Coating and Transforming the Y(OH)CO3 Shell on Upconversion Nanoparticles
Dongmei Liu, Xiumei Chen, Ze Yuan, Min Lu, Lisha Yin , Xiaoji Xie , Ling Huang
Institute of Advanced Materials, Nanjing Tech University, Nanjing 211816, P.R.China.
Abstract:Along with the promising applications of lanthanide doped upconversion nanomaterials in diverse fields such as biology, anticounterfeiting, and lasering, the demand for multifunctional upconversion nanomaterials is increasing.One effective means of obtaining these nanomaterials is to fabricate upconversion nanomaterial-based heterostructures, which may provide superior properties as compared to the sum of the parts.However, obtaining heterostructured upconversion nanomaterials remains challenging mainly because of the crystal lattice mismatch between upconversion nanomaterials and other materials.Typically used strategies for synthesizing upconversion nanomaterial-based heterostructures are applicable only to limited types of materials.Alternatively, transformation of the intermediate layer is a promising strategy used to obtain these heterostructures.Nevertheless, this method remains in its infancy and, to date,only a few intermediate layers have been developed.New types of intermediate layers are therefore highly desirable.In this study, we show that amorphous Y(OH)CO3 can be a promising candidate as an intermediate layer for fabricating upconversion nanoparticle-based heterostructures.As a proof-of-concept experiment, ligand-free NaGdF4:Yb/Tm upconversion nanoparticles were first prepared as core nanoparticles.The Y(OH)CO3 shell was then directly coated on the NaGdF4:Yb/Tm upconversion nanoparticles in an aqueous solution using urea and Y(NO3)3, by a homogeneous precipitation approach.The thickness of the resulting Y(OH)CO3 shell could be tuned by adjusting the amounts of either urea or Y(NO3)3.The as-coated Y(OH)CO3 shell could be easily converted to YOF by heating at 300 °C, yielding NaGdF4:Yb/Tm@YOF core-shell heterostructured nanoparticles.In addition, we found that the NaGdF4 core could be transformed to lanthanide oxide fluoride if the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles were heated at 350 °C.We also observed that treating the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles at even higher temperatures (e.g.,400 °C) produced aggregations of nanoparticles without regular morphologies.The transformation of the shell can be attributed to the decomposition of Y(OH)CO3 and reactions between the Y(OH)CO3 shell and NaGdF4 core.Meanwhile,the transformation of the NaGdF4 core at relatively high temperatures could be primarily due to the reactions between Y(OH)CO3 and NaGdF4.Notably, in this study, the core-shell structured nanoparticles, with either a Y(OH)CO3 or YOF shell, maintained the photon upconversion properties of NaGdF4:Yb/Tm upconversion nanoparticles.In addition, the method used here could be extended to the coating of other shells such as Tb(OH)CO3 and Yb(OH)CO3 on upconversion nanoparticles.Moreover, the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles could be transformed to other nanoparticles with novel structures such as yolk-shell nanoparticles.These results can pave the way for preparing upconversion nanoparticle-based heterostructures and multifunctional composites, thus promoting new applications of upconversion nanoparticles.
Key Words:Lanthanide;Doping;Luminescence;Core-shell;Nanomaterials

1 Introduction
Heterostructured core-shell nanomaterials have interested many researchers in various fields, including biology1,2,catalysis3,4, and optoelectronics5,6, due to their distinctive properties generated by the combination of core and shell materials7.Typically, heterostructured core-shell nanomaterials can possess improved or new properties when compared with either the core or the shell materials.Among diverse heterostructured core-shell nanomaterials, lanthanide doped upconversion nanoparticles with core-shell heterostructures,which can convert two or more low-energy photons into highenergy photons, recently have received considerable attention because of their merits and promising applications6,8-17.Specifically, heterostructured core-shell upconversion nanoparticles can improve the luminescent properties of conventional upconversion nanoparticles, giving strong and tunable upconversion emission9,18. Furthermore,heterostructured core-shell upconversion nanoparticles can realize multifunctions, including photocatalysis19-21,theranostics22-25, and highly efficient energy transfer8,26-29,which is difficult to be achieved using bare core or homostructured core-shell upconversion nanoparticles.Despite the advantages of heterostructured core-shell upconversion nanoparticles, only several types of heterostructured core-shell upconversion nanoparticles, such as NaYF4@CaF226,NaYF4@TiO221,30,31, and NaYF4@Fe3O432core-shell nanoparticles, have been developed thus far8,33.The lack of heterostructured core-shell upconversion nanoparticles should be mainly due to the difficulties in synthesizing these nanoparticles1,9,34.
To prepare heterostructured core-shell upconversion nanoparticles, several strategies6,8-10,34, such as electrospinning35,interlayer mediation36, and epitaxial growth37,38, have been developed.However, these strategies still suffer from some limitations.Taking the most widely used epitaxial growth strategy as an example, this strategy usually requires small lattice mismatch between the core and shell materials, which limits this strategy to a few types of materials8.Furthermore, the shell material could also prefer to grow on certain crystallographic planes of core upconversion nanoparticles39,yielding imperfect core-shell nanoparticles.
Alternatively, coating a shell by converting an intermediate layer recently emerges as a promising route for preparing heterostructured core-shell upconversion nanoparticles33.In this strategy, an amorphous layer, serving as an intermediate layer, is first coated on the core nanoparticles, and then transformed to a desired shell under controlled conditions.Thus, heterostructured core-shell upconversion nanoparticles with a large lattice mismatch can be facilely obtained.For example, NaYF4@ZnO and NaYF4@ZnxCd1-xS core-shell upconversion nanoparticles can be synthesized by usingas an intermediate layer40.Nevertheless, limited intermediate layers have been explored thus far, and furthermore current methods for coating an intermediate layer are still complex6,33.Consequently,continuous efforts are needed to be devoted to this strategy for constructing new heterostructured core-shell upconversion nanoparticles.
In this work, using NaGdF4:Yb/Tm upconversion nanoparticles as an example, we develop a simple method to directly coat upconversion nanoparticles with an amorphous Y(OH)CO3 layer.The amorphous Y(OH)CO3 can be converted to other types of materials with crystalline structure, such as YOF, making heterostructured materials on the basis of upconversion nanoparticles.The results shown here should offer a new route to fabricate upconversion nanoparticle-based heterostructures.
2 Experimental and computational section
2.1 Materials
Gadolinium acetate hydrate (Gd(CH3COO)3, 99.9%),ytterbium acetate hydrate (Yb(CH3COO)3, 99.9%), thulium acetate hydrate (Tm(CH3COO)3, 99.9%), yttrium nitrate hexahydrate (Y(NO3)3, 99.9%), oleic acid (90%), 1-octadecene(90%), and NaOH ( > 97%) were obtained from Alfa Aesar.NH4F ( ≥ 98%) was purchased from Sigma-Aldrich.Urea(H2NCONH2, AR) and all other chemicals (AR grade) were obtained from Sinopharm Chemical Reagent Beijing Co., Ltd.All the chemicals were used as received unless otherwise noted.
2.2 Synthesis of NaGdF4:Yb/Tm@Y(OH)CO3 coreshell nanoparticles
NaGdF4:Yb/Tm (49/1 mol%, mole fraction) upconversion nanoparticles were first synthesized according to previous reports41.Briefly, to a two-neck round-bottom flask, oleic acid(4 mL) and an aqueous solution (2 mL) containing Gd(CH3COO)3(0.2 mmol), Yb(CH3COO)3(0.196 mmol), and Tm(CH3COO)3(0.004 mmol) were added, and the resulting mixture was stirred (300 r∙min-1) at 130 °C for 30 min.The mixture was then heated at 150 °C for 30 min, followed by the addition of 1-octadecene (6 mL).After the mixture was kept at 150 °C for another 30 min, the mixture was cooled to room temperature.Subsequently, a methanol solution (5 mL) of NaOH(1.0 mmol) and NH4F (1.2 mmol) was added to the flask, and the resulting mixture was stirred at room temperature (~23 °C) for 1 h.The mixture was then heated to 100 °C, and the reaction system was vacuumed with an oil pump for ~10 min.Next, the reaction mixture was heated to 280 °C within ~22 min and kept for ~1.5 h under a N2atmosphere.After the mixture was cooled to room temperature, the NaGdF4:Yb/Tm upconversion nanoparticles were precipitated by ethanol, collected by centrifugation, and washed with ethanol.Finally, the NaGdF4:Yb/Tm nanoparticles were stored in cyclohexane (4 mL) for further use.
The oleic acid ligands, on the surface of NaGdF4:Yb/Tm nanoparticles, were then removed by HCl treatment41.Typically, the obtained NaGdF4:Yb/Tm nanoparticles in cyclohexane (1 mL) were first collected by the addition of ethanol (0.8 mL) and centrifugation (16000 r∙min-1, 5 min).The collected nanoparticles were dispersed in ethanol (0.8 mL) by ultrasonication, followed by the addition of HCl (1 mol∙L-1, 0.8 mL).The mixture was then sonicated for ~30 s, and the nanoparticles were collected by centrifugation (16000 r·min-1,10 min).Thecollected nanoparticles were further washed with a mixture of ethanol (0.8 mL) and HCl (1 mol∙L-1, 0.2 mL) in a similar manner.Finally, the ligand-free NaGdF4:Yb/Tm nanoparticles were obtained and redispersed in deionized water.
To coat Y(OH)CO3on NaGdF4:Yb/Tm nanoparticles, ligandfree NaGdF4:Yb/Tm nanoparticles (5 mg) were first dispersed in water (10 mL) and sonicated for 30 min at room temperature.To the dispersion, an aqueous solution of Y(NO3)3(0.1 mol∙L-1,0.75 mL) and urea (0.225 g) were added, and the resulting mixture was stirred (400 r∙min-1) at room temperature for 30 min.Subsequently, the flask containing the mixture was kept at 90 °C for 4 h under steady stirring (400 r∙min-1).After the reaction mixture was cooled to room temperature, the NaGdF4:Yb/Tm@Y(OH)CO3core-shell nanoparticles were collected by centrifugation (5000 r∙min-1, 5 min), washed with ethanol and water twice, and finally redispersed in water (2 mL)for further use.Pure Y(OH)CO3 was synthesized according to the same procedure without the addition of NaGdF4:Yb/Tm nanoparticles.
2.3 Transformation of the Y(OH)CO3 shell of the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles
To transform the Y(OH)CO3shell layer, the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles were first dried at 70 °C in a vacuum oven, and the resulting dry powder of the nanoparticles was then placed in a tube furnace.The tube furnace was then purged with Ar for 10 min.Afterwards, the nanoparticles were heated to desired temperature with a rate of 5 °C⋅min-1, and then kept at the temperature for 3 h under an Ar atmosphere.Finally, the nanoparticles were cooled to room temperature naturally and collected for further use.
2.4 Characterization
Transmission electron microscopy (TEM) measurements were performed on a JEM-1400Plus (JEOL, Japan) transmission electron microscope operated at an acceleration voltage of 120 kV.High resolution TEM, dark-field scanning transmission electron microscopy (STEM), elemental mapping, and energydispersive X-ray spectroscopy (EDX) measurements were conducted on a JEM-2100F (JEOL, Japan) transmission electron microscope, equipped with an energy-dispersive spectrometer(Oxford, UK), at an acceleration voltage of 200 kV.Powder X-ray diffraction (XRD) patterns were recorded using CuKαradiation (λ= 1.5406 Å, 1 Å = 0.1 nm) in a Rigaku Smartlab (9 kW, Japan) X-ray diffractometer.Fourier transform infrared(FTIR) spectra were recorded on a compact FTIR spectrometer(Alpha, Bruker, Germany).Upconversion luminescence spectra were obtained on a fluorescent spectrometer (FLS1000,Edinburgh, UK) coupled with a power tunable diode laser (980 nm).Note that the luminescence spectra were presented without adding the intensity correction of the photodetector.Thermogravimetric (TG) analysis was performed on a TG analyzer (TGA2, Mettler-Toledo, Switzerland) under the N2 atmosphere at a heating rate of 5 °C∙min-1.
3 Results and discussion
To coat the Y(OH)CO3shell layer on upconversion nanoparticles, we first synthesized NaGdF4:Yb/Tm (49/1 mol%,mole fraction) upconversion nanoparticles, with oleic acid ligands on the surface, by the well-documented thermal coprecipitation method, because of the intriguing properties of NaGdF4:Yb/Tm nanoparticles37.As revealed by the transmission electron microscopy (TEM) analysis (Fig.1a and Fig.S1 (Supporting Information (SI))), the as-synthesized NaGdF4:Yb/Tm nanoparticles are polyhedrons with a size of near 40 nm (Fig.S1b) and a thickness about 12 nm (Fig.S1c)according to the different orientations of the nanoparticles on the TEM grid.Both the powder X-ray diffraction (XRD) and high-resolution TEM analyses show that the obtained nanoparticles hold the single crystalline nature with a hexagonal NaGdF4crystal structure (Fig.1b and j).

Fig.1 (a) TEM image of the as-synthesized NaGdF4:Yb/Tm (49/1 mol%) upconversion nanoparticles with oleic acid ligands.(b) High resolution TEM image of a NaGdF4:Yb/Tm nanoparticle shown in (a) (upper panel) and corresponding Fourier transform diffraction pattern (lower panel).(c, d) TEM and dark-field STEM images of the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles, respectively.(e-i) Corresponding elemental mapping of the core-shell nanoparticles shown in (d).Scale bars are 50 nm for panels (d-i).Note that the element maps of (e, h) are overlapped with the STEM image for comparison.(j) XRD patterns of the oleic acid capped NaGdF4:Yb/Tm nanoparticles, Y(OH)CO3, and NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles.Note that the diffraction pattern at the bottom of (j) is the literature reference for hexagonal NaGdF4 crystals (JCPDS 27-0699).
Considering that Y(OH)CO3 was typically synthesized in aqueous solutions, we then removed the oleic acid ligand on NaGdF4:Yb/Tm nanoparticles by thorough acid treatment in order to make the nanoparticles hydrophilic41.TEM image (Fig.S2a (SI)) shows that, after ligand removal, the nanoparticles,named ligand-free NaGdF4:Yb/Tm nanoparticles, remained monodispersed.Although the size of the nanoparticles became slightly smaller after acid treatment (Fig.S2c) because of acid etching41, the resulting ligand-free NaGdF4:Yb/Tm nanoparticles maintained their crystallinity and crystal structure(Fig.S2b and d).Our further Fourier transform infrared (FTIR)spectroscopy studies confirmed the successful removal of oleic acid ligands (Fig.S3 (SI)).In addition, the ligand-free nanoparticles exhibited almost the same upconversion luminescence properties as those of the oleic acid capped nanoparticles (Fig.S4 (SI)).Upconversion emission of both Tm3+and Gd3+ions, mainly centred at ~310 (Gd3+:6P7/2→8S7/2),345 (Tm3+:1I6→3F4), 360 (Tm3+:1D2→3H6), 450 (Tm3+:1D2→3F4), 475 (Tm3+:1G4 →3H6), and 800 (Tm3+:3H4 →3H6) nm,could be observed under 980 nm excitation (Fig.S4).
Next, we tried to directly coat the Y(OH)CO3shell on ligandfree NaGdF4:Yb/Tm nanoparticles by mixing the nanoparticles with Y(NO3)3and urea in an aqueous solution.Y(OH)CO3coated nanoparticles, with the size of ~65 nm, were obtained as indicated by the TEM images (Fig.1c and Fig.S5 (SI)), although the core-shell structure could not be clearly revealed by TEM.We then used dark-field scanning transmission electron microscopy (STEM) to confirm the core-shell structure of the Y(OH)CO3coated nanoparticles.As shown in Fig.1d and Fig.S6 (SI), the sharp contrast in the dark-field STEM images clearly reveals the core-shell structure of the Y(OH)CO3coated nanoparticles.Meanwhile, according to the elemental mapping of the Y(OH)CO3coated nanoparticles (Fig.1e-i), together with energy-dispersive X-ray spectroscopy (EDX) analysis (Fig.S7(SI)), we found that F, Gd, and Yb elements mainly located in the centre of the nanoparticles, while Y and O elements distributed almost evenly.These results confirm the core-shell structure of the Y(OH)CO3 coated NaGdF4:Yb/Tm nanoparticles, and thus we here denote the core-shell nanoparticles as NaGdF4:Yb/Tm@Y(OH)CO3.
We then studied the crystal structure and upconversion properties of the NaGdF4:Yb/Tm@Y(OH)CO3core-shell nanoparticles.Typically, nano/micro-sized Y(OH)CO3is amorphous42,43, and consistently, bare Y(OH)CO3, synthesized under our experimental conditions, is also amorphous as indicated by the broad band in the XRD pattern (Fig.1j).After Y(OH)CO3was coated on NaGdF4:Yb/Tm nanoparticles, we only found diffraction peaks of NaGdF4(Fig.1j), indicating the amorphous feature of the Y(OH)CO3shell.Benefiting from the amorphous feature of the Y(OH)CO3 shell, the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles maintained the upconversion properties of the NaGdF4:Yb/Tm core nanoparticles (Fig.2).It should be mentioned that the upconversion emission of the core-shell nanoparticles becomes weaker when compared with that of core nanoparticles (Fig.S8(SI)), which could be due to the scattering of light caused by the thick shell.
On a separate note, we found that the thickness of Y(OH)CO3shell could be tuned by adjusting the amounts of Y(NO3)3and urea during the reaction (Figs.S9 and S10 (SI)).Specifically, in the presence of excess urea, the shell thickness increased with the increase of Y(NO3)3 (Fig.S9).If the amount of Y(NO3)3 was fixed, the Y(OH)CO3 shell first became thicker and then remained almost unchanged when more urea was added (Fig.S10).These results indicate that Y(NO3)3mainly determines the thickness of the Y(OH)CO3shell on NaGdF4:Yb/Tm core nanoparticles under our experimental conditions.
One benefit of the amorphous Y(OH)CO3 shell is that Y(OH)CO3 can be facilely transformed to other materials42,43.In the following sets of experiments, considering that Y(OH)CO3can be easily decomposed under heating, we tried to transform the amorphous shell to oxides.In order to select the proper heating temperature, thermogravimetric (TG) analysis of NaGdF4, Y(OH)CO3 and NaGdF4:Yb/Tm@Y(OH)CO3 coreshell nanoparticles was performed (Fig.3a).We found that NaGdF4nanoparticles were relatively stable under a N2atmosphere even heated to 800 °C.In contrast, both Y(OH)CO3and NaGdF4:Yb/Tm@Y(OH)CO3continuously lost weight during heating, and we attributed the weight loss below 200 °C to the loss of H2O.Accordingly, temperatures higher than 200 °C were selected for further shell transformation.
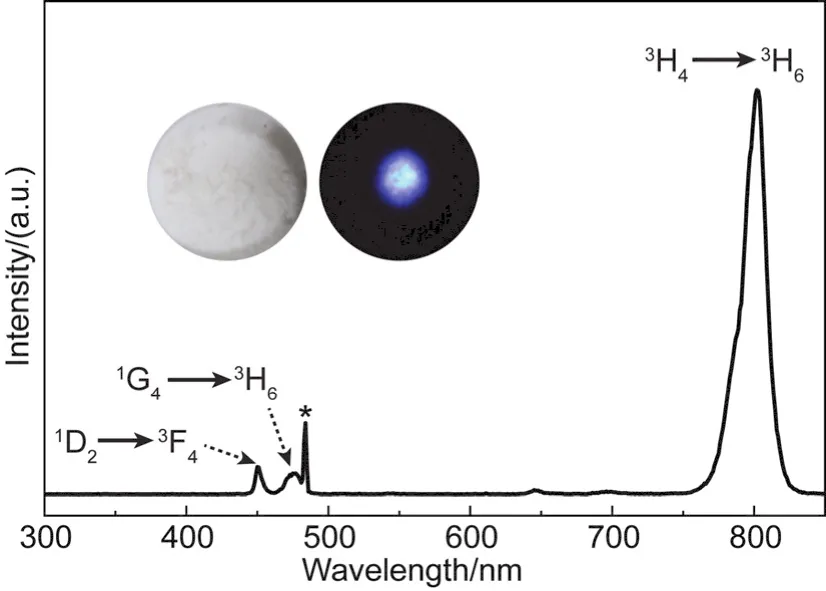
Fig.2 Upconversion luminescence spectrum of the powder of NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles under a 980 nm laser excitation.Note that the peak marked by asterisk at ~490 nm should be due to the scattering of excitation laser.The insets in the figure are photos of the core-shell nanoparticle powder under daylight (left) and the excitation of a 980 nm laser (right).
In an attempt to transform the Y(OH)CO3shell, we heated the NaGdF4:Yb/Tm@Y(OH)CO3core-shell nanoparticles, with a shell of ~8 nm, at temperatures higher than 200 °C.We found that the nanoparticles remained their core-shell structure after being heated at 300 and 350 °C as revealed by dark-field STEM images (Fig.3b, c).In particular, some small low-contrast areas,in the dark-field STEM images, were observed in the core of the heat-treated nanoparticles.Our further elemental mapping analysis revealed that some F element diffused to the shell layer(Fig.3d-i and Fig.S11 (SI)).These results indicate that certain reactions occur between core and shell materials during the thermal treatment.Meanwhile, treating the core-shell nanoparticles at even higher temperatures, such as 400, 450 and 500 °C, made the nanoparticles aggregate and lose their morphologies (Fig.S12 (SI)).In addition, the core and shell materials looked fused together according to the elemental analysis (Fig.S13 (SI)), where all elements almost distributed evenly.

Fig.3 (a) Thermogravimetric analysis curves of Y(OH)CO3, ligandfree NaGdF4:Yb/Tm and NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles.(b-d) Dark-field STEM images of the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles after heated at(b, d) 300 and (c) 350 °C, respectively.(e-i) Corresponding elemental mapping of the nanoparticles shown in (d).Scale bars are 50 nm for panels (d-i).Note that the element maps of (e, h) are overlapped with the STEM image for comparison.
Subsequently, we analysed the heat-treated core-shell nanoparticles by XRD (Fig. 4a). For the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles treated at 300 °C, characteristic diffraction peaks of NaGdF4 crystals were observed.Meanwhile, some new diffraction peaks that can be assigned to YOF crystals appeared.In stark contrast, no diffraction peak of NaGdF4crystals was recorded, when the core-shell nanoparticles were treated at 350 °C for 3 h.Only characteristic diffraction peaks of YOF crystals could be found.Similar results were also found when the core-shell nanoparticles were treated at even higher temperatures (e.g.,400, 450 and 500 °C, Fig.S14a (SI)).Taking the dark-field STEM images and elemental mapping results into account (Fig.3b-i and S11), these observations indicate that the Y(OH)CO3shell can decompose and react with the NaGdF4 core at 300 °C,giving YOF shell coated core-shell nanoparticles.In addition, at relatively high temperature (e.g.350 °C), we deduce that the NaGdF4:Yb/Tm core is also transformed to lanthanide oxide fluoride, yielding core-shell structured lanthanide oxide fluoride.It should be mentioned that GdOF and YbOF have similar crystal structures to that of YOF (Fig.S14b).We reason that the formation of lanthanide oxide fluoride in the core should be due to the reaction between NaGdF4:Yb/Tm and oxygen containing species in the shell at high temperature.

Fig.4 XRD patterns of the NaGdF4:Yb/Tm@Y(OH)CO3 core-shell nanoparticles after heated at 300 and 350 °C for 3 h.Note that the diffraction patterns at the top and bottom are the literature references for hexagonal NaGdF4 (JCPDS 27-0699) and YOF (JCPDS 25-1012)crystals, respectively.(b, c) High resolution TEM images (left panel)and corresponding Fourier transform diffraction patterns (right panel)of the shell layer of the core-shell nanoparticles after treated at(b) 300 °C and (c) 350 °C for 3 h, respectively.
In a parallel set of experiments, we studied the transformed shell by high-resolution TEM (Fig.4b, c).Lattice fringes can be clearly observed in the shell of the heat-treated core-shell nanoparticles, while the shell exhibited polycrystallinity.Besides, the transformed core-shell nanoparticles maintained the upconversion properties (Fig.S15 (SI)).Taking aforementioned results together, we can conclude that the Y(OH)CO3 shell can be converted to YOF, forming heterostructured core-shell nanoparticles.Furthermore, the NaGdF4core nanoparticles can react with shell material under proper conditions, transforming to core-shell structured lanthanide oxide fluoride nanoparticles.In addition, considering the amorphous features of Y(OH)CO3and the polycrystallinity of the YOF shell, we deduce that the shell of the obtained NaGdF4:Yb/Tm@YOF core-shell heterostructured nanoparticles may possess porous structures,which can be useful for drug loading and delivery.
One advantage of our strategy shown here is its versatility.For example, different shells, such as Tb(OH)CO3and Yb(OH)CO3,can be facilely coated on NaGdF4:Yb/Tm upconversion nanoparticles under almost the same conditions (Fig.S16 (SI)).Meanwhile, other core materials, like NaYF4:Yb/Er upconversion nanoparticles, can also be used for shell coating(Fig.S16) and further transformation of shell.Moreover, the NaGdF4:Yb/Tm@Y(OH)CO3core-shell nanoparticles can be further transformed to nanoparticles with other novel structures.Representatively, yolk-shell structured nanoparticles can be obtained after the NaGdF4:Yb/Tm@Y(OH)CO3core-shell nanoparticles were hydrothermally treated (Fig.S17 (SI)), which can be a potential candidate for drug delivery.
4 Conclusions
In conclusion, we have fabricated heterostructured core-shell upconversion nanoparticles by using amorphous Y(OH)CO3as an intermediate shell layer.The Y(OH)CO3shell can be converted to YOF through thermal treatment.Meanwhile, the core materials can be converted through thermal treatment due to the reactions between the core and shell materials.We believe that the amorphous Y(OH)CO3 shell can also be converted to fluorides or phosphates under proper conditions.Moreover, the results shown here should shed light on the preparation of upconversion nanoparticle-based heterostructures, widening the potential applications of upconversion nanomaterials.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.

