An ultra-sensitive and easy-to-use assay for sensing human UGT1A1 activities in biological systems
Y-Di Zhu,Hui-Lin Png,Qi-Hng Zhou,Zi-Fi Qin,Qing Jin,Mosh Finl,Yi-Nn Wng,Wi-Wi Qin,Yin Lu,Dn-Dn Wng,**,Gung-Bo G,*
aInstitute of Interdisciplinary Integrative Medicine Research,Shanghai University of Traditional Chinese Medicine,Shanghai,201203,China
bJiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Materia Medica,School of Pharmacy,Nanjing University of Chinese Medicine,Nanjing,210023,China
cSchool of Life Science and Medicine,Dalian University of Technology,Panjin,124221,China
dDepartment of Pharmacy,The First Affiliated Hospital of Zhengzhou University,Zhengzhou,China
eDivision of Pharmaceutical Chemistry and Technology,Faculty of Pharmacy,University of Helsinki,00014,Finland
fDepartment of Pharmacy,Huashan Hospital,Fudan University,Shanghai,China
Keywords:
UGT1A1
LC-FD
N-butyl-4-(4-hydroxyphenyl)-1,8-naphthalimide
Modulators
ABSTRACT
The human UDP-glucuronosyltransferase 1A1(UGT1A1),one of the most essential conjugative enzymes,is responsible for the metabolism and detoxification of bilirubin and other endogenous substances,as well as many different xenobiotic compounds.Deciphering UGT1A1 relevance to human diseases and characterizing the effects of small molecules on the activities of UGT1A1 requires reliable tools for probing the function of this key enzyme in complex biological matrices.Herein,an easy-to-use assay for highly-selective and sensitive monitoring of UGT1A1 activities in various biological matrices,using liquid chromatography with fluorescence detection(LC-FD),has been developed and validated.The newly developed LC-FD based assay has been confirmed in terms of sensitivity,specificity,precision,quantitative linear range and stability.One of its main advantages is lowering the limits of detection and quantification by about 100-fold in comparison to the previous assay that used the same probe substrate,enabling reliable quantification of lower amounts of active enzyme than any other method.The precision test demonstrated that both intra-and inter-day variations for this assay were less than 5.5%.Furthermore,the newly developed assay has also been successfully used to screen and characterize the regulatory effects of small molecules on the expression level of UGT1A1 in living cells.Overall,an easy-to-use LC-FD based assay has been developed for ultra-sensitive UGT1A1 activities measurements in various biological systems,providing an inexpensive and practical approach for exploring the role of UGT1A1 in human diseases,interactions with xenobiotics,and characterization modulatory effects of small molecules on this conjugative enzyme.
1.Introduction
UDP-glucuronosyltransferases1A1(UGT1A1)is one of the most essential enzymes responsible for the biotransformation and detoxification of the endogenous toxin bilirubin,and metabolic clearance of a variety of crucial therapeutic drugs and other xenobiotics[1,2].Multiple evidences have indicated that dysfunction or potent inhibition of hepatic UGT1A1 may result in a disorder of bilirubin metabolism,causing different degrees of hyperbilirubinemia,liver disorders,and other diseases[3,4].In addition,reduced expression/activities of UGT1A1 may trigger clinically relevant drug/herbs-drug interactions by affecting the pharmacokinetic behaviors of the UGT1A1-substrate drugs[5-7].Due to the vital roles of UGT1A1 in biotransformation of both endogenous compounds(particularly bilirubin conjugation)and drugs,the European Medicines Agency(EMA)and the US Food and Drug Administration(FDA)have recommended that the inhibition potency of new drug candidates or phytochemical products on UGT1A1 activities should be tested before marketing approval[8].
A panel of analytical methods have already been reported for studying the inhibition potency of different drug candidates or phytochemical products on the human UGT1A1,either employing the recombinant enzyme or liver preparations,such as microsomes[9].However,most of the previously reported methods had considerable limitations in sensing UGT1A1 activities in biological samples that vary from instability of the substrate under the assay conditions,to poor selectivity towards UGT1A1 over other hepatic UGTs,to low sensitivity unless liquid chromatography-tandem mass spectrometry(LC-MS/MS)is used as the analytical tool[10,11].Usually,the LC-MS/MS based assays are costly and laborintensive,because the expensive instruments and skillful operators are always required.
Recently,we have developed two isoform-specific fluorogenic substrates for the human UGT1A1,which provide alternative methods for high-throughput detection of this key enzyme,using fluorescence-based assays[12,13].In particular,a recent investigation has clearly demonstrated that N-butyl-4-(4-hydroxyphenyl)-1,8-naphthalimide(NHPN)is an isoform-specific fluorogenic probe for UGT1A1(Fig.1A),which binds at the bilirubin binding site on this key enzyme,is highly suitable for replacing bilirubin for measuring UGT1A1 activities and investigating UGT1A1-ligand interactions in tissue preparations[13,14].Nevertheless,even though the specificity of NHPN for UGT1A1 is high,the fluorogenic properties of this probe are not good enough,mainly due to short emission wavelength of its O-glucuronide(<520 nm).This is a problem since some endogenous compounds and phytochemicals emit fluorescence signals at visible wavelengths(400-600 nm),leading to a high background fluorescence that may strongly affect the detection of NHPN O-glucuronide,lowering its sensitivity and accuracy.Hence,it is necessary to develop a practical and accurate method for highly selective and ultra-sensitive probing of UGT1A1 activities in complex biological matrix.The practical approach is to take advantage of the high specificity of NHPN and combine it with improved analytical assays that include advanced fluorescence detection,allowing taking full benefits of this newly developed fluorogenic substrate for UGT1A1.

Fig.1.The chemical structure of NHPN and its mechanism for sensing UGT1A1 activity(A).Liquid chromatography- fluorescence detection(LC-FD)chromatograms of NHPN and NHPNG(B).(a)NHPN only,(b)NHPN was co-incubated with UDPGA in the presence of activated HLM(0.5 mg/mL)at 37℃ for 20 min,(c)NHPNG only,(d)NHPN was co-incubated with HLM(0.5 mg/mL)but without UDPGA,(e)buffer only.The fluorescence signals of NHPN and NHPNG were recorded using excitation wavelength of 370 nm and emission wavelength of 520 nm.
In the present study,a reliable and easy-to-use assay for high sensitivity and selectivity probing of UGT1A1 activities in various biological systems is presented in details.To this end,NHPN was used as the fluorogenic substrate,while liquid chromatography with fluorescence detection(LC-FD)was used to separate the target analytes without interference from the endogenous matrix.The assay was fully validated in terms of sensitivity,specificity,precision,quantitative linear range and stability.Furthermore,the newly developed LC-FD based assay was successfully used to measure UGT1A1 activities in tissue preparations,as well as to screen and characterize the regulatory effects of small molecules on this conjugative enzyme in living cells.Collectively,these findings provided a practical and reliable assay for probing UGT1A1 activities,which would strongly facilitate UGT1A1-associated studies in both academic and industrial fields.
2.Methods and experimental
2.1.Reagents and materials
The fluorogenic probe substrate N-butyl-4-(4-hydroxyphenyl)-1,8-naphthalimide(NHPN)and its O-glucuronide(NHPNG)were synthesized and purified(purity≥98%)according to the previously reported scheme[13].Nilotinib and chrysin were from Dalian Meilun Biotech Co.,Ltd.(Meilun,Dalian,China).The tissue preparations,including pooled human liver microsomes(HLM),pooled human intestine microsomes(HIM),pooled human kidney microsomes(HKM),and pooled human lung microsomes(HLuM)were purchased from Celsis Inc.(Baltimore,MD,USA).Recombinant human UGT1A1 was from BD Biosciences(Woburn,MA,USA).Stably transfected HeLa cells(named HeLa-UGT1A1 cells)were constructed as previously reported [15-17].The uridine diphosphate-glucuronic acid(UDPGA),polyethylene glycol hexadecyl ether(Brij 58)and anti-GAPDH antibody(G8795)were purchased from Sigma-Aldrich(St.Louis,MO,USA).Anti-UGT1A1 antibody(ab194697)was obtained from Abcam(Cambridge,MA).The reagents for the SimpleWestern blotting system were purchased from ProteinSimple(San Jose,CA).Ultrapure water purified byMilli-Q®Integral Water Purification System(Millipore,USA)was used throughout,while LC grade methanol,acetonitrile,and formic acid were ordered from Tedia(Fair field,USA).
2.2.Chromatography and analytical conditions
Both NHPN and NHPNG were analyzed by a UFLC system(Shimadzu,Kyoto,Japan),equipped with a CBM-20A communications bus module,two LC-20AD pumps,a DGU-20A5R vacuum degasser,a SIL-20AC autosampler,a CTO-20AC column oven,and an RF-20A fluorescence detector.A Shim-pack VP-ODS C18column(4.6 mm×150 mm,5μm,Shimadzu)was used in this study,and the column temperature was maintained at 40℃.The fluorescence signals of NHPN and NHPNG were recorded with the excitation wavelength at 370 nm,while the emission wavelength was set at 520 nm[13].The mobile phase was a mixture of acetonitrile(A)and 0.2%formic acid(B).The following gradient elution program was used:0-0.5 min,40%-20%B;0.5-1.8 min,20%-5%B;1.8-3.8 min,5%B;3.8-5 min,20%-40%B;and 5-6 min,balance to 40%B.
2.3.Method validation
In this study,the limit of detection(LOD),the limit of quantification(LOQ),the linear range for UGT1A1 activities detection,matrix effects,as well as the precision and stability of the newly developed LC-FD based assay were carefully investigated.LOD and LOQ were determined as the fluorescence signals of NHPNG that produces three and ten folds of the baseline noise,respectively.Standard curves were constructed by using seven concentrations of both NHPN and NHPNG.The linearity of the method was evaluated by linear regression analysis.The inter-day and intra-day precision of the newly identified LC-FD based method was assessed by analyzing three different batches of samples and different concentrations of NHPN(0.2 μM,1 μM and 8 μM)and NHPNG(2 nM,20 nM,80 nM).To assess intra-day precision,the NHPN and NHPNG concentrations in each sample were measured within 24 h.For inter-day assays,NHPN and NHPNG in each sample were quantified for three consecutive days.The overall precision was expressed using relative standard deviation(%,RSD).Stability was evaluated by analyzing the concentrations of NHPNG in UGTs reaction mixtures stored for 24 h or 48 h at 4℃.
2.4.Enzymatic activities and kinetic analyses
The activities and kinetic behavior of NHPN-O-glucuronidation were investigated in both recombinant human UGT1A1 and pooled HLM.Prior to kinetic analyses,the linear fluorescent responses with respect to protein concentration and reaction time in each type of sample were investigated.After that,the kinetic plots of recombinant UGT1A1-mediated NHPN-O-glucuronidation were constructed using the following NHPN concentrations 0,0.1,0.5,1,2,and 5μM.The protein concentrations were 0.01 mg/mL for both recombinant UGT1A1 and HLM.The relationship between the substrate concentration[S]and the rate of formation NHPNG[V]was plotted,and the Hill kinetic equation(Eq.1)was employed to calculate the kinetic parameters.

Here,V is the O-glucuronidation rate,Vmaxis the maximum O-glucuronidation rate,S is the NHPN concentration,S50is the NHPN concentration resulting in 50%of Vmax,and n is the Hill coefficient.
2.5.Determination of UGT1A1 activities and protein level in biological samples
The UGT1A1 activities in tissue preparations from different human samples,including HLM,HIM,HKM and HLuM,were measured by the newly developed LC-FD assay.Tissue preparations(0.2 mg/mL, final protein concentration)were first activated by preincubation with Brij 58 on ice for 20 min.Subsequently,a total volume of 90μL incubation system consisting of NHPN(5μM, final concentration),Tris-HCl buffer(50 mM,pH 7.4),MgCl2(5 mM),and the tissue preparation(mixed with Brij 58)was pre-incubated at 37℃ for 3 min,and then reaction was initiated by the addition of UDPGA(dissolved in water)to a final concentration of 4 mM.Following incubation at 37℃ for 30 min,100 μL ice-cold acetonitrile was added to terminate the reaction.Following centrifugation at 20,000 g for 20 min,the supernatant was subjected to LC-FD analysis.Meanwhile,the UGT1A1 protein levels in HLM,HIM,HKM and HLuM could be assayed by the SimpleWestern blotting system.For the latter,in brief,3μL of total protein lysate(0.4 mg/mL, final concentration)was loaded into a SimpleWes assay plate(12-to 230-kDa,ProteinSimple,USA)and 400 nL of each sample was withdrawn through a capillary according to the manufacturer’s instruction.
2.6.UGT1A1 inhibition assays using various enzyme sources
Nilotinib(a known human UGT1A1 inhibitor)was used for testing the efficiency of the newly developed LC-FD based assay to monitor the O-glucuronidation rates of NHPN in UGT1A1 inhibition assays by various biological systems[18,19].HLM(0.05 mg/mL, final concentration)was first pre-incubated with the same concentration of Brij 58 on ice for 20 min and then at 37℃ for 3 min.Thereafter,an incubation system was conducted as described in Section 2.5 with or without nilotinib(DMSO only).Following incubation for 30 min at 37℃,100 μL ice-cold acetonitrile was added to the incubation system to terminate the reaction.Subsequently,the reaction mixture was centrifuged at 20,000 g for 20 min.The supernatant was subjected to LC-FD analysis.As for UGT1A1 inhibition assays in living cells,Hela-UGT1A1 cell lines were grown in DMEM/high glucose medium supplemented with 10%fetal bovine serum(FBS),in a humidified atmosphere(95%air and 5%CO2)at 37℃.Hela-UGT1A1 cells were seeded in 96-well plates.When cells in 96-well plates were about 50%con fluent,they were treated with nilotinib for 1 h.After that,cells were treated with NHPN(50μM,final concentration)for 3 h,then terminated by adding the equal volume of ice-cold acetonitrile.The reaction mixture was centrifuged at 20,000 g for 20 min.The supernatant was subjected to LCFD analysis.The IC50values of nilotinib on UGT1A1-mediated NHPN-O-glucuronidation in HLM and in recombinant UGT1A1 were estimated by using GraphPad Prism V6.0(Graphpad Software Inc.,La Jolla,USA).
2.7.UGT1A1 induction assays in living cells
Chrysin was used as a positive inducer of UGT1A1[20,21].Here we used it to try and induce UGT1A1 in living cells.In brief,Caco-2 cells were cultured in DMEM/high glucose medium,supplemented with 10%FBS and 1%penicillin-streptomycin solution,in a humidified atmosphere(95%air and 5%CO2)at 37℃.The cells were seeded in 6-well plates and 96-well plates,respectively.Chrysin was added into the cell culture system,at the final concentrations of either 2 μM,10μM,or 25 μM.Following 72 h co-incubation with chrysin,the cells were collected for mRNA assay and UGT1A1 activities assay.For mRNA level assay,the cells were collected with RNAiso Plus reagent and the total RNA was extracted as previously described[22].The cDNA was synthesized from total RNA using RNA PCR kit.Real-time PCR experiments were performed on an ABI 7500 real-time PCR System.The primers used are listed in Table S1[23,24].For UGT1A1 activities assay,after the final day of induction treatment,the cell medium was discarded and the cultured cells were incubated with 50μM NHPN in a serum-free medium.After 3 h incubation,50μL of the medium in each well was mixed with ice-cold acetonitrile(50μL).The mixture was then centrifuged at 20,000 g for 20 min,and the supernatant was subjected to LC-FD analysis.
3.Results and discussion
3.1.Method development

Fig.2.The standard curves of NHPN(A)and NHPNG(B)in Tris-HCl and acetonitrile(1:1,v/v),by using LC-FD based assay.The standard curves of NHPN(C)and NHPNG(D)in Tris-HCl and acetonitrile(1:1,v/v),by using microplate reader based assay.

Table 1 The linear range,LOD,and LOQ of NHPNG using microplate reader and LC-FD.
As mentioned above,we are looking for a way to avoid the interference of endogenous substances and some tested drugs or other xenobiotics which may affect the fluorescence output of the substrate NHPN and its NHPNG[13].To this end,an LC-FD based assay was developed via integration of the advantages of both chromatographic separation and fluorescence detection.As shown in Fig.1B,at the excitation wavelength of 370 nm,both NHPN and NHPNG exhibited good fluorescence response and could be well separated within 3.0 min.The chromatographic separation using acetonitrile(A)and 0.2%formic acid-water(B)as the mobile to generate the mobile phase and the suitable gradient resulted in very high resolution and separation of NHPN and NHPNG(Fig.S1).Notably,most of the endogenous compounds could already be eluted from the ODS column within the first minute(near the dead time),which greatly reduced the interference from endogenous matrix during fluorescence analysis of the two aim analytes(the retention times of the aim analytes were 2.51 min and 1.53 min,for NHPN and NHPNG,respectively).The specificity of the newly developed LC-FD method for detection of NHPN or NHPNG is very high.By the way,as shown in Fig.S2,no interference peaks weredetected in blank tissue samples in the retention time of NHPN or NHPNG.
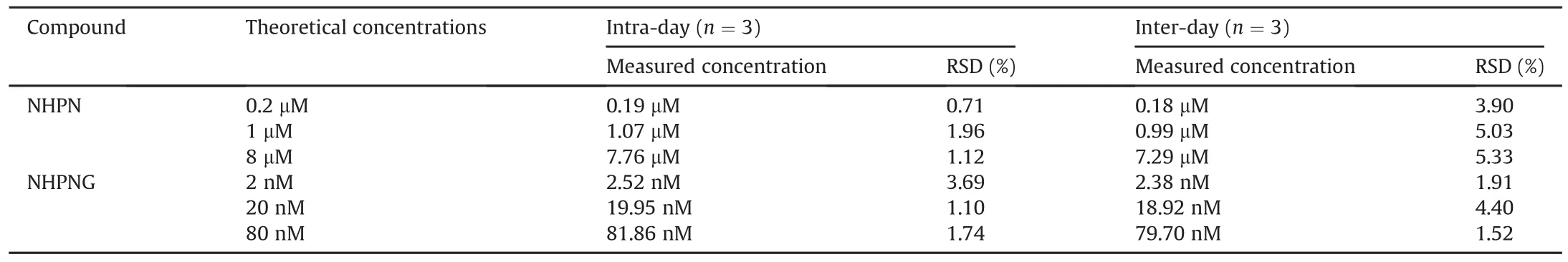
Table 2 Intra-and inter-day variability of the LC-FD based assay for quantitative determination of NHPN and NHPNG.

Table 3 Stability of the main product NHPNG in reaction mixtures.

Fig.3.Enzymatic kinetic plots of NHPN-O-glucuronidation in HLM(A)and in recombinant human UGT1A1(B).
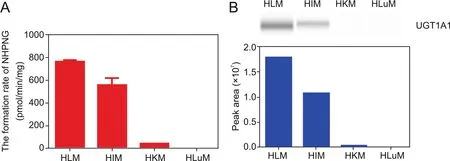
Fig.4.NHPN-O-glucuronidation activities(A)and protein levels(B)of UGT1A1 in HLM,HIM,HKM and HLuM.
3.2.Method validation
The newly developed LC-FD based assay was rigorously validated in terms of linear range,precision,accuracy and stability.As depicted in Figs.2A and B,the calibration curves of both NHPN and NHPNG exhibited good linearity between peak areas and concentrations while using this newly developed LC-FD based assay,within the linear range of 0.1-20μM and 2-100 nM,for NHPN and NHPNG,respectively.Meanwhile,as shown in Figs.2C and D,the calibration curves were also constructed for NHPN and NHPNG and it showed satisfactory linearity between the fluorescence response and the concentration within the range of 0-25μM and 0-16 μM,respectively.The linear fluorescence responses with increasing concentrations of recombinant hUGT1A1 were also investigated and are presented in Fig.S3.Notably,the LOQ of NHPNG was calculated as low as 1 nM(Table 1),which was equivalent to that of the generated NHPNG in 0.16μg/mL of hUGT1A1 under optimized conditions(1μM NHPN was incubated with hUGT1A1 for 30 min at 37℃).To the best of our knowledge,the described LC-FD based method is currently the most sensitive UGT1A1 activities assay.The intra-day and inter-day variabilities of this assay were also determined via quantification of the two main analytes in different samples.As shown in Table 2,the relative standard deviation(RSD)for quantification of the two analytes was less than 5.5%and 5.0%,for NHPN and NHPNG,respectively.Furthermore,as shown in Table 3,the stability of NHPNG in different reaction mixtures was tested by analyzing the samples stored at 4℃ for different storage time(0 h,24 h and 48 h).The results clearly showed that NHPNG in the reaction mixture was stable after storage of 48 h,with over 90%recovery.These findings suggested that the newly developed LC-FD based assay is highly sensitive and reliable,making it is suitable for sensing UGT1A1 activities in biological samples.
3.3.Kinetic analyses using the LC-FD based assay
Enzymatic kinetic behavior of the maker reaction is an important indication for sensing the real activities of the target enzyme in biological samples[25].Unfortunately,kinetic plots of NHPN-O-glucuronidation could hardly be examined by the microplate reader-based assay,owing to the limited sensitivity at low substrate concentrations.In the present study,this problem has been solved and the kinetic plots of NHPN-O-glucuronidation in HLM and in recombinant hUGT1A1 were carefully analyzed using the newly developed LC-FD based method.The kinetic curves of NHPN-O-glucuronidation in both recombinant hUGT1A1 and HLM are displayed in Fig.3.It is clear that the kinetic behaviors of NHPN-O-glucuronidation in both HLM and UGT1A1 are well-fitted to the Hill equation,and the derived kinetic constants are listed in Table S2.Hence,NHPN-O-glucuronidationin both HLM and UGT1A1 exhibited similar kinetic behaviors,with quite similar S50values(0.27μM in recombinant UGT1A1 and 0.41μM in HLM)and similar Vmaxvalues(330.1 pmol/min/mg protein in recombinant UGT1A1 and 307.6 pmol/min/mg protein in HLM).These findings clearly demonstrated that NHPN is a very high-affinity substrate for UGT1A1,with an S50value of less than 0.5μM,which is far below the S50value of other UGT1A1 probe substrates,such as etoposide(S50=285 μM in HLM)or NCHN(S50=365 μM in HLM)[12,26].
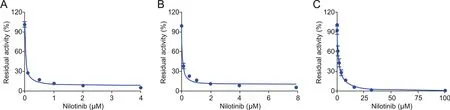
Fig.5.The dose-inhibition curves of nilotinib against UGT1A1-catalyzed NHPN-O-glucuronidation in various enzyme sources,including recombinant UGT1A1(A),HLM(B)and Hela-UGT1A1 living cells(C).
Fig.6.Induction of UGT1A1 by different doses of chrysin(0 μM,2 μM,10 μM,25 μM)in living Caco-2 cells.(A)The UGT1A1 mRNA levels were monitored by real-time PCR,and(B)the UGT1A1 activity was determined by the LC-FD based assay.
3.4.Detection of UGT1A1 activities in tissue preparations
To further explore the practicability of this newly developed LCFD based assay for sensing UGT1A1 activities in real samples,this assay was applied for sensing UGT1A1 activities in real samples.As shown in Fig.4A,the UGT1A1 activities in microsomes from HLM,HIM,HKM and HLuM were determined by the LC-FD,using NHPN as the probe substrate.In addition,the protein levels of UGT1A1 in the same tissue preparations were assayed using Western blotting.The results clearly demonstrated that UGT1A1 was abundant in the human liver and the small intestine,barely detected in the kidney,and was undetectable in lung microsomes(Fig.4B).These findings were highly consistent with those of several previous reports regarding UGT1A1 expression in the human body[27]and with the protein levels of UGT1A1 listed in the protein atlas database(https://www.proteinatlas.org/).It is worth noting that the determined UGT1A1 activities in the different tissue preparations are in good agreement with the(native)UGT1A1 protein levels in these biological samples,suggesting that the LC-FD based assay for sensing UGT1A1 is reliable and can be used for sensing the real activities of this key enzyme in complex biological samples.
3.5.UGT1A1 inhibition assays
The newly developed LC-FD based method was also applied in screening and characterization of UGT1A1 inhibitors,which is very useful for assessing and preventing potential risks of drug/herbdrug interactions.In this study,we used the leukemia drug nilotinib as a known UGT1A1 inhibitor to test the inhibitory effects of nilotinib on UGT1A1 mediated NHPN-O-glucuronidation in recombinant hUGT1A1,pooled HLM and HeLa-UGT1A1 cells.As shown in Fig.5,UGT1A1-mediated NHPN-O-glucuronidation in all the tested enzyme sources could be strongly inhibited by nilotinib in a dose-dependent manner.The IC50values for nilotinib,however,varied between the enzyme sources and they were 29.2 nM,53.1 nM and 1494.0 nM,for recombinant UGT1A1,HLM and HeLa-UGT1A1 cells,respectively(Table S3).The data showed that the inhibition potency of nilotinib against UGT1A1 in living cells is much weaker than in recombinant UGT1A1 and in the isolated microsomal fraction of liver cells.Notably,the much weaker inhibition of UGT1A1 by nilotinib in intact cells,as revealed by our results(Table S3)was in good agreement with the approval of nilotinib as drug and that hyperbilirubinemia was not its main side effect.In addition,the new inhibition results were consistent with those in the previous reports regarding UGT1A1 inhibition[19],and also suggested that the newly developed LC-FD based assay is suitable for characterization and screening UGT1A1 inhibitors in different enzyme sources.
3.6.UGT1A1 induction assays
The new LC-FD based assay was subsequently used to evaluate the induction of the UGT1A1 gene by small molecules in living cells.In this study,Caco-2 cells were used as model cells,while real-time PCR and NHPN-based biochemical methods were used to measure the mRNA level and catalytic activities of UGT1A1,respectively[20].As shown in Fig.6,both the mRNA level and O-lucuronidation activities of UGT1A1 were significantly enhanced by the presence of chrysin in the incubation medium in a dose-dependent manner.Furthermore,the increase in UGT1A1 activities(determined by the new LC-FD assay with NHPN as the substrate)matched quite well the relative abundance of UGT1A1 mRNA levels(detected by realtime PCR).The only slight difference was the relatively high activities seen already at the control sample,which could be attributed tothe fact that UGT1A1 had already been expressed before the start of the experiment.Taking all the results in Fig.6 into account,the results clearly demonstrated that the newly developed UGT1A1 activities assay provided a practical assay for screening and characterization UGT1A1 inducers via monitoring the formation of NHPNG in living cells.
4.Conclusion
In summary,a reliable and easy-to-use LC-FD based assay has been developed and validated for ultra-sensitive UGT1A1 activities assays in various biological systems,using NHPN-O-glucuronidation as the probe reaction.The newly developed LC-FD based assay shows several important advantages over previously reported UGT1A1 activities assays,including high selectivity and sensitivity,reliable and ease of use,as well as strong antiinterference ability.The LOQ of the LC-FD based assay is much lower than that of previously reported UGT1A1 activities assays,making this assay more suitable for sensing UGT1A1 activities in real samples.Furthermore,the new assay system has been successfully applied to quantify UGT1A1 activities in human tissue preparations and even to screening the regulatory effects of small molecules on UGT1A1 in living cells.Collectively,this study provides a reliable and easy-to-use method for sensing UGT1A1 activities in biological systems,which will be very useful for exploring the relevance of this conjugative enzyme to human diseases,as well as for rapid screening and discovery of effective modulators,both inhibitors and inducers of this key enzyme.
Conflicts of interest
The authors declare that there are no Conflicts of interest.
Acknowledgments
This work was finically supported by the NSF of China(81773687,81922070,81973286,81703604),the National Key Research and Development Program of China(2017YFC1700200,2017YFC1702000),the Open Project Program of Jiangsu Key Laboratory for Pharmacology and Safety Evaluation of Chinese Materia Medica(No.JKLPSE-201803),the Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions(PAPD),Program of Shanghai Academic/Technology Research Leader (18XD1403600), Drug Innovation Major Project(2018ZX09731016),Shuguang Program(18SG40)supported by Shanghai Education Development Foundation and Shanghai Municipal Education Commission,and the Graduate Innovation Project of Shanghai University of Traditional Chinese Medicine(Y2019063).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2020.05.005.
 Journal of Pharmaceutical Analysis2020年3期
Journal of Pharmaceutical Analysis2020年3期
- Journal of Pharmaceutical Analysis的其它文章
- Current status and future directions of high-throughput ADME screening in drug discovery
- Current LC-MS-based strategies for characterization and quantification of antibody-drug conjugates
- Current status of in vivo bioanalysis of nano drug delivery systems
- Ultra-sensitive bioanalysis of the therapeutic peptide exenatide for accurate pharmacokinetic analyses at effective plasma concentrations utilizing UPLC-MS/MS
- Software-aided detection and structural characterization of cyclic peptide metabolites in biological matrix by high-resolution mass spectrometry
- Quantitation of DNA by nuclease P1 digestion and UPLC-MS/MS to assess binding efficiency of pyrrolobenzodiazepine
