Less and shrunken pollen 1 (LSP1) encodes a member of the ABC transporter family required for pollen wall development in rice (Oryza sativa L.)
To Luo , Ting Zou , Guoqing Yun , Zhiyun He , Wenjie Li , Yng To ,Miomio Liu , Dn Zhou , Hongfeng Zho , Jun Zhu , Yueyng Ling , Qiming Deng , Shiqun Wng , Aiping Zheng , Huinin Liu , Lingxi Wng , Ping Li ,b,Shungcheng Li ,b,*
aState Key Laboratory of Crop Genetic Resource Exploitation and Utilization in Southwest China, Sichuan Agricultural University, Chengdu 611130, Sichuan, China
bState Key Laboratory of Hybrid Rice, Rice Research Institute, Sichuan Agricultural University, Chengdu 611130, Sichuan, China
Keywords:Rice Pollen fertility Pollen wall ABCG transporter OsABCG3
ABSTRACT Pollen fertility is an agronomic trait that strongly influences rice yield.Recent studies have revealed that the development of the pollen wall is required for pollen fertility and is regulated by several genes. However, the mechanisms underlying pollen and pollen wall development in rice remain largely unknown. In the present study, a point mutation in a gene on chromosome 1 was identified that resulted in the production of less and shrunken pollen(LSP)and led to defects in pollen wall formation.This gene was named LSP1 and was found to encode a member of the adenosine triphosphate-binding cassette (ABC)transporter G subfamily, OsABCG3. Two other loss-of-function mutants of LSP1/OsABCG3,generated using CRISPR/Cas9 technology, showed the same male sterile phenotype. The LSP1/OsABCG3 gene showed a spatio-temporal expression pattern in the developing anthers, and is an ortholog of the Arabidopsis genes AtABCG1 and AtABCG16, which play an important role in pollen wall development. Mutation of LSP1/OsABCG3 affected the expression of several genes involved in pollen and pollen wall formation. These results suggest that LSP1/OsABCG3 is critical for normal pollen fertility and shed light on the molecular mechanisms underlying rice pollen wall development.
1.Introduction
Rice (Oryza sativa L.) is a monocotyledonous model system plant and is one of the most important food crops worldwide[1].Pollen fertility in rice strongly influences yield.The use of pollen-sterile germplasm in producing hybrid rice seeds eliminates the need to conduct the difficult process of emasculation [2]. The formation of mature pollen grains involves a series of developmental processes. Briefly, pollen mother cells (PMCs) and the four-layer anther wall (from the inside to the outside: tapetum, middle layer, endothecium,and epidermis) are differentiated from anther primordial cells. PMCs undergo meiosis to release microspores, which then develop into mature pollen grains via formation of pollen walls and two additional mitotic divisions[3].
Mature rice pollen is surrounded by a finely structured wall called the pollen wall [4]. The integrity of the pollen wall is important for the maintenance of normal pollen function.For example, the pollen wall protects male gametophytes from various environmental stresses and microbial diseases,while also promoting pollen germination.The pollen wall is divided primarily into the exine and the inner intine layers [5]. The exine structure consists of the tectum (outermost structure),the bacula, and the flat nexine (innermost structure). The pollen exine is composed predominantly of a lipid-rich sporopollenin. However, the composition of the pollen intine is quite different,being similar in composition to the primary cell walls of somatic cells, which are composed mainly of pectin polymers,cellulose,and hemicellulose[4].
The ABC transporter protein family is found in a wide range of prokaryotes and eukaryotes[6].The ABCG subfamily is the largest subfamily of ABC transporters, is found in both Arabidopsis and rice [7], and is known to contribute to the translocation of various substrates (e.g. hormones and primary and secondary metabolites) across cell membranes [6].Recently,several plant ABCG transporters have been reported to be involved in the development of pollen or the pollen wall[8]. In Arabidopsis, AtABCG1 and AtABCG16 function together to deliver nexine and intine precursors from the tapetal cells to the anther locules for pollen wall development [9,10].AtABCG9 and AtABCG31 coordinate to play a role in the deposition of steryl glycosides for pollen coat maturation[11].AtABCG26 functions in Arabidopsis to transport sporopollenin precursors across the tapetum into the anther locules for exine formation [12,13]. The rice ortholog of this protein,OsABCG15, is also essential for pollen fertility [14]. Unlike OsABCG15, which occupies a polar location on the inner side of the tapetal cell membrane and participates primarily in the distribution of sporopollenin precursors from the tapetum to the anther locule, OsABCG26 transports anther cuticle precursors from the tapetum to the anther surface and is located on the membranes of the epidermis, endothecium, and tapetum [8,15]. OsABCG26 is an ortholog of AtABCG11, and mutation of the OsABCG26 gene caused pollen sterility [15].However, the biological roles of the ABCG transporters in pollen or pollen wall development in rice remain largely unknown.
In the present study, a new male-sterile mutant, lsp1 (less and shrunken pollen 1), which displayed defective pollen wall formation, was discovered. Genetic analysis and mapping indicated that a single nucleotide substitution in an ABC transporter encoded by LSP1 co-segregated with the sterility phenotype. This ABC transporter is also known as OsABCG3 and belongs to the G subfamily. Knocking out the LSP1/OsABCG3 gene resulted in a male-sterile phenotype similar to that of the lsp1 mutant. LSP1/OsABCG3 is an ortholog of the Arabidopsis genes AtABCG1 and AtABCG16, and showed a spatio-temporal expression pattern in the developing anthers that corresponded to its function in pollen development and the time at which phenotypic defects occurred in the lsp1 mutant. These results suggested that LSP1/OsABCG3 functions similarly to its Arabidopsis orthologs in pollen and pollen wall development in rice.
2. Materials and methods
2.1. Plant materials and growth conditions
The lsp1 mutant was sourced from an ethyl methanesulfonate(EMS)-induced mutant library of the indica cultivar 9311. The CRISPR/Cas9-targeted genome editing tool was used to generate loss-of-function mutants of LSP1/OsABCG3 in the japonica cultivar Nipponbare (Nipp) as previously described[16].All plants were grown in paddy fields at the Rice Research Institute of Sichuan Agricultural University (Chengdu, Sichuan, China) and Lingshui (Hainan, China) under normal cultivation conditions.
2.2. Characterization of the mutant phenotype
The phenotypes of whole plants and floral organs were photographed with an EOS 1200D digital camera (Canon,Tokyo,Japan).Mature pollen grains were stained with a 1%I2-KI solution and then photographed with an Axio Lab.A1 microscope (Zeiss, Oberkochen, Germany) to analyze pollen fertility. Anther semi-thin sectioning, scanning electron microscopy (SEM), and transmission electron microscopy(TEM)were performed as previously described[17].
2.3. Gene mapping and phenotype association assay
The lsp1 mutant was backcrossed with its progenitor wild-type line 9311 (WT), and the resulting BC1F1plant, named lsp1/WT,was further self-pollinated to generate a BC1F2population.Fifty male sterile plants from the BC1F2population were randomly selected for DNA extraction(Plant Genomic DNA Kit,TIANGEN,Beijing, China), and equal amounts of DNA were pooled and subjected to next generation sequencing with a HiSeq 2500 instrument (Illumina,San Diego, CA,USA). Sequencing results were analyzed to calculate single-nucleotide polymorphism(SNP) indices as previously described in the MutMap method[18].To verify the association between the candidate mutation in LOC_Os01g61940 and the male-sterile phenotype of the lsp1 mutant, 78 fertile plants and 22 male-sterile plants in the F2population from lsp1/WT were further genotyped using direct sequencing of the PCR products amplified by the primer set LSP1-1 (Table S1). The target site sequences of all the CRISPR/Cas9-edited transgenic plants were also observed via the direct or cloned sequencing of PCR products amplified by the primer set LSP1-2 or LSP1-3(Table S1).
2.4. Quantitative real-time PCR
Total RNA from various rice tissues was extracted using an RNeasy Plant Mini Kit(QIAGEN,Dusseldorf,Germany)and was reverse transcribed using the SuperScript First-Strand Synthesis System(Life Technologies,Carlsbad,CA,USA).Quantitative real-time PCR (qPCR) was performed, with corresponding primer sets and soAdvanced SYBR Green Supermix (Bio-Rad,Hercules,CA,USA),using the CFX96 Real-time PCR System(Bio-Rad) according to the manufacturer's instructions. OsACTIN1 was used as the internal standard gene to which the cDNA levels of the target gene were normalized.Three replicates were used for each sample. The relative expression levels were measured as previously described[17].
2.5. Histochemical activity assay of GUS (β-glucuronidase)
To visualize the expression of the LSP1/OsABCG3 gene, a 2.3-kb DNA fragment of the LSP1/OsABCG3 promoter(upstream of the start codon ATG) was amplified from Nipp using the primer set LSP1-P (Table S1) and was used to construct the pLSP1/OsABCG3::GUS plasmid as previously described[17].The construct was then introduced into the Agrobacterium tumefaciens strain EHA105 and subsequently transferred into Nipp. The histochemical activity detection of GUS in transgenic plants followed a previously described method [17].After staining, samples were cleared with a 70% (v/v) ethanol solution and photographed with the EOS 1200D digital camera and Axio Lab.A1 microscope.
2.6. Phylogenetic analysis and three-dimensional model analysis
The LSP1/OsABCG3-related protein sequences from both monocot and dicot species were identified using the full-length amino acid sequence of LSP1/OsABCG3 as a BLAST query in the Phytozome database (https://www.phytozome.net/) using the default parameters. The retrieved peptide sequences were aligned using ClustalW [19]. The phylogenetic trees were generated using the neighbor-joining method with the default parameters, with the exception of 1000 bootstrap replications,in MEGA 5 software[20]and edited with the Interactive Tree of Life online tool(http://itol.embl.de/)[21].
For molecular three-dimensional(3D)simulation analysis,the full-length protein sequence of LSP1/OsABCG3 in the WT and the inferred protein sequence,with an amino acid substitution of Ser to Asn in lsp1,were modeled using the same template in SWISSMODEL (https://swissmodel.expasy.org/). The modeling results were then further analyzed with Swiss-Pdb Viewer[22].
3. Results
3.1.The lsp1 mutant exhibits abnormal pollen and pollen wall formation
Screening of the EMS-induced mutant library (in the background of indica cultivar 9311)resulted in the identification of the male sterile mutant lsp1. In comparison to the WT, lsp1 showed normal vegetative organs (Fig. 1-A) and floral organs(Fig.1-B),while producing less and shrunken pollen(Fig.1-C).

Fig.1-Phenotypic characteristics of the lsp1 mutant.(A)WT and lsp1 mutant plants after heading.Bars = 20 cm.(B)Spikelets(upper),and spikelets with palea and lemma removed(lower).Bars = 2 mm.(C)Pollen grains with I2-KI staining.Bars =200 μm.
To investigate the cytological differences between the WT and the lsp1 mutant, transverse sections of the anthers at different developmental stages were examined. The anther developmental stages were classified according to a previous study[3].As shown in Fig.2,from stages 8a to 10,microspores released from tetrads vacuolated and enlarged normally in the lsp1mutant, with no detectable defects being observed in the anther compared to the WT.At stage 11 to stage 12 in the WT,the microspores underwent mitosis twice and developed into deep-stained and round-shaped mature pollen, and the tapetum degenerated rapidly (Fig. 2). In contrast, the tapetal cell layers of the lsp1 mutant were slightly larger than those of the WT at stage 11 (Fig. 2). At stage 12, although the tapetum layer in both the WT and the lsp1 mutant had disappeared,only shrunken empty pollen grains remained in anther locules of the lsp1 mutant(Fig.2).

Fig. 2 - Comparison of transverse sections to assess anther-development differences between the WT and lsp1 mutant. Six stages (S) of anther development in the WT and lsp1 were compared. Dp, deformed pollen; E, epidermis; En, endothecium; Ml, middle layer; Mp, mature pollen; Msp, microspore; PMC, pollen mother cell; Tds, tetrads; T, tapetum. Bars = 20 μm.
To observe in detail the defects in the lsp1 mutant, the anther and pollen grains were examined by SEM and TEM. In comparison with the WT,the cuticle on the epidermis surface(Fig. 3-B), the Ubisch bodies along the inner surface of the tapetum (Fig. 3-C), and the tectum on the exine surface appeared normal in the lsp1 mutant (Fig.3-E, G), whereas the pollen grains in the lsp1 mutant had a deformed and deflated morphology (Fig. 3-D, F). The lsp1 mutant pollen grains showed the tectum and nexine layers, but showed an abnormal bacula and lacked the intine and cytoplasm, in comparison to those of the WT (Fig. 3-G). These results suggested that the defects in pollen wall formation accounted for the male-sterile phenotype of the lsp1 mutant.
3.2. Cloning of the LSP1 gene
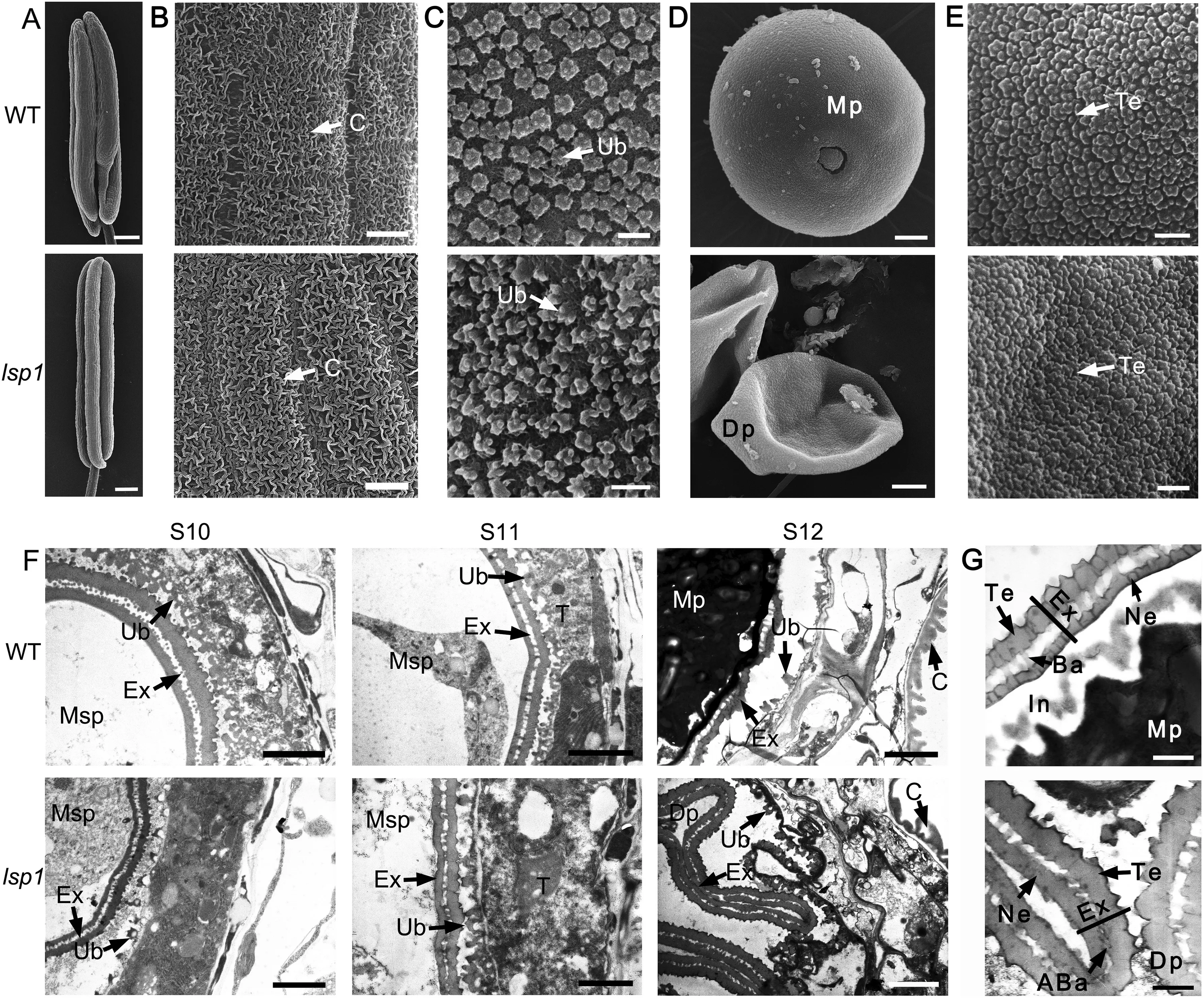
Fig.3- Scanning electron microscopy (SEM)and transmission electron microscopy (TEM)observations for the WT and lsp1 mutant anthers and pollen.(A-E)SEM surface analysis of anthers and pollen grains in the WT and lsp1 mutant at stage 12.(A)Whole anthers.Bars = 200 μm.(B)The outer surface of the anther epidermis.Bars = 2 μm.(C)The inner surface of the tapetum.Bars = 2 μm.(D)Pollen grains.Bars = 2 μm.(E) The outer surface of the pollen grain.Bars = 500 nm.(F and G)TEM observations of the anthers and pollen wall in the WT and lsp1 mutant at different anther stages.(F)Ultrastructure of the anthers.Bars = 2 μm.(G) Ultrastructure of pollen walls.Bars = 500 nm.ABa,abnormal bacula;Ba,bacula;C,cuticle;Dp,deformed pollen;Ex,exine;In,intine;Mp,mature pollen; Msp,microspore; Ne,nexine;Ub,Te,tectum;Ub,Ubisch body.
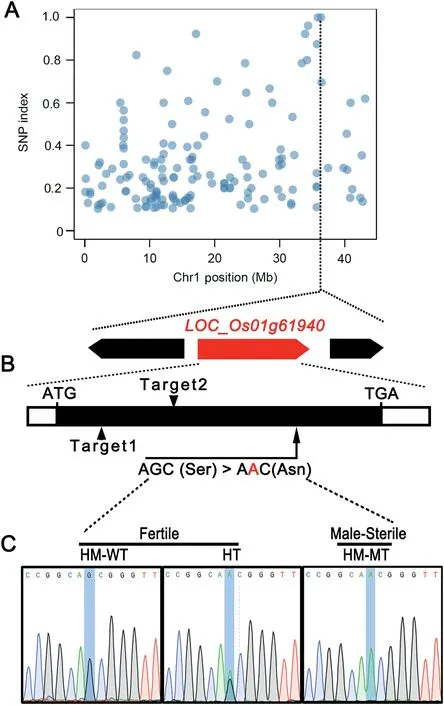
Fig. 4 - Mapping of the LSP1/OsABCG3 gene. (A) Distribution of SNP sites on chromosome 1. (B) Structure of the LSP1/OsABCG3 gene. Arrows indicate the mutation site of LSP1/OsABCG3 in the lsp1 mutant and two independent sgRNA targeting sites(Targets) of the CRISPR/Cas9 system. Details of the mutated site are listed below. The coding region is shown as a black box. 5′or 3′ untranslated regions are shown as white boxes. (C) Identification of the mutation site of the LSP1/OsABCG3 gene in the lsp1/9311 BC1F2 population by sequencing. HM-WT, HT, and HM-MT indicate the homozygous wild-type genotype, the heterozygous genotype, and the homozygous mutant, respectively.
The lsp1 mutant was backcrossed with the parental WT to generate a BC1F2population. The segregation of fertile and sterile plants followed a 3:1 ratio (231:69, χ2= 0.329 < χ2= 3.84) in the BC1F2generation, indicating that a single recessive gene was responsible for the phenotype of the lsp1 mutant. To map the causal gene, next-generation sequencing and MutMap cloning approaches were applied [18]. SNPs differentiating the WT and the mutant pool were identified (Fig. S1). To identify a causal SNP, these SNPs were filtered in three steps. The first step involved the deletion of SNPs with a SNP index <0.9 and extraction of SNPs with a SNP index of 1,which are considered to be homozygous SNPs and theoretically cosegregated with the mutant phenotype. As a result, 11 candidate SNPs were obtained(Table S2). Among these SNPs,two on chromosome 1 formed a cluster, while the other nine SNPs were distributed on chromosomes 5, 6, 7, 8, 10, and 12(Fig. S1, Table S2). The second step removed SNPs located in intergenic regions and SNPs that resulted in synonymous mutations.The third step retained SNPs that showed G to A or C to T transitions, which are the most frequent changes caused by EMS-induced mutagenesis [18,23,24]. Finally, two SNPs located in two candidate genes (LOC_Os01g61940 and LOC_Os01g62790) on chromosome 1 were obtained (Fig. 4-A,Table S2).
The SNP (a G-to-A transition) in LOC_Os01g61940 was localized to the exon of this gene and represented a nonsynonymous mutation (Fig. 4-B, Table S2).LOC_Os01g61940 encodes the ABC transporter- OsABCG3[6,7]. OsABCG3 is a homolog of AtABCG1 and AtABCG16,which are required for pollen wall development in Arabidopsis[9,10].However,the other SNP on chromosome 1 was localized to the intron of LOC_Os01g62790 (Table S2), and may exert a smaller effect on the function of this gene than LOC_Os01g61940. Further sequencing results showed that the mutation of LOC_Os01g61940 co-segregated with the male sterile phenotype of the lsp1 mutant (Fig. 4-C, Table S3). These findings suggested that the SNP of LOC_Os01g61940 may play a major role in producing the defects in the lsp1mutant, and that LOC_Os01g61940 was the most appropriate candidate for LSP1.
3.3. The LSP1 gene encodes a half-size ABCG transporter
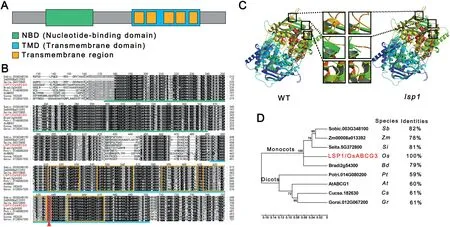
Fig.5- Protein sequence analysis of LSP1/OsABCG3.(A)Schematic representation of the conserved nucleotide-binding and transmembrane domains in the LSP1/OsABCG3 protein.The domains were detected using the Simple Modular Architecture Research Tool(http://smart.embl-heidelberg.de/).(B) Multiple sequence alignment of the conserved domains from LSP1/OsABCG3-related proteins.The green line indicates the nucleotide-binding domain region.The blue line indicates the transmembrane domain region.The orange frames indicate transmembrane regions.The red arrow and frame indicate the conserved amino acid that was mutated in the lsp1 mutant. (C)Comparison of the predicted protein model of LSP1/OsABCG3 between the WT and lsp1 mutant.The black frames indicate possible structural alterations. (D)Phylogenetic tree of LSP1/OsABCG3 and its homologs in other plant species.The numbers at the nodes are bootstrap values.The percentage identities represent the sequence similarity between the corresponding proteins and LSP1/OsABCG3.At, Arabidopsis thaliana;Bd,Brachypodium distachyon;Cs, Cucumis sativus; Gr,Gossypium raimondii;Os,Oryza sativa;Pt,Populus trichocarpa;Sb,Sorghum bicolor;Si,Setaria italica;Zm, Zea mays.
Like its Arabidopsis homologs AtABCG1 and AtABCG16 [9,10], the OsABCG3 protein encoded by LOC_Os01g61940 belongs to the half-size ABCG transporter subfamily (Fig. S2). A protein sequence analysis showed that OsABCG3 contains two typical domains of the half-size ABCG transporter, a nucleotidebinding domain (NBD), also known as ATP-binding domain, and a transmembrane domain (TMD) in its N-terminus and Cterminus, respectively (Fig. 5-A). The mutation in the OsABCG3 gene of the lsp1 mutant resulted in the change of the 588th serine(Ser)to an asparagine(Asn)(Fig.4-B),which is conserved in OsABCG3-related proteins from different plant species and is proximal to the fourth transmembrane region(Fig.5-B).This amino acid substitution resulted in a structural change in one beta sheet and two alpha helices (Fig. 5-C),suggesting that the function of OsABCG3 in the lsp1 mutant was disrupted. The phylogenetic analysis showed that OsABCG3 was closely related to its homologs in monocots,and shared high similarity with its plant orthologs(Fig.5-D).
3.4. Knocking out the LSP1/OsABCG3 gene also resulted in male sterility
To determine the role of OsABCG3 in rice pollen fertility, the CRISPR/Cas9 genome-editing tool was used to disrupt the function of its associated protein in Nipp. Two independent targets within the gene were designed (Fig. 4-B). Five positive transgenic lines with homozygous mutations in the corresponding target sites were obtained (Fig. 6-A). All of these mutations caused premature truncation of the OsABCG3 protein (Fig. S3). No obvious differences in vegetative growth(Fig. S4-A) and floral organ development (Figs. 6-B, S4-B)between Nipp and these knockout (ko) lines were observed.The development of microspores during stages 8 to 10 was also observed to be normal, whereas the ko lines displayed a swollen tapetum at stage 11 and shrunken and empty pollen grains at stage 12(Fig.S4-C),mimicking the phenotype of the lsp1 mutant. These results supported the inference that OsABCG3 was LSP1 and suggested that LSP1/OsABCG3 is critical for rice pollen fertility.
3.5. Expression pattern of the LSP1 gene
To further elucidate the function of LSP1/OsABCG3, its expression profile in different organs was examined by qPCR and in transgenic plants carrying the pLSP1/OsABCG3::GUS construct. LSP1/OsABCG3 transcripts, or GUS signals, were detected in the roots (Figs. 7-A, S5) and developing anthers(Fig.7-A-C),with the anthers at stage 9 showing the strongest expression (Fig. 7-A, B). Sectioning GUS-stained anthers showed that the expression of LSP1/OsABCG3 occurred in both anther somatic cell layers and microspores at stage 9(Fig.7-D).However,from late stage 10 to early stage 11,when the pollen exine was almost completely formed and the intine began to develop, GUS signals were observed exclusively in microspores (Fig. 7-E). These results suggested that LSP1/OsABCG3 follows a spatio-temporal expression pattern in developing anthers.
3.6. Expression analyses of genes involved in pollen wall development
Several genes have been reported to be involved in pollen wall formation[5].In the tapetum of rice,CYP703A3 and CYP704B2,two cytochrome P450 family members, participate in the synthesis pathway of sporopollenin precursors required for pollen wall formation [25-27]. DPW and DPW2 encode a fatty acid reductase and a BAHD acyltransferase, respectively, and both play essential roles in the biosynthesis of key components of the pollen wall [28,29]. Two ABCG transporter members, OsABCG15 and OsABCG26 are also involved in pollen development by affecting the formation of the anther cuticle and pollen wall [14,15,30-33]. Two tapetum-specific lipid transport genes OsC4 and OsC6, have fundamental functions in the formation of the pollen wall during pollen development[34,35].
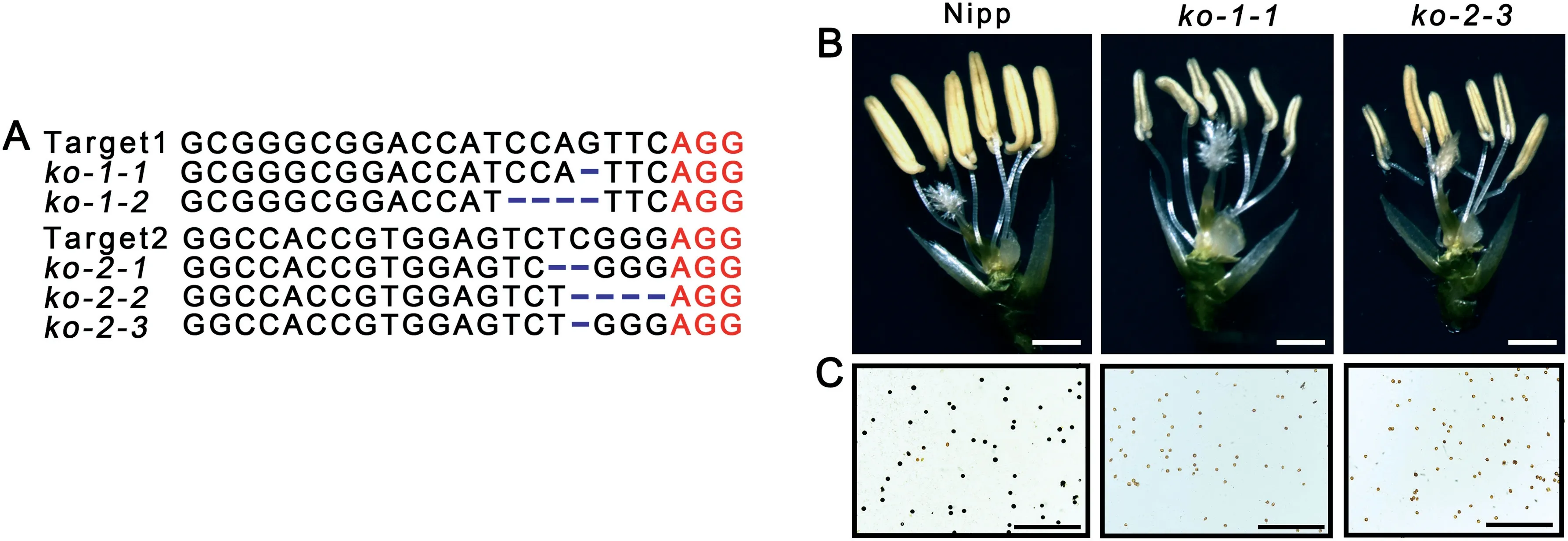
Fig. 6 - Functional analysis of the LSP1/OsABCG3 gene using the CRISPR/Cas9 genome editing system. (A) Sequence information of Nipp and the homologous mutants within the target 1 and target 2 regions in the knockout (ko) plants with the Nipp background. Protospacer Adjacent Motif (PAM) sequences and mutations are shown in red and blue, respectively. (B) Spikelets with palea and lemma removed. Bars = 2 mm. (C) Pollen grains with I2 -KI staining. Bars = 500 μm.
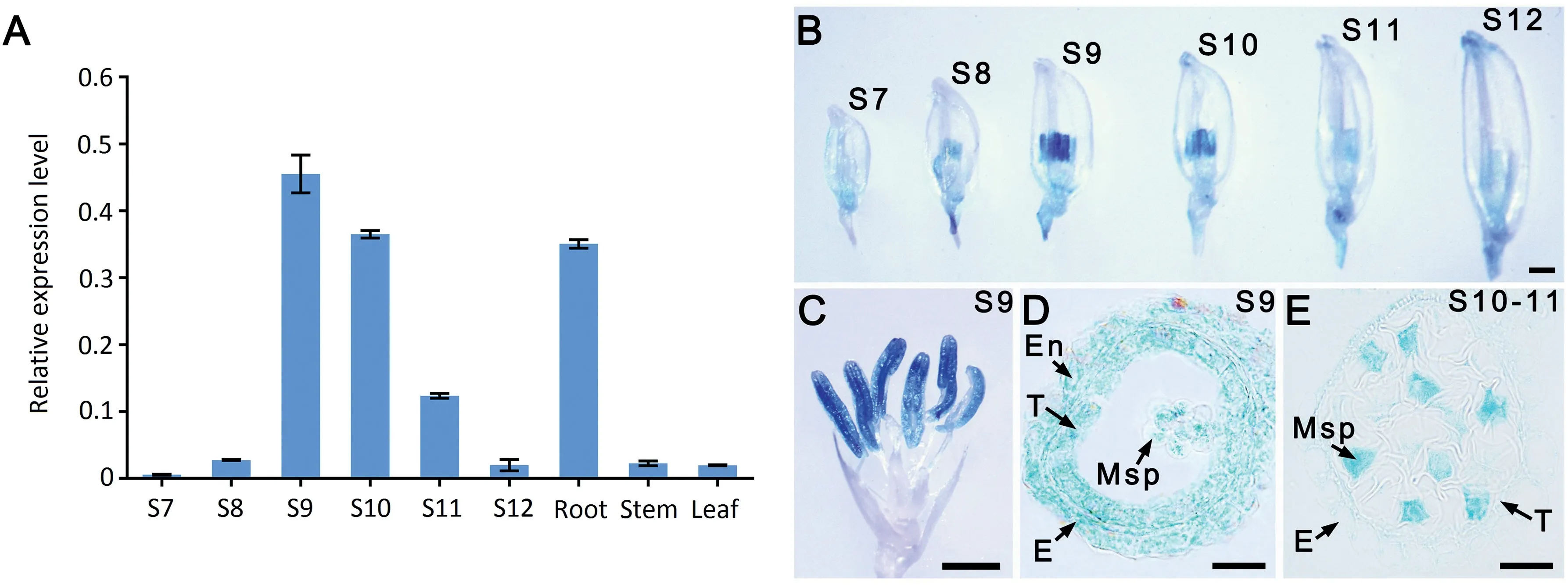
Fig. 7 - Expression profiles of the LSP1/OsABCG3 gene in different tissues. (A) qPCR analysis of LSP1/OsABCG3 in roots, stems, leaves, and anthers at different developmental stages (S) of the WT. The OsACTIN1 gene was used as the normalized reference.(B-E) GUS expression (blue staining) patterns in different organs of pOsABCG3/LSP1::GUS transgenic plants. (B) Spikelet at different anther developmental stages. Bars = 2 mm. (C) Spikelet with palea and lemma removed. Bars = 2 mm. (D and E) Section of a GUS-stained anther. Bars = 20 μm.
Based on the observed pollen wall development defects in the lsp1 mutant,the expression profiles of these genes in rice anthers at different development stages were investigated(Fig. 8). Among these genes, the expression patterns of CYP704B2,OsABCG15,and OsABCG26 in developing anthers of the lsp1 mutant were similar to those of the WT, whereas CYP703A3 showed stronger expression in the lsp1 mutant than in the WT. Furthermore, although the transcription levels of DPW2, OsC4, and OsC6 in both the WT and the lsp1 mutant reached their peak at stage 10, their levels were severely reduced in the lsp1 mutant in comparison to the WT.These results suggested that the mutation of the LSP1/OsABCG3 gene affects the expression of additional essential genes involved in pollen wall development.
4. Discussion
Pollen fertility is a critical yield-determining trait in rice. Correct pollen wall development is essential for the production of viable pollen [36]. Recent genetic studies have revealed the functions of several genes that participate in the development of pollen and the pollen wall in rice, such as CYP703A3 [26,27], CYP704B2 [25], DPW [29], DPW2 [28], OsABCG15 [14,31-33], OsABCG26 [15,30], OsACOS12 [37-39],OsC4 [34,35], OsC6 [35], OsPKS1 [40-42], OsPKS2 [17,43], and OsSTRL2 [44]. All these genes are specifically or highly expressed in anthers. Mutations in these genes result in defective pollen wall formation leading to pollen sterility.
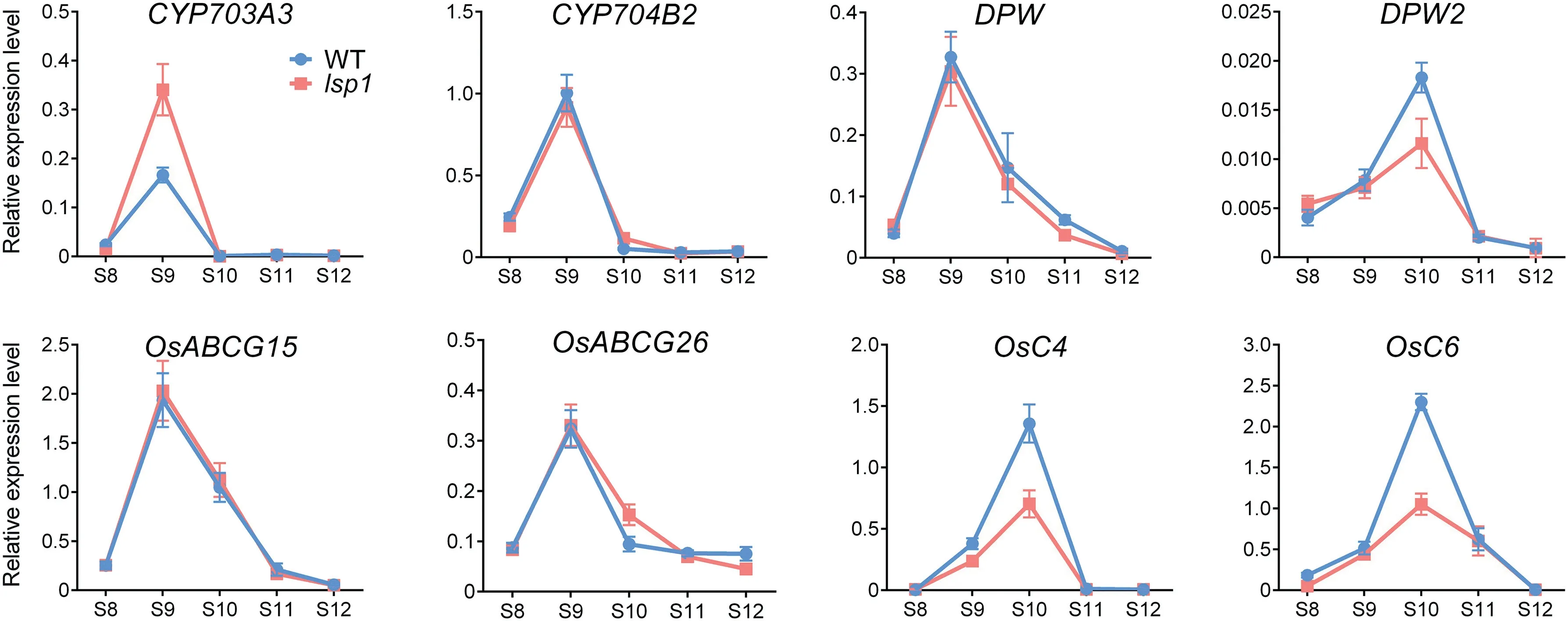
Fig. 8 - Expression profiles of genes involved in pollen and pollen wall development. The corresponding transcript levels in anthers during stages (S) 8 to 12 were measured by qPCR. The OsACTIN1 gene was used as the normalized reference.
In the present study, a male sterile mutant lsp1 (in the genetic background of indica rice cultivar 9311, Fig. 1), that produced less and shrunken pollen with abnormal pollen wall formation was obtained, and the responsible gene LSP1/OsABCG3 (LOC_Os01g61940) was cloned (Fig. 4). Knockout of the LSP1/OsABCG3 gene using the CRISPR/Cas9 approach also resulted in the production of shrunken and empty pollen grains(in the genetic background Nipp,Fig.6),confirming that LSP1/OsABCG3 was essential for pollen fertility.qPCR and GUS assay showed that LSP1/OsABCG3 followed a spatial and temporal expression pattern in developing anthers (Fig. 7).Coincidentally, the development of microspores was normal until stage 10, but the morphological defects in the microspores became apparent at the later anther stages in the lsp1 mutant (Fig. 2). These results suggested that the expression pattern of the LSP1/OsABCG3 gene in the anthers corresponds to its function in pollen and pollen wall development and also to the timing of the appearance of phenotypic defects in the lsp1 mutants.
Recently, another two allelic mutants, osabcg3-1 and osabcg3-2,of LSP1/OsABCG3 in the genetic background of indica rice cultivar Huanghuazhan were reported to show complete male-sterility phenotypes [45]. Careful comparison of the anther sections and electron microscopy results of lsp1 and osabcg3-1 revealed similar defects of anther and pollen development, including the swollen tapetum at the stage 11,completely degenerated pollen intine and cellular contents,and deformed bacula at stage 12. No obvious difference in anther and pollen development between these two mutants was found. The expressions of DPW2, OsC4, and OsC6, which were decreased in osabcg3-1, were also downregulated in the lsp1 mutant,suggesting a similar effect of OsABCG3 deficiency on the expression of genes involved in pollen wall formation among these two alleles. These findings collectively show that LSP1/OsABCG3 plays an important and conserved role in normal male reproductive development in diverse rice genetic backgrounds.
Although the morphology of the pollen surface is very diverse between Arabidopsis and rice, their pollen walls have similar structural features [46]. Their genetic and developmental pathways are also conserved [47,48]. In Arabidopsis,CYP703A2 catalyzes the in-chain hydroxylation of medium chain fatty acids [49], whereas CYP704B1 catalyzes ωhydroxylation of long-chain fatty acids[50].The hydroxylated fatty acids produced by CYP703A2 and CYP704B1 are further converted by the ACOS5 protein to CoA esters [51], which are subsequently used as substrates for LAP5/PKSB and LAP6/PKSA to synthesize sporopollenin precursors[52,53].Single or double mutants of genes encoding these proteins have been shown to have abnormal pollen walls[52,53].In rice,a similar pathway of pollen wall formation has been characterized[5,41]. CYP703A3 [26,27], CYP704B2 [25], OsACOS12 [37-39],OsPKS1 [40-42], and OsPKS2 [17,43] in rice are orthologs of CYP703A2, CYP704B1, ACOS5, LAP6/PKSA, and LAP5/PKSB in Arabidopsis, respectively, and were reported to share similar enzymatic functions with their Arabidopsis homologs. The loss-of-function mutations in these rice genes also showed defects in pollen wall formation, suggesting that the molecular mechanisms of pollen wall development in Arabidopsis and rice are highly conserved.LSP1/OsABCG3 is an ortholog of the Arabidopsis genes AtABCG1 and AtABCG16 (Fig. S2).
AtABCG1 and AtABCG16 transport the precursors or components required for pollen wall formation, and abcg1 abcg16 double-mutant plants released shriveled pollen that lacked the intine of the pollen wall[9,10].An ultrastructural analysis showed that the intine of the lsp1 mutant pollen wall was also missing (Fig. 3-G). These findings suggest that the LSP1/OsABCG3 gene may share similar functions with the Arabidopsis genes AtABCG1 and AtABCG16 in pollen wall development.
OsABCG15 and OsABCG26 are two further ABCG transporters that function in pollen wall formation [14,15,30,33].Both the osabcg15 mutant and the osabcg26 mutant failed to generate viable pollen and displayed severely abnormal degradation of the tapetal cell layer, an undeveloped anther cuticle,a deformed Ubisch body,and a defective pollen exine.However, the lsp1 mutant produced shrunken and empty pollen grains with a relatively complete pollen exine structure, and showed a slightly swollen tapetum with normal anther cuticle formation and Ubisch body development, but lacked the pollen intine and its inner cytoplasm (Figs. 1-C, 2,3), defects different from those observed in the osabcg15 or osabcg26 mutants. The expression patterns of OsABCG15 and OsABCG26 in the lsp1 mutant also showed no differences from those in the WT(Fig.8).Phylogenetic analysis of the rice halfsize ABCG transporters grouped LSP1/OsABCG3, OsABCG15,and OsABCG26 into distinct classes (Fig. S2). Together, these results suggest that the pathway of pollen wall development in which LSP1/OsABCG3 is involved may be different from those of OsABCG15 and OsABCG26.
The core unit of a half-size ABCG transporter is composed of one NBD and one TMD[54].NBDs participate in binding and hydrolyzing ATP, which provides the driving force for transport, while TMDs are responsible for substrate recognition and translocation across the lipid bilayer [6,7]. OsABCG3 belongs to the half-size ABCG transporter family (Fig. S2).The point mutation of the OsABCG3 gene in the lsp1 mutant led to an amino acid substitution near the fourth transmembrane region of the TMD (Fig. 5-B). Three-dimensional molecular simulation showed that the amino acid substitution resulted in marked structural changes in one beta sheet and two alpha helices (Fig. 5-C). We accordingly propose that disruption of the function of OsABCG3 in the lsp1 mutant caused the male-sterile phenotype in the mutant. However,testing this hypothesis will await further investigation of the biochemical functions of OsABCG3.
It has been assumed that half-size ABC proteins cannot function alone, but can form a dimer complex to achieve the full ABC transporter function of delivering substrates across biological membranes [6,7,54]. In Arabidopsis, AtABCG11 and AtABCG12 interact with each other to play roles in lipid export, although only AtABCG11 homodimers were detected[55]. LSP1/OsABCG3 forms a homodimer and can heterodimerize with RCN1/OsABCG5 in rice [56]. RCN1/OsABCG5 is required for the formation of the apoplastic barrier in roots and for closure of stomata [56,57], but is not expressed in reproductive tissues [58,59]. It is thus plausible that LSP1/OsABCG3 participates in pollen wall development via the formation of homodimers or via heterodimerization with other half-size ABCG proteins other than RCN1/OsABCG5.
These results shed light on the mechanisms underlying pollen fertility in rice and suggest a potential target for the manipulation of male fertility in hybrid rice breeding. However,the function of this ABCG transporter in formation of the pollen wall remains unclear. Elucidating the cellular function of LSP1/OsABCG3 awaits further identification of its potential substrates and other interactions.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31570004), the Open Research Fund of State Key Laboratory of Hybrid Rice, Hunan Hybrid Rice Research Center (2016KF10), the Sichuan Province Science and Technology Support Program (2016NZ0103 and 2017NZDZX0001), and the National Key Research and Development Program of China(2017YFD0100201).
Declaration of competing interest
Authors declare that there are no conflicts of interest.
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2019.09.001.
- The Crop Journal的其它文章
- Base editing in plants:Current status and challenges
- Increasing fidelity and efficiency by modifying cytidine base-editing systems in rice
- Improving the efficiency of the CRISPR-Cas12a system with tRNA-crRNA arrays
- Developing high-efficiency base editors by combining optimized synergistic core components with new types of nuclear localization signal peptide
- Highly efficient CRISPR-SaKKH tools for plant multiplex cytosine base editing
- Intron-targeted gene insertion in rice using CRISPR/Cas9: A case study of the Pi-ta gene

