Construction of Pt-M (M = Co, Ni, Fe)/g-C3N4 Composites for Highly Efficient Photocatalytic H2 Generation
Liang Wang , Chenglu Zhu , Lisha Yin ,2,*, Wei Huang
1 Institute of Advanced Materials, Nanjing Tech University, Nanjing 211816, P.R.China.
2 School of Materials Science and Engineering, Nanyang Technological University, Singapore 639798.
Abstract:Platinum (Pt) is recognized as an excellent cocatalyst which not only suppresses the charge carrier recombination of the photocatalyst but also reduces the overpotential for photocatalytic H2 generation.Albeit of its good performance, the high cost and low abundance restricted the utilization of Pt in large-scale photocatalytic H2 generation.Pt based transition metal alloys are demonstrated to reveal enhanced activities towards various catalytic reactions,suggesting the possibility to substitute Pt as the cocatalyst.In the present work,Pt was partially substituted with Co, Ni, and Fe and Pt-M (M = Co, Ni, and Fe)/g-C3N4 composites were constructed through co-reduction of H2PtCl6 and transition metal salts by the reductant of ethylene glycol.The crystal structure and valence states were measured by X-ray diffractometer (XRD) and X-ray photoelectron spectrometer (XPS), respectively.The higher degree of XRD peaks and larger binding energies for Pt 4f5/2 and Pt 4f7/2 after incorporating Co2+ ions indicated that Co was successfully introduced into the lattice of Pt and Pt-Co bimetallic alloys was attained through the solvothermal treatment.The morphology was subsequently observed by transmission electron microscope (TEM), which showed a good dispersion of Pt-Co nanoparticles on the surface of g-C3N4.Meanwhile, the shrinkage of lattice fringe after introducing cobalt salt further confirmed the presence of Pt-Co bimetallic alloys.The UV-Vis absorption spectra of g-C3N4 and Pt, Pt-Co deposited g-C3N4 were subsequently performed.It was found that the absorption edges were all consistent for all three samples as anticipated, implying that the band gap energy was maintained after hybridizing with Pt or Pt-Co alloys.Furthermore, the photocatalytic H2 generation was carried out over the as-prepared composites with triethanolamine (TEOA) as sacrificial reagent.Under visible-light illumination, the1%(w) Pt2.5M/g-C3N4 (M = Co, Fe, Ni) composites all exhibited higher or comparable activity towards photocatalytic H2 generation when compared to 1% (w) Pt loaded counterpart.In addition, the atomic ratios of Pt/Co and the loading amount of Pt-Co cocatalyst were modified to optimize the photocatalytic performance, among which, 1% (w) Pt2.5Co/g-C3N4 composite revealed the highest activity with a 1.6-time enhancement.Electrochemical impedance spectra (EIS) and photoluminescence (PL) spectra indicated that the enhancement might be attributed to improved charge transfer from g-C3N4 to Pt2.5Co cocatalyst and inhibited charge carrier recombination in the presence of Pt2.5Co cocatalyst.Therefore, the present study demonstrates the great potential to partially replace Pt with low-cost and abundant transition metals and to fabricate Pt based bimetallic alloys as promising cocatalysts for highly efficient photocatalytic H2 generation.
Key Words:Photocatalytic H2 generation;Composite;Cocatalyst;g-C3N4;Pt-Co alloy
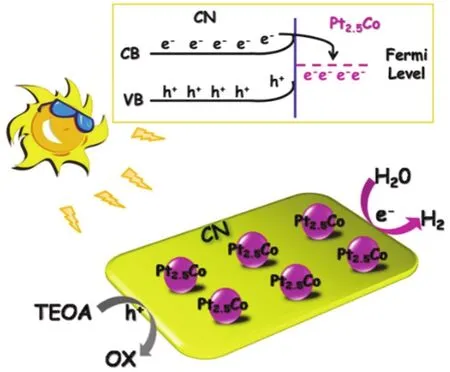
1 Introduction
Photocatalytic water splitting is a promising strategy to generate clean and sustainable hydrogen as an energy carrier.The core challenge in this process is the choice of a suitable photocatalyst.An ideal photocatalyst should possess appropriate band structure to respond to visible light as well as produce photoexcited electrons with sufficient reduction potential to split water into H21.Besides, the photocatalyst should be chemically stable, low-cost and non-toxic.From this point of view, g-C3N4is a potential candidate which could meet the requirements aforementioned2.Therefore, g-C3N4has attracted enormous attention since the first report of its application in photocatalytic water splitting3,4.Nevertheless, the photocatalytic performance of g-C3N4is moderate under visible light due to rapid recombination of photogenerated e-/h+pairs and high overpotential2,5,6.On the one hand, the lifetime of the photogenerated e-/h+pairs is only a few nanoseconds (ns)7.If they are not able to migrate to the surface of g-C3N4within this duration, they would recombine.On the other hand, the weak van der Waals’ forces between the layers and the hydrogen bonds between the domains of g-C3N4structure would inhibit the migration and transfer of photogenerated charge carriers as well.Consequently, only a small proportion of photogenerated e-/h+pairs would reach the surface reactive sites through tri-s-triazine unit connected planes and participate in the following redox reactions.
Metal nanoparticles are of great significance in the applications such as catalysis, electronics, and biology, etc.which account for two thirds of all elements in the periodic table8-10.Loading of noble metals (e.g.Pt, Pd, Au and Ag) as cocatalysts is reported to be an efficient approach to enhance the photocatalytic performance of various photocatalysts11-13.Generally, Pt and other noble metals show much larger work functions (> 5.0 eV)as compared to the semiconductor photocatalysts.For noble metal/semiconductor composites, the mismatch of Fermi levels would drive the electrons to migrate from n-type semiconductor to the metal, resulting in band bending and formation of Schottky barrier which would then hinder the recombination of photogenerated e-/h+pairs14.Moreover, the presence of metal nanoparticles as cocatalysts could also reduce the overpotential from several hundred millivolts (mV) to tens of mV for the surface redox reaction, further enhancing the photocatalytic performance15.Meanwhile, cocatalysts would accelerate photocatalytic reactions by inhibiting backward reactions16.
Pt is the model cocatalyst that works efficiently on a majority of photocatalysts for photocatalytic hydrogen generation due to its high work function (5.6-6.1 eV)17.For example, Yuet al.reported that photodeposited Pt on TiO2nanosheets with exposed (001) facet could exhibit a rate as high as 333.5µmol⋅h-1for photocatalytic H2generation at 2% (w) loading amount, albeit TiO2alone is inactive under UV light illumination ascribed to rapid charge carrier recombination18.Xu and coworkers found that platinum were loaded onto the surface of CdS in the form of Pt0and Pt2+, respectively, after photochemical reduction from H2PtCl6in alkaline solution and acidic/neutral solution19.Madea and co-workers further demonstrated that the nonionic complex (bis(1,5-cyclooctadiene)platinum complex)led to a better dispersion of Pt on g-C3N4than ionic H2PtCl6and hence an enhanced activity was attained20.Despite of its high efficiency, the rareness and high cost limit the implementation of Pt in large-scale photocatalytic H2generation in the future.Therefore, low-cost and highly efficient cocatalysts composed of abundant elements should be explored.
Recently, Pt based transition metal alloys have been demonstrated to show excellent performances in a variety of catalytic reactions21.For instance, Pt3Co, Pt3Ni and Pt3Fe alloys revealed higher activity than pure Pt for oxygen reduction reaction (ORR)22-25.PtCo-Cu2ZnGeS4hetero-structured nanoparticles could facilitate the reduction of I-3to I-in dyesensitized solar cells and exhibited an impressive power conversion efficiency of 8.12% as a counter electrode, which was higher than the Pt counterpart (7.69%)26.In case of photocatalytic H2generation, Yu and co-authors achieved almost doubled photocatalytic activity upon loading Pt3Co on CdS as compared to the Pt-loaded counterpart27.However, the study of Pt3Co and other Pt based transition metal alloys as cocatalysts in photocatalysis is still on the way.Herein, we report the substitution of Pt with Co, Ni or Fe and preparation of Pt-M(M = Co, Ni, Fe) bimetallic alloys, which arein situanchored on the surface of g-C3N4with high dispersion and good stability through simultaneous reduction of Pt4+and Co2+/Ni2+/Fe3+by ethylene glycol.Results indicated that Pt-M (M = Co, Ni, Fe)cocatalysts could remarkably enhance the photocatalytic performance of g-C3N4towards hydrogen generation.Among the three composites, Pt-Co/g-C3N4sample with Pt/Co ratio of 2.5/1 and loading amount of 1% (w) exhibited 1.6-fold enhancement in comparison with Pt/g-C3N4counterpart under the same conditions, providing a good example for the development of low-cost and highly efficient photocatalytic systems.
2 Experimental
2.1 Materials
Melamine (99%), dihydrogen hexachloroplatinate (IV)hexahydrate (H2PtCl6⋅6H2O, 8% in water), cobalt nitrate hexahydrate (Co(NO3)2⋅6H2O, 98%), ethylene glycol(≥ 99%),ferric chloride hexahydrate (FeCl3⋅6H2O, 97%), nickel acetate tetrahydrate (Ni(CH3COO)2⋅4H2O, 98%), and triethanolamine(TEOA, ≥ 98%) were purchased from Sigma-Aldrich and used directly without further purification.
2.2 Samples preparation
2.2.1 Preparation of g-C3N4
g-C3N4(denoted as CN from hereafter) was preparedviapolycondensation method using melamine as the precursor according to our previous work28.
2.2.2 Preparation of Pt/g-C3N4, Co/g-C3N4and Pt-M/g-C3N4(M = Co, Fe, Ni) composites
Pt-Co alloys were anchored onto the surface of CNviaa polyol process in which H2PtCl6⋅6H2O, and Co(NO3)2⋅6H2O were utilized as Pt and Co precursor, respectively.In a typical synthesis of 1% (w) PtxCo/g-C3N4composite (wherexdonated as the atomic ratio of Pt/Co and the sample was labelled as PtxCoCN), a desired amount of H2PtCl6⋅6H2O and Co(NO3)2⋅6H2O (the resulting PtxCo alloys were all 3 mg), and 297 mg CN were subsequently added into 20 mL ethylene glycol at the pH of 11 (adjusted by 1 mol∙L-1NaOH aqueous solution).Then the mixture was sonicated and stirred to get a homogeneous solution before transferred into a 40-mL Teflonlined stainless steel autoclave.Afterwards, the autoclave was kept at 473 K for 10 h.Finally, the obtained sample was washed with distilled water and absolute ethanol for three times, and dried in the vacuum oven.
For other loading amount of PtxCoCN samples, the amount of PtxCo alloy was maintained at 3 mg whereas the amount of CN varied accordingly.
For 1% (w) Pt (or Co) loaded sample, only Pt precursor (or Co precursor) was added with a final metal product of 3 mg.
In the case of 1% (w) Pt2.5Fe/g-C3N4 and Pt2.5Ni/g-C3N4(donated as Pt2.5FeCN and Pt2.5NiCN, respectively), FeCl3⋅6H2O and Ni(CH3COO)2⋅4H2O were used as the transition metal precursors, respectively, while the other conditions were kept the same.
2.3 Characterizations
The crystallographic structures of the resulting samples were characterized by a Shimadzu XRD-6000 X-ray diffractometer(Japan) with CuKαsource under 2-theta mode from 10° to 80°at a scan rate of 1 (°)·min-1.The morphologies were examined by JEOL JEM-2010 and JEOL JEM-2100F transmission electron microscope (TEM, Oxford, UK).The UV-Vis diffuse reflectance spectra were recorded by Lambda 750UV/Vis/NIR spectrophotometer (Perkin-Elmer, USA).The photoluminescence(PL) spectra were measured by using 325 nm excitation on the Shimazu RF-5310PC fluorometer (Japan).
2.4 Photocatalytic H2 generation
A 45-mL closed glass tube reactor was applied in this experiment.In a typical photocatalytic H2generation test, 10 mg of the obtained sample was dispersed into 10 mL of 15% (vol.)TEOA aqueous solution in the reactor.Subsequently, the reactor was sealed with a rubber seal, and the system was purged with nitrogen gas under stirring to drive away the residual air.Afterwards, the reactor was illuminated under 300-W xenon lamp (MAX-302, Asahi Spectra, USA) coupled with a UV cutoff filter (λ> 420 nm).In this process, the suspension was stirred at 750 r·min-1to maintain a homogeneous illumination environment.The generated H2in this process was quantified by a gas chromatograph (GC-7890A, Agilent) with a TCD detector at an interval of 1 h.
2.5 Photoelectrochemical analyses
The photoelectrochemical (PEC) analyses were carried out in an electrochemical cell with a standard three-electrode apparatus on an electrochemical analyzer (Solartron Instruments SI287).A Pt wire and an Ag/AgCl electrode were employed as the counter electrode and the reference electrode, respectively.Prior to the fabrication of the working electrode, a stock solution contained a mixture of 50 µL Nafion (Sigma-Aldrich), 1 mL ethanol and 4 mL DI water was prepared.Then 8 mg of the as-prepared samples were added into 2 mL of the stock solution and sonicated for 2 h to obtain a homogeneous suspension. Afterwards, the desired working electrode was obtained by dispersing the suspension onto the fluorine-doped tin oxide(FTO) glass piece with an exposed area of 0.25 cm2.During the measurement, Xe arc lamp was employed as light source whereas potassium phosphate buffer solution (KPi, 1 mol∙L-1,pH = 7) was used as electrolyte.The Nyquist plots of electrochemical impedance spectra (denoted as EIS) were measured by employing the same setup.During the measurement, the perturbation signal was set at 20 mV and the frequency varied from 10 mHz to 100 kHz.
3 Results and discussion
3.1 Structural characterizations
The polyol reduction method has been extensively used to prepare various alloys and alloy composites, for instance Pt-Co and Pt3Co loaded CdS and TiO227,29-32.In this work, the deposition of Pt and Pt2.5Co nanoparticles onto CN were also achieved by co-reduction of H2PtCl6and Co(NO3)2in ethylene glycol under high-temperature and high-pressure treatment.XRD analyses were performed for the as-prepared 1% (w) PtCN and 10% (w) Pt2.5CoCN to confirm the crystallographic phase of Pt and Pt2.5Co in the composites and to evaluate the influence of Co-substitution in the Pt crystal structure (Fig.1).Two characteristic peaks for CN (13.0° and 27.4°) were observed for both samples as expected.Besides, a small peak at 39.8° was observed for Pt loaded sample, which is assigned to (111) plane of standard Pt (JCPDS 70-2431).However, this peak shifted to 40.5° for the Pt2.5Co loaded sample as indicated in the inset of Fig.1, in good accordance with that reported by Yuet al.27.The Pt 4f5/2and Pt 4f7/2peaks in the XPS measurements (Fig.S1,Supporting Information (SI)) also shifted to higher binding energies after the introduction of Co, in consistent with that reported by Choiet al.33.The shifts both imply the successful preparation of Pt-Co alloy since the smaller size of Co atom could lead to a contraction of lattice fringe and consequently a higher diffraction peak and binding energy.In addition, similar peak shifts towards higher degree were observed for Pt2.5FeCN and Pt2.5NiCN composites (Fig.S2 (SI)), suggesting the successful deposition of Pt2.5Fe and Pt2.5Ni on the surface of g-C3N4.
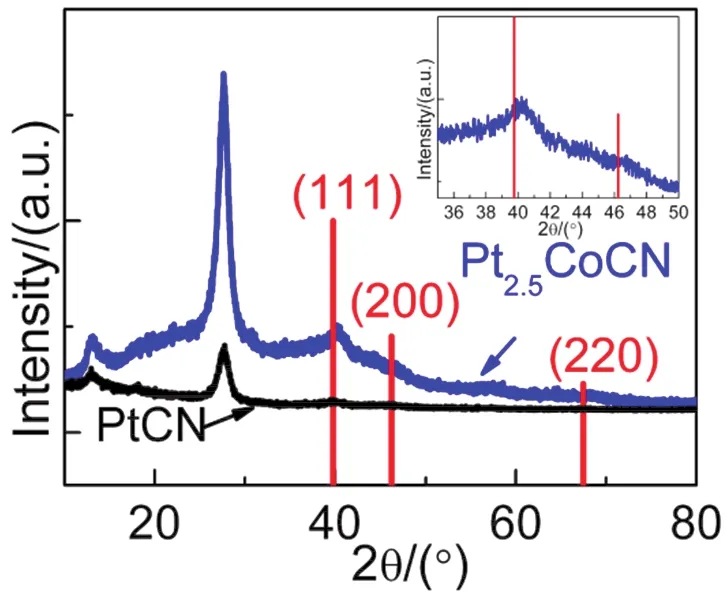
Fig.1 XRD patterns of 1% (w) PtCN (black) and 10% (w) Pt2.5CoCN (blue and inset).
The morphology of as-prepared 1% (w) PtCN and 1% (w)Pt2.5CoCN were examined under TEM and high resolution TEM(HRTEM) as displayed in Fig.2.The bright field TEM image of PtCN (Fig.2a) and dark field TEM image of Pt2.5CoCN (Fig.2b)indicated that the metal cocatalyst nanoparticles were highly dispersed on the surface of CN.The HRTEM image further revealed the lattice fringe of Pt and Pt2.5Co were respectively 0.234 and 0.222 nm, corresponding to (111) facet of Pt.The shrinkage of the lattice fringe after partially replacing Pt with Co further confirmed the successfully preparation of Pt2.5Co alloy,consistent with the XRD patterns.Besides, the energy dispersive spectrum (EDS) (Fig.S3 (SI)) also revealed the presence of C,N, Pt, Co, providing another evidence for the successful deposition of Pt2.5Co alloy on the surface of g-C3N4.
The UV-Vis diffuse reflectance spectra of pristine CN, 1% (w)PtCN and 1% (w) Pt2.5CoCN were shown in Fig.3.In comparison to pristine CN, enhanced light absorption between 460 and 800 nm was observed for the Pt and Pt2.5Co loaded sample, which might be ascribed to light absorption and scattering by the cocatalyst particles since both Pt and Pt2.5Co are black.
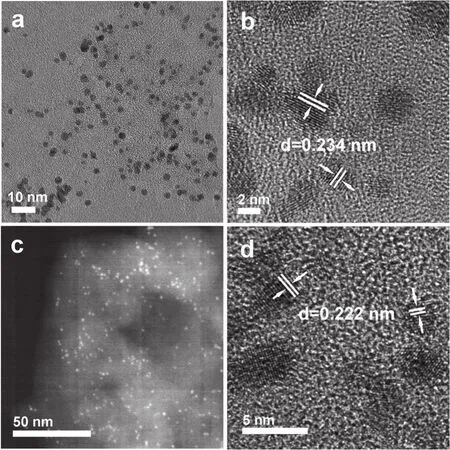
Fig.2 TEM images of 1% (w) PtCN (a, b) and 1% (w) Pt2.5CoCN (c, d).
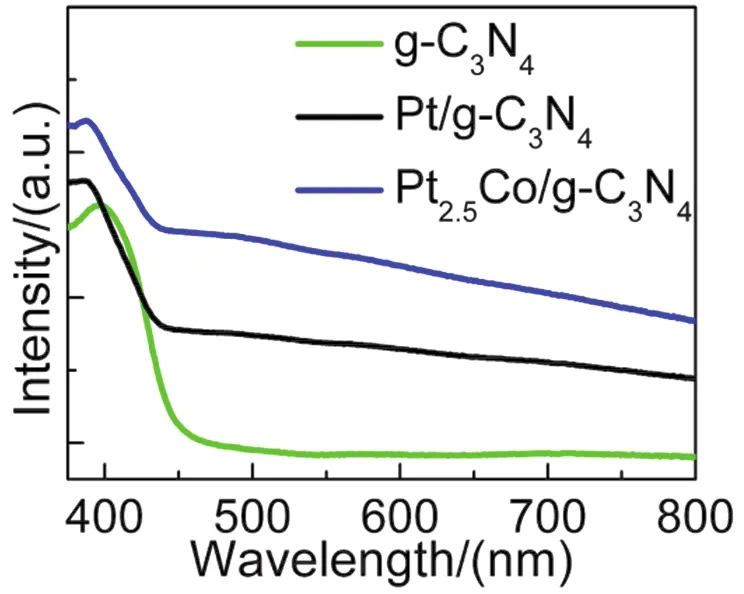
Fig.3 UV-Vis diffuse reflectance spectra of pristine g-C3N4 (green),1% (w) Pt/g-C3N4 (black), 1% (w) Pt2.5Co/g-C3N4 (blue).
3.2 Photocatalytic performance
The photocatalytic activities for H2production over the CN samples loaded with 1% (w) cocatalyst (Pt or Pt-Co alloys of different atomic ratios) were evaluated under visible light illumination in an aqueous solution of 15% (vol.) TEOA as hole scavenger.As indicated in Fig.4a, b, the PtCN showed a medium H2evolution rate of 2.95 µmol⋅h-1(equal to 295 µmol⋅h-1⋅g-1).The introduction of Co into Pt and formation of Pt-Co alloys resulted in a significant improvement in photocatalytic H2evolution rate, demonstrating a vital influence of the Co content in the alloys on the photocatalytic activity.In the presence of a small amount of Co (Pt3Co), the activity over Pt3Co loaded CN was enhanced to 3.91 µmol⋅h-1(equal to 391 µmol⋅h-1⋅g-1).When the Co content further increased to Pt2.5Co, the rate over the composite could ramp up to 4.63 µmol⋅h-1(equal to 463µmol⋅h-1⋅g-1), which is 1.6 times in comparison to pure PtCN.Further increasing Co content in the Pt-Co alloy led to gradual decrease in photocatalytic H2evolution rate.Pt2CoCN exhibited a rate of 3.27 µmol⋅h-1(equal to 327 µmol⋅h-1⋅g-1), slightly higher than that of PtCN.In the case of large ratio of Co, the rate dropped dramatically to 0.49 µmol⋅h-1(equal to 49µmol⋅h-1⋅g-1) for PtCo6CN.Pure Co loaded CN showed a negligible activity of 0.05 µmol⋅h-1(equal to 5 µmol⋅h-1⋅g-1),much lower than that of pure Pt loaded counterpart, indicating Co is a less effective cocatalyst compared with Pt.Among all the samples, 1% (w) Pt2.5CoCN showed the highest activity for H2generation.
Subsequently, the photocatalytic H2production activities over Pt2.5CoCN of various loading amounts were investigated under the same conditions, as shown in Fig.4c, d.Compared to the 1%(w) counterpart, the 0.5% (w) loaded sample showed a slightly lower activity, whereas the activities of 3% (w) and 10% (w)loaded samples were much lower.At high loading amount, the excessive cocatalyst nanoparticles on the CN surface would block the visible light absorption of CN and lead to decreased photocatalytic activities.These results indicated that 1% (w)Pt2.5CoCN exhibited the highest photocatalytic H2production rate under current conditions.Furthermore, the stability of 1%(w) Pt2.5CoCN sample for H2generation was verified through continuous visible-light irradiation for 25 h (Fig.4e).A linear plot of photocatalytic H2evolution rateversustime was obtained, indicating a constant H2production rate.Hence, the asprepared sample exhibited very good stability under the present test conditions.
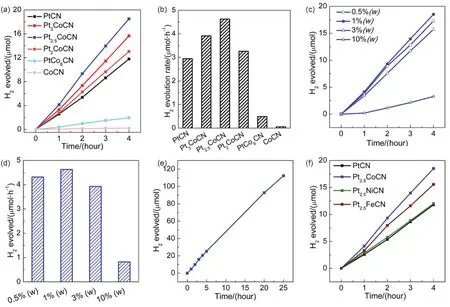
Fig.4 Photocatalytic H2 evolution performance over Pt, Co and Pt-Co alloys of different atomic ratios loaded CN at 1% (w) loading amount (a, b)and over Pt2.5Co CN at various loading amounts (c, d) and stability test over 1% (w) Pt2.5CoCN (e) and photocatalytic H2 evolution rate over Pt based bimetallic cocatalysts loaded CN at 1% (w) loading amount (f).
In addition, other Pt-M (M = Ni, Fe) bimetallic alloys were also synthesized through similar approaches and applied for photocatalytic H2evolution.Fig.4f showed that 1% (w)Pt2.5FeCN exhibited a higher rate than the 1% (w) Pt loaded sample, while 1% (w) Pt2.5NiCN exhibited a similar rate compared to the Pt counterpart.The better performance of Pt2.5Co cocatalyst might be ascribed to the smaller particle size and better dispersion on g-C3N4surface.As indicated in Fig.S4 and S5 (SI), the average size for Pt2.5Co is 1.72 nm, whereas the mean sizes are 2.64 and 2.59 nm for Pt2.5Fe and Pt2.5Ni,respectively.Moreover, aggregation is observed for both Pt2.5FeCN and Pt2.5NiCN composites, but not for Pt2.5CoCN composite.Nevertheless, our studies demonstrate that these Pt based bimetallic alloys (Pt2.5Co, Pt2.5Fe, Pt2.5Ni) are promising Pt substitute as cocatalysts for photocatalytic H2evolution for the consideration of cost and efficiency.
3.3 Proposed mechanism
A tentative mechanism is proposed for the high photocatalytic H2generation activity over 1% (w) Pt2.5CoCN, as illustrated in Fig.5.Upon illumination of visible light, the valence band (VB)electrons of CN are excited and transported to the conduction band (CB).In the presence of Pt2.5Co, the electrons on CB of CN could rapidly migrate to Pt2.5Co from CN attributed to lower Fermi level.These electrons would then participate in the proton reduction to produce H2.
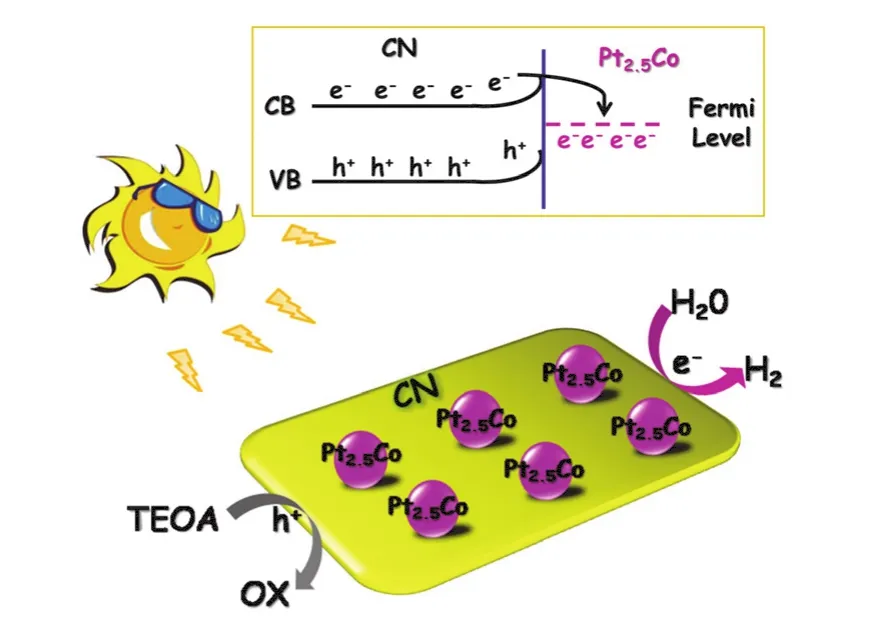
Fig.5 A tentative mechanism proposed for enhanced photocatalytic performance of Pt2.5CoCN.
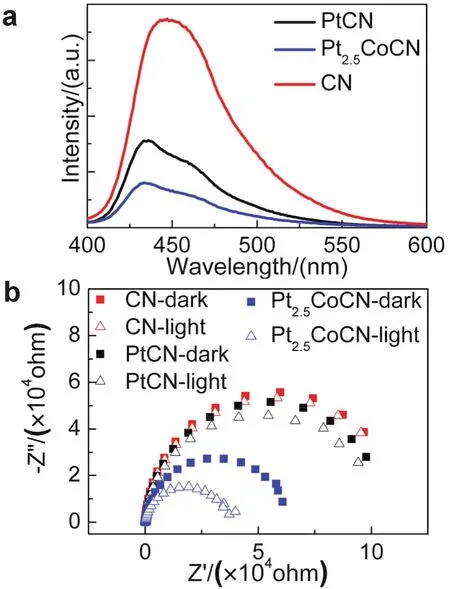
Fig.6 (a) PL spectra of pristine CN, Pt and Pt2.5Co loaded CN.(b) Nyquist plots of EIS with pristine CN (red, solid: in the dark,hollow: under light), Pt (black, solid: in the dark, hollow: under light)and Pt2.5Co (blue, solid: in the dark, hollow: under light) loaded CN working electrodes at voltage of 0.3 V (vs RHE).
PL spectra were performed to verify this charge transfer process (Fig.6a).A quenched PL intensity was observed from pristine CN to PtCN, and subsequently Pt2.5CoCN, suggesting that the electrons transfer from CN to Pt2.5Co is more effective than that from CN to Pt.Moreover, Nyquist plots of EIS measurement were conducted to study the interfacial charge transfer properties of FTO/CN, FTO/CN/Pt, FTO/CN/Pt2.5Co.It was found that the charge transfer resistance decreased in the order of CN > PtCN > Pt2.5CoCN, indicating that the electrons transfer are more efficient at CN/Pt2.5Co interfaces and the charge recombination is effectively suppressed in the presence of Pt2.5Co cocatalyst.
Why do electrons and holes migrate more effectively from CN to Pt2.5Co? According to Wakisaka and co-workers, the 5delectron density of Pt decreased when alloying with a second component34.This might allow the photogenerated electrons to be trapped for a longer time on Pt-Co alloys.On the other hand,Pt could catalyse the back reaction of generated hydrogen due to its robust adsorption ability35.As such, comparing to pure Pt,the Pt-Co alloys exhibit weaker binding with the produced hydrogen36, which reduces the chance of back reaction (H2to H2O) on the metal surface.Therefore, the Pt-Co alloy allow for higher activity of photocatalytic H2generation owing to the promoted charge transfer from excited CN and the reduced rate of back reaction (H2 oxidation).Certainly, excessive Co in the Pt-Co alloy would play negative roles since Pt is the major part to lower down the energy barrier of H+reduction into H atoms.As such, there is an optimal ratio of Pt/Co.In our studies, the optimal ratio (Pt2.5Co) is based on the experimental observation.
4 Conclusions
To summarize, Pt-MCN (M = Co, Fe, Ni) composites were developed by a polyol reduction method with ethylene glycol as the reducing agent.The Pt based bimetallic alloys were all demonstrated to be lower-cost and efficient cocatalysts on the surface of g-C3N4.Remarkably, the 1% (w) Pt2.5CoCN exhibited the highest photocatalytic H2evolution rate, which was even higher than that of 1% (w) PtCN under the same conditions.To explain the enhancement in photocatalytic activity, PL spectra and EIS spectra were conducted.The results indicated that more effective electrons/holes transfer was achieved for Pt2.5CoCN,which was the main reason for the enhancement.Thus, this work provides a universal approach for Pt based transition metal alloys as a class of promising cocatalysts superior to Pt for photocatalytic H2 generation in the future.
Supporting Information:available free of chargeviathe internet at http://www.whxb.pku.edu.cn.

