarder;U6-2,ZmU6 PolIII promoter;Ubi,Zm-Ubiquitin 1 promoter;UGI,Uracil Glycosylase Inhibitor;XTEN,residue peptides;nCas9(D10A),SpCas9 variants of D10A,Asp10Ala,a nickase Cas9 without RuvC activity.(C)Evaluation of CT-nCas9 targeting base editing activity on ZmALS1 and ZmALS2 in maize mesophyll protoplasts.Cytosine nucleotide at site 7 and 8 within expecting window from 4 to 8 were targeted.
As shown in Fig. 1-A, the objective of the design was to precisely edit the ALS gene to leave low or no affinity for sulfonylurea herbicide but retain the synthase activity of branched amino acids. Toward this end, a maize baseediting vector was constructed (Fig. 1-B) based on a similar design from a mammalian system. The rat cytidine deaminase enzyme APOBEC1 was to the N terminus of SpCas9-D10A,which is a DNA nickase variant without RuvC activity owing to Asp10Ala (D10A) substitution. ZmALS-sgRNA was designed to target both the ZmALS1 and ZmALS2 genes and sgRNA was expressed using our previously characterized PolIII promoter [29]. In order to achieve the C-to-T baseediting activity similar to that reported [10], a 16-residue XTEN linker was inserted between nCas9 (D10A) and APOBEC1. This linker provides an efficient cytidine deamination activity window of approximately 5 nt,typically from positions 4 to 8 within the protospacer, counting the base distal from the protospacer-adjacent motif(PAM)as position 1 (Fig. 1-C). A uracil DNA glycosylase inhibitor (UGI) was fused to the C terminus of nCas9 (D10A) to subvert baseexcision repair initiation at the local nicking site.The nuclear localization signal sequence of SV40 and nucleoplasmin were embedded at either end of APOBEC1-XTEN-nCas9(D10A)-UGI. This APOBEC1-XTEN-nCas9 (D10A)-UGI fusion gene driven by the maize ubiquitin-1 (Ubi) promoter was incorporated into a CUB backbone vector[6,8].The guide RNA expression cassette along with an additional Bar expression cassette for bialaphos selection was used. In short, all those elements were embedded as our CT-nCas9 vector,which was expected to achieve target C-to-T base editing within the designed activity window.
3.2. Evaluating base-editing activity of CT-nCas9 in maize protoplasts
Targeted C-to-T base editing activity was assessed for 2 Cs in the target window from site ranges of 4-8 in both ZmALS1 and ZmALS2 (Fig. 1-C). Maize mesophyll protoplast cells transiently transformed with the CT-nCas9 vector were harvested for DNA extraction and genotyped using next-generation sequencing(NGS). As shown in Fig. 1-C, a C-to-T transition was achieved within the“deamination window”,accounting for 2.6%at C7and 3.4%at C8of the total sequenced reads on ZmALS1.However,the base-editing efficiency in ZmALS2 was 0.6%at C7and 1.7%at C8,much lower than in ZmALS1. Some unexpected mutations, for example C-to-G and C-to-A transversions, were also found in fewer than 0.02%of reads(Table S3).

Fig.2-Targeted base editing of ZmALS1 conferred chlorsulfuron herbicide resistance in maize.(A)The target site for ZmALS by CT-nCas9.The target sequence of ZmALS-sgRNA is shown and the PAM DNA sequences are underlined.Gray-shaded sequences indicate an introduced NruI restriction site derived from the expected edited C7 →T7 substitution mutation.The nucleotide number is counted from the end distal to the protospacer-adjacent motif(PAM)as position 1 in the sgRNA associated region).T(G),T SNP at −17 site indicates ALS1 and G SNP indicates ALS2 allele.(B) PCR-RE(NruI)assay for efficient identification of T2 homozygous C7 →T7 substitution mutant plants.Only the homozygous mutation sequences were cleaved into two bands;the heterozygous mutations sequence were partially cleaved.Other mutations and the wild type were not cleaved.+,positive control of artificial PCR created C7 →T7 single based substitution mutation M,DNA size marker;WT,wild type negative control.(C)The observed four different base-edited mutations and their frequencies among the 16 mutant plants.The expected target C →T base editing activity window is indicated in light blue shading.(D)Chlorsulfuron resistance experiment among T2 mutant individuals(on left in pods)harboring homozygous C7 →T7 mutation and wild type plants(on right in pods)in six-leaf-stage seedlings.Scale bar,10 cm.(E)In vitro specific ALS activity was characterized in mutant and wild-type plants using 10 nmol L−1 chlorsulfuron.
3.3. Recovery of ZmALS1-targeted C-to-T mutant plants and mutant phenotype characterization
To generate base-edited maize plants for breeding and assess in vivo target mutation efficiency, we introduced the CT-nCas9 construct into the maize inbred line ZC01 to produce stable transgenic plants using Agrobacteriummediated immature embryo transformation as described previously [8]. CT-nCas9 was designed to edit both ZmALS1 and ZmALS2 simultaneously, given that the two non-allelic ALS genes can be discriminated using a T/G SNP located at the −16 site,16 bp upstream of the 1st protospacer(Figs.1-C,2-A, -C). Thirty-eight independent T0 transgenic plants(Table S4) were obtained and around 500 T1 individuals were generated. A targeted C7to T7substitution within the base-editing window is expected to produce an Nru I restriction enzyme site for PCR-RE screening assay (Fig. 2-A).The PCR-RE assay was implemented by digesting the PCR products containing the target region using NruI to screen for homologous C7to T7base-edited mutants in T2 individuals.The genotype of each individual can be identified from three different restriction patterns: the wild type (uncut), the homozygous mutant (cut) and the heterozygous mutant(uncut and cut mixture) (Fig. 2-B). The PCR-RE assay permitted efficient isolation of the intended mutant from a large number of segregants.
To evaluate the targeted mutation efficiency, T1 family individuals (Table S5) were subjected not only to PCR-RE screening but also Sanger sequencing to identify the target mutation genotypes. Four types of base substitution at C7and C8sites were induced in the target region (Figs. 1-C, S1). Target sequences were examined in 115 T1 plants.Targeted base-editing frequency was 13.9% (16 of 115) in ZmALS1. Targeted C7to T7substitution accounted for most (13/16, 81%) of these substitutions (Fig. 1-C). These lines were used to isolate T2 plants homozygous for the mutated allele but free of CT-nCas9 (Table S5). Based on specific for both ALS1 and ALS2 and T/G SNPenabled discrimination between them(Figs.2-A,S1),we failed to detect any targeted mutation -n ALS2 in 115 individuals. This observation of low mutation efficiency on ZmALS2 is consistent with the in vitro protoplast data.
To isolate progeny harboring homozygous mutations but lacking CT-nCas9, we recovered T2 individuals derived from T1 families. Sanger sequencing identified 27 lines harboring the target homozygous mutations (Fig. S2) but lacking both Bar and Cas9 elements as verified by PCR (primers listed in Table S1). These results indicated that the created targeted base-editing mutation is heritable. Phenotypic evaluation with variable herbicide concentrations (Fig. S3) showed that 500 mL of 100 mg L−1chlorsulfuron per pod killed all plants of wild type inbred ZC01 after 3-4 weeks following treatment at the 4-6 leaf stage,the most susceptible stage of maize[32].To score the mutant phenotype, the herbicide at this dose was applied. Interestingly, T2 lines harboring homogenous C7-to-T7, C7-to-G7, or C7C8-to-T7G8with different amino acid substitutions (Table S5) all showed herbicide tolerance (Fig.2-D).The in vitro specific ALS activity subjected to 10 nmol L−1chlorsulfuron treatment was employed.Significantly differed from wild type, whose ALS specific activity decreased significantly by around 50% when subjected to chlorsulfuron treatment; however, the synthase activities for the three different types of mutants were shown at the same level compared to non-treatment(Fig.2-E).
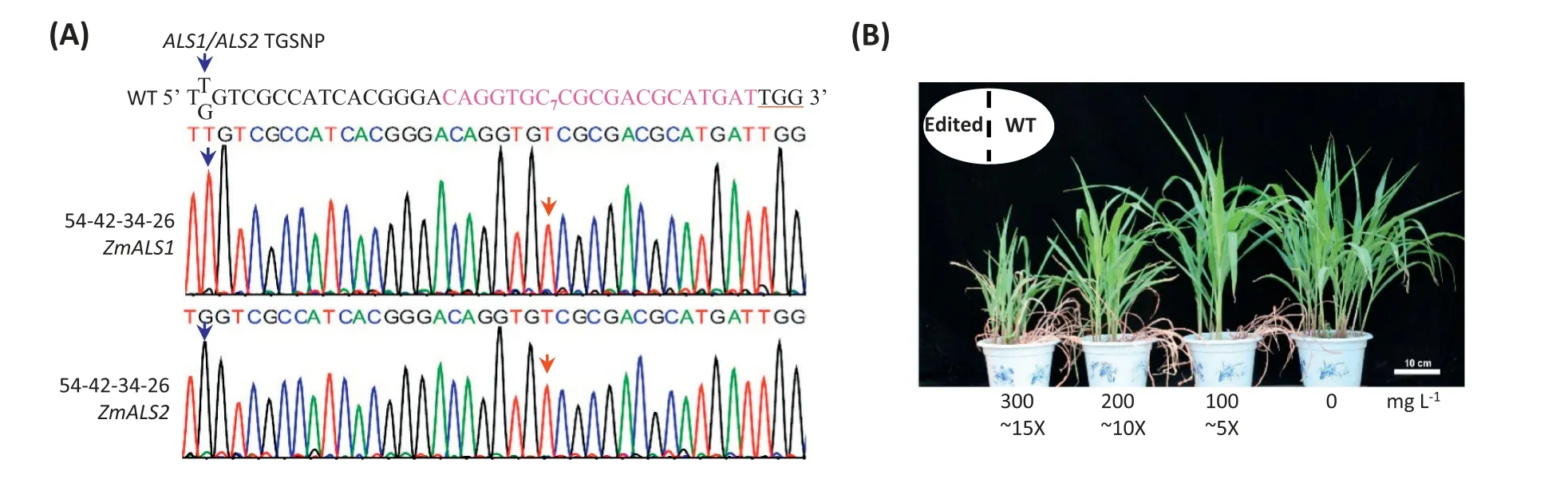
Fig.3-Targeted base editing in two non-allelic ALS genes,ZmALS1 and ZmALS2,conferred high-dose chlorsulfuron resistance in maize plants.(A)Sequencing profiles identified a T3 line,54-42-34-26,with homologous thymine mutations in both ZmALS1 and ZmALS2 at target site 7.Blue arrows indicate the T/G SNP discriminating ZmALS1 and ZmALS2;red arrows indicate the induced target mutation of cytosine to thymine. (B)Three chlorsulfuron concentration level treatments in both ZmALS1 and ZmALS2 targeted CT base edited mutants(on left in pods) and wild-type control(on right in pods)of ZC01.Photographs were made 30 days after herbicide(chlorsulfuron)treatments.Treatments with 500 mL of 0(empty control),100,200,and 300 mg L−1 chlorsulfuron were applied in each pot of this test.Each pot contained 6 mutant plants on the left and 6 WT plants on the right.5×,10×,and 15×indicate the received chlorsulfuron dosage for plants:5,10,and 15 times the recommended field application dose,respectively.Scale bar,10 cm.
3.4. Recovery of precise mutations in both non-allelic acetolactate synthase genes and identification of its resistance doses
To obtain plants with mutations in both ZmALS1 and ZmALS2,we increased the population size of 54-42,which was a T1 line harboring both the CT-nCas9 transgene and the ZmALS1 C7-to-T7 mutation(Table S6).However, we identified no ZmALS2 homozygous mutants. However, we identified 29 plants that contained ALS2 targeted heterozygous mutations (Table S6).Among them in the T3 generation, we identified one line,54-42-34-26, in the negative plants. Sequencing showed that it harbored homozygous C7-to-T7 mutations in both ZmALS1 and ZmALS2 (Fig. 3-A), the only two non-allelic acetoacetate synthase genes in maize.
Given that the herbicide-resistance mechanism of the target mutation is to release the inhibiting “substrate channel” for herbicide,we speculated that high resistance could be achieved in a mutant harboring both non-allelic enzyme mutations. To test this hypothesis, we used three high-dose chlorsulfuron herbicides.In field maize production,the recommended chlorsulfuron dose range received per plant at seedling stage is between 0.2 and 0.8 mg.We tested doses of 5,10,and 15 times the recommended upper limit.The calculated amounts of received chlorsulfuron per maize plant were 4.2, 8.4, and 12.6 mg (Fig. 3-B). Mutant plants survived after 30 days,while the wild-type plants were killed.The growth of mutants subjected to 5 times the upper limit of herbicide treatment was similar to that of the wild type without herbicide treatment. Thus, targeted base editing of both nonallelic acetolactate synthase genes conferred resistance to at least 5 times the upper-limit dose of chlorsulfuron,and maize mutant plants survived at doses even 15 times higher. We did not have sufficient amounts of seeds, including ALS1 mutants, ALS2 mutants,and both ALS1 and ALS2 mutants,to determine whether there were agronomic performance differences under nonherbicide-stress conditions.We have since observed the doublemutant individuals from germination to 10 days after pollination and found no differences in germination,early vigor,plant height,ear height,plant architecture,leaf numbers,flowers,or flowering time.A full set of 14 types of ALS1/ALS2 mutant combinations is being developed for evaluating both their herbicide-resistance phenotypes and their agronomic performance.
4. Discussion
4.1. Mutation types, frequencies and their bias at two loci
Our study was designed to achieve C-to-T base-editing in an activity window,NGS genotyping and later stable transformation base editing genotyping both verified that the majority of the mutations happened as expected.However,both protoplast NGS and the in vivo system showed a bias between mutant frequencies in ZmALS1 and ZmALS2, such that the frequency in ZmALS1 was at least three times that in ZmALS2.This bias is unlikely to be explained by the sequence contexts of the two genes,which differ only by a few SNPs. An explanation might be the chromatin states of their locations. We found heritable target mutations in ZmALS2 in a larger number of progeny. Our results show that stable base-edited plants can be generated.
In addition to the base substitutions,InDels were observed in both the NGS and Sanger sequencing results.Interestingly,using as an example site T15, two plants were found to carry this T deletion, which is the target nucleotide of the nCas9 nicking site. This mutation was even homozygous in the T2 generation (data not shown), suggesting that the ZmALS1 knockout mutation had no lethal effect. In other words,ZmALS1 and ZmALS2 should play redundant roles in maize.InDel mutations may also be introduced by Cas9 (D10A)during nicking of the non-edited strand. This frequency was<0.8%,in agreement with Komor et al. [10].
4.2. Off-target mutations
Off-target effects of in vivo gene editing systems have always been of concern.Recently,substantial genome-wide off-target effects were found in a C-to-T base-editing system whether sgRNA was present or absent [33]. This finding exacerbates concerns about off-target effects of gene editing systems,particularly single-base editing in human gene therapy. We also collected some NGS data on high off-target potential loci(Table S2). Neither C-to-T conversion nor InDel mutations were found with the significant difference with baseline mutation. Further evidence of in vivo genome-wide off-target effects in genome wide awaits high-coverage (50×) wholegenome NGS analysis.
4.3. Agronomic performance of mutants and wild type
Another concern is whether induced target mutations cause agronomic performance reduction under non-herbicide-stress conditions. The two ALSs are vital for maize because they catalyzes the initial common step in the biosynthesis of branched-chain amino acids including valine, leucine, and isoleucine. If the induced mutation led to impaired agronomic performance, it would be of limited agricultural value. We recorded agronomic traits in all four types of T1 homozygous mutant lines. The mutants showed no significant differences from the wild-type control in plant height, ear height and hundred-kernel weight(Fig.S4).Among these traits,plant height and ear height are strongly associated with plant architecture and biomass production and hundred-kernel weight reflects grain production. Plot grain yields and grain yield of individual plants could also be collected to address this concern. All mutants including the double mutant with mutations in both non-allelic ALS genes could be studied in an open-pollinated environment mimicking actual maize production conditions.
4.4. Herbicides and the resistance dose
Acetolactate synthase(ALS)is the target enzyme of the sulfonylureas, imidazolinones, triazolopyrimidine sulfonanilides, and pyrimidinyl oxybenzoates[18]. These types of herbicides are the most widely used in agricultural production. Thus, generating ALS enzyme insensitive to herbicides holds industrial application potential.In this study,we tested only tolerance to chlorsulfuron,one of the sulfonylurea herbicides. In agriculture, the use of pesticides such as herbicides is extensive.Improper management and overapplication[34]or soil residue[35]lead to crop damage,and yield loss often occurs. In the present study, the ZmALS1 single-gene mutants were resistant to 5×the recommended dose limit and the double non-allelic mutant survived at up to 15×the recommended dose limit. Maize cultivars with such resistance levels should pose low risks in agricultural production. The present study did not evaluate the comparative herbicide resistance of ALS1 mutants, ALS2 mutants, and ALS1 and ALS2 double mutants. Such evaluation would reveal whether the two non-allelic ALSs mutations confer additive resistance.
Declaration of competing interest
Authors declare that there are no conflicts of interest.
Acknowledgments
This work was supported by the Key Area Research and Development Program of Guangdong Province(2018B020202008), the National Natural Science Foundation of China (31771808), Beijing Municipal Science and Technology Project (D171100007717001), the National Key Research and Development Program of China (2016YFD0101803), and National Engineering Laboratory for Crop Molecular Breeding.
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2019.10.001.
- The Crop Journal的其它文章
- Base editing in plants:Current status and challenges
- Increasing fidelity and efficiency by modifying cytidine base-editing systems in rice
- Improving the efficiency of the CRISPR-Cas12a system with tRNA-crRNA arrays
- Developing high-efficiency base editors by combining optimized synergistic core components with new types of nuclear localization signal peptide
- Highly efficient CRISPR-SaKKH tools for plant multiplex cytosine base editing
- Intron-targeted gene insertion in rice using CRISPR/Cas9: A case study of the Pi-ta gene

