Prophylactic and therapeutic roles of oleanolic acid and its derivatives in several diseases
Alaattin Sen
Alaattin Sen, Department of Molecular Biology and Genetics,Faculty of Life and Natural Sciences,Abdullah Gul University,Kayseri 38080,Turkey
Abstract
Key words:Oleanolic acid;Prophylactic;Anti-inflammatory;Anti-diabetics;Neuroprotective;Hepatoprotective
INTRODUCTION
Oleanolic acid(OA:3β-hydroxyolean-12-en-28- oic acid,Figure 1)is a biologically active natural pentacyclic triterpenoid compound that is present in over 2000 plant species,as well as numerous food and medicinal plants[1].The compound is particularly common in the Oleaceae family,among which olive(Olea europaea),the plant species after which the compound was entitled,is still the primary supply of mercantile OA.
OA is plentiful in apple skin,papaya fruit,persimmon fruit and leaf,plum,loquat,soybeans,filamentous fungi(Table 1)[2-4].Several medicinal herbs such as ginseng contain OA as one of the active ingredients.The concentrations of OA are often as high as 1% in olive fruit,apple skin,ginseng,papaya fruit and dark plums[5].It is not solely present as a free compound but also occurs as an aglycone precursor for triterpenoid saponins,in which it is bonded to one or more sugar chains[1,5].As a triterpenoid,OA belongs to an oversized cluster of structurally diverse natural products,including sterols,steroids,and triterpenoid saponins[6].
The artificial modification of OA on its three ‘‘reactive'' regions;the C3-OH,the C12=C13double bond,and the C28-COOH,has led to a series of new synthetic oleanane triterpenoids[7-9].Compared to OA,some of these compounds showed increased biological activity such as anti-inflammatory and hepatoprotective activities.One such compound with increased biological activity is 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid(CDDO)or its C-28 methyl ester(CDDO-Me;Figure 2)[1,7,10,11].
PHARMACOLOGY
OA and its derivatives have plenty of useful effects;including remarkable antioxidant,anti-inflammatory,antiviral,and anti-diabetic effects.They are efficacious against proliferation in tumour-bearing mice,such as breast cancer.
Anti-inflammatory effects
Inflammatory processes are characterised by extreme reactive oxygen species(ROS)levels and are related to many pathological conditions,including ulcerative colitis,AD,PD and cancer[12-14].Table 2 summarises the recent studies investigating thein vivoanti-inflammatory effects and related mechanisms of action of OA and its natural or synthetic derivatives[14-26].A proposed potential strategy is to examine the roles of OA and its derivatives in preventing inflammatory responses involving the nuclear factor erythroid-2-related factor 2(NRF-2)and nuclear factor-κB(NF-κB)pathways[15,27](Figure 3).
OA significantly inhibited DSS-induced colitis,as verified by the inhibition of Th17 cells and the downregulation of the expression of interleukin(IL)-1,NF-ĸB,MAPK and RORγt in the colon,whilst the FOXP3 and IL-10 expression,macroscopic score,colon shortening,and myeloperoxidase activity increased.Thus,OA prevents and relieves inflammatory diseases such as colitis[14].Similarly,a multifunctional semisynthetic OA-derivative,i.e.,CDDO-Me prevented the high-fat diet(i.e.,modelling obesity)-induced chronic low-grade inflammation in the rodent colon.It reduced the expression of F4/80,CD11c,COX-2,IL-6,KI67,NF-B,and tumour necrosis factor(TNF)-α but increased CD206 and IL-10,showing an anti-inflammatory mechanism[16].Likewise,another synthetic OA derivative 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28- oyl] imidazole(CDDO-Im)inhibited IL-6 and IL-17 and relieved DSS-induced colitis in mice.CDDO-Im also notably inhibited the signal transducer and activator of transcription-3 activation.Thus,OA and its derivatives have a unique anti-inflammatory potential as pharmacological therapies for inflammatory bowel disease[14,17].
Acetylated and methylated derivatives of OA isolated fromSyzygium aromaticum L.generated a better anti-inflammatory response in models of inflammation in male Wistar rats than did OA[18,19].Another natural OA derivative isolated from the leaves ofCostus igneusshowed anti-inflammatory action in a carrageenan-provoked rat model.This derivative inhibited inflammation-associated enzyme activities such as COX,LOX,MPO and NOS[20].Maslinic acid and 3-epi-maslinic acid were assessed for their capacity to repress inflammatory gene expression in a mouse model of 12-Otetradecanoylphorbol-13 acetic acid(TDPAA)-induced skin inflammation.All examined compounds had the capacity to repress the expression of at least one or more inflammatory genes provoked by TDPAA in mouse skin,which were more effective than the OA[21].These results suggest that OA could be a potential prophylactic and therapeutic agent for the treatment of induced inflammatory responses[22-29].

Figure 1 Chemical structure and properties of oleanolic acid.
Neuroprotective effects
Considering the pervasiveness of ageing-related diseases,studies investigating the neuroprotective impacts of natural compounds and their derivatives have become popular during recent years.The signalling pathways engaged with neuroprotection are the focus of studies their mechanism of the activity and intervene in their pleiotropic prophylactic action against neuronal harm.In the present review,the molecular mechanisms of the neuroprotection provided by OA and its derivatives are revised.By acting upon various systems simultaneously,OA is the highlight as a promising multi-targeting operator.
Several studies have shown that OA possesses neuroprotective effects(Table 3)[30-41].The prophylactic role of OA and its derivatives has been examined using differentin vivomodels of hydroxydopamine-induced neurodegeneration,Aβ25-35 injectioninduced memory deficit in Alzheimer's disease models,Parkinsonian rat models,stem cell differentiation,and brain slice model of neurodegeneration and ischemic stroke(Table 3 and Figure 4).
OA amazingly advanced the migration and proliferation of neural stem cells(NSCs).Differentiation included the increased expression of MAP-2,neuron-explicit marker tubulin-bIII and Mash1,while the astrocyte-explicit marker glial fibrillary acidic protein and Nestin diminished significantly.Moreover,both the phosphorylation of GSK-3β at Ser9 and β-catenin expression were promoted by OA[42-44].In a DNA microarray investigation,OA was found to differentially controlled 183 genes,and 87 of which were anticipated to share typical NKX-2.5 binding sequences[42].These outcomes demonstrated that OA is a viable inducer of NSCs differentiation into neuronsviaNKX-2.5 related components to some extent.Additionally,OA and its derivatives induce neural differentiation and synapse plasticity through a pathway involving histone deacetylase(HDAC)5 phosphorylation[45].These results strongly suggest that OA might be a significant therapeutic for the treatment of neurodegenerative diseases under normal conditions or in response to tissue damage.
Animals treated with 6-hydroxydopamine(HDA)showed functional deficiency in a forelimb use asymmetry test and had less dopamine in the striatum,these effects were improved with OA treatment 7-d pre-injury and 1-d post-injury.In addition,pre- or post-injury OA treated rats recovered from HDA-caused membrane depolarisation,indicating that that pre-administration of OA protects dopamine neurons from the toxic effects of HDA[31,32].Similarly,OA exerted neuroprotective effects on HDA-induced PD in rats by alleviating microglial activation[46,47].In addition,OA derivatives displayed neuroprotective actions by repressing the expression of α-synuclein and the generation of ROS provoked by rotenone treatment.Additionally,an autophagy biomarkeri.e.,microtubule-associated protein 1A/1Blight chain 3(LC3II),was increased significantly.These results suggest that OA and its derivatives could be a new class of prophylactic or therapeutic compounds for PD therapy[48].

Table 1 The oleanolic acid contents of some fruits[2-4]
OA injection during the last 14 d of fluoride treatment considerably recuperated the fluoride-induced brain injury by modulating brain metabolism.The beneficial neuroprotective impacts of OA in ischemic brain injury suppressed glial activities that promote neurotoxicity while raising glial activities that promote neuronal survival[30,33,47].
The pretreatment of rats with OA before the induction of cortical hypoxia by cobalt chloride injection produced a decreased neuronal degeneration and glial activation and improved brain injury[30].Moreover,OA mitigated the neuronal degeneration and synaptic changes produced by Aβ25-35 in an AD model.OA treatment significantly increased the expression levels of brain-derived neurotrophic factor(BDNF),CaMKII,cAMP response element-binding(CREB)NMDAR2B,PKC and TRKB in an AD model.Thus,the ameliorative effect of OA was displayed as to maintain synaptic plasticity of the hippocampus in the Aβ-induced memory loss of AD rats[34].
Furthermore,it was reported that OA significantly hinders the Aβ23-35induced differentiation of NSCs into astrocyte by down-regulating the JAK/STAT signalling pathway through increasing NGN1 expression.These outcomes suggest that OA might impede the progress of AD[44].Finally,OA confers specific neuroprotection against amyloid precursor protein and TAU-induced neurodegeneration and ischemic injury modelled by oxygen-glucose deprivation in organotypic brain slice models[35].
OA mitigated the memory deficits in a cholinergic blockade-induced cognitive deficit mouse model.A single injection of OA significantly improved the latency in a passive avoidance learning assay,spontaneous alternation behaviour in the Y-maze and the exploration time on the novel object recognition assay.These behavioural results implied that OA reverses the cognitive impairment caused by scopolamine.At the molecular level,it was revealed that OA intensified CREB protein and extracellular-signal-regulated kinase 1/2(ERK1/2)phosphorylation and BDNF expression in the hippocampus[36].Similarly,augmented ERK/2,CREB and BNDF phosphorylation which was associated with the upregulation of miR-132 was reported for the antidepressant-like effect of OA.Yiet al[37]showed that a 3 wk of OA treatment in a chronic unpredictable mild stress model attenuated anhedonic and anxiogenic behaviours.All these studies confirm that OA might be a potential therapeutic means for the treatment of cognitive deficits and depression.
OA treatment inhibited the development of experimental autoimmune encephalomyelitis(EAE)in mice by reducing the activation of microglial cells,protecting blood-brain barrier(BBB)integrity,and preventing the infiltration of inflammatory cells into the CNS[26,49-51].EAE mice treated with OA exhibited decreased levels of TNF-α and cytokines in CNS tissue without toxicity[52-56].Similar results were also observed with a natural derivative isolated from caper[57].OA and its derivatives improved neuroinflammation by suppressing the secretion of pro-inflammatory cytokines CCL-5,CXCL-9,CXCL-10,IL-6,IL-1β,NF-κB and TNF-α[57-59].Additionally,the expression of genes involved in myelination/remyelination was increased significantly.Therefore,these studies have shown that OA possesses neuroprotective effects)[30-59].
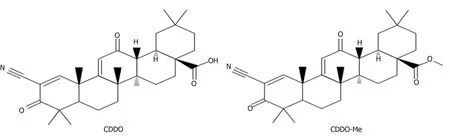
Figure 2 Structures of 2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid and its C-28 methyl ester.CDDO:2-cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid;CDDO-Me:C-28 methyl ester of CDDO.
Hepatoprotective effects
One of the most remarkable pharmacological impacts of OA and its derivatives is hepatoprotection(Figure 5).OA protects against diverse range of hepatotoxic agents,including metals,alcohol,bile acids,natural and synthetic toxins,drugs,viral or microbial agents and ischaemic perturbations.OA and its derivatives perform important protective roles in the instigation of acute liver injury induced by alcohol,carbon tetrachloride(CCl4),acetaminophen(APAP)and phalloidin(Table 4)[59-70].
The hepatoprotective eects of OA and its derivatives against CCl4-caused liver injury involved decreasing the increased serum levels of alanine aminotransferase(ALT),lactic dehydrogenase,aspartate aminotransferase(AST)and hepatic malondialdehyde(MDA)levels and increasing SOD and GPX activities.These biochemical attenuations were further supported by histochemical analyses[61-63].
Esculentoside A(EsA)is an OA derivative that treatment attenuated CCl4- and GalN/LPS-induced acute liver damage in mice.The prophylactic impact of EsA involved the inhibition of the inflammatory response such as IL-1β,IL-6 and TNF-α and oxidative stress,and the underlying mechanism included the peroxisome proliferator-activated receptor(PPAR)-γ,NF-κB and ERK signalling pathways[63].EsA also exhibited protective eects against APAP,which is known to account for overdose toxicity for the majority of acute liver failure cases.EsA treatment attenuated APAPinduced serum AST and ALT levels and stimulated NRF-2 activation and glutathione(GSH)production.Additionally,it significantly increased the phosphorylation of AMP-activated protein kinase(AMPK)and serine/threonine kinase(Akt),as well as glycogen synthase kinase-3 beta(GSK-3β)suggesting that EsA potentiates the NRF-2-controlled survival process through the AMPK/AKT/GSK-3β pathway[71].Similarly,the induction of antioxidant defence and suppression of ER stress and inflammatory responses by the NRF-2 battery as an OA-induced protection against phalloidininduced hepatotoxicity were reported[64].OA reduced the liberation of inflammatory agents and liver enzymes and prevented ConA-induced liver injury.OA treatment decreased the phosphorylation of cJUN NH2-terminal kinase(JNK)and increased the expression levels of PPAR-α[72].Another NRF-2 mediated protective role of OA was reported against LCA-induced hepatotoxicity and obstructive cholestasis,whereby NRF-2-mediated upregulation of multidrug resistance-associated proteins was possibly involved[65,66].
Alcoholic liver disease(ALD)is one of the main causes of death worldwide,and oxidative stress was found to be an important factor in the pathogenesis of ALD damage.OA plays an important role in preventing alcohol-induced oxidative injury by decreasing the upregulation of serum AST,ALT and ATP levels while increasing the reduced hepatic GSH level and SOD and CAT activity.The protective effect of OA involved the uprising of anti-oxidative pathways such as NRF-2,HO-1,SOD-1 and GR expression and the suppression of pro-inflammatory cytokines,for instance,TNFα and IL-6[60].One of the important enzymes in alcohol-instigated toxicity is CYP2E1,which produces both toxic aldehydes and free radicals from ethanol and is suppressed by OA[73].

Table 2 In vivo anti-inflammatory effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives(2014-2020)
Non-alcoholic fatty liver disease(NAFLD)is another highly prevalent liver disease involving disrupted metabolism.It was found that the neonatal administration of OA exhibited hepatoprotective effects on the subsequent development of dietary fructoseinduced NAFLD in adulthood,as evidenced by lower NAFLD scores for inflammation and steatosis and liver lipid content[74].In addition,OA significantly inhibited the transactivation of liver X receptor α and its target genes,resulting in the selective decrease in hepatocellular lipid content,which is beneficial in the treatment of NAFLD[75].In addition,OA enhanced the phosphorylation of AMPK in hepatocytes.Similarly,3-Acetyl-OA(AOA)exerted a protective effect on hyperlipidemia in NAFLD ratsviaAMPK-regulated pathways[67].Thus,OA shows prophylactic and therapeutic effects against NAFLD complications and shows great promise as a possible natural therapeutic agent for the treatment of liver diseases[60-70,76].
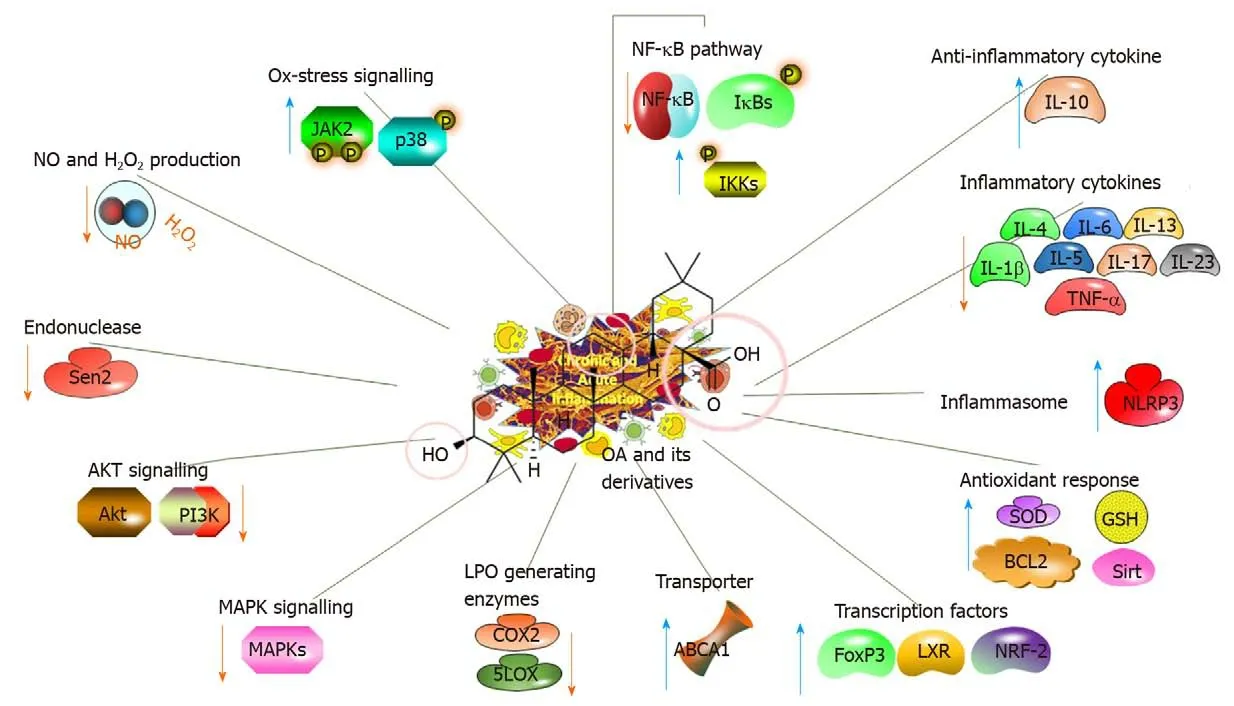
Figure 3 Anti-inflammatory impacts of oleanolic acid and its derivatives,illustrating the molecular mechanisms.OA:Oleanolic acid;NF-κB:Nuclear factorκB;IL:Interleukin;TNF-α:Tumour necrosis factor-α;Akt:Serine/threonine kinase;GSH:Glutathione;LXR:Liver X receptor;NRF-2:Nuclear factor erythroid-2-related factor 2.
Anti-diabetic effects
Diabetes is a complicated,progressive and chronic disorder that results from impaired insulin secretion or sensitivity.Type 2 diabetes(T2DM)is a common form of diabetes that is described as hyperglycaemia resulting from either insulin resistance or insufficient insulin secretion by pancreatic β-cells.Increasing evidence illustrates that T2DM is correlated with obesity,as well as with the development of several comorbidities,including cardiac,hepatic,and renal disorders.It is also consolidated with different metabolic complications affecting organs such as the arteries,eyes,kidney and nerves(Figure 6)[77-79].
Plant-derived OA alleviated hyperglycaemia by decreasing HBA-1c and EPO concentrations in streptozotocin(STZ)-induced diabetic rats.Furthermore,it notably increased RBC count and other RBC indices,increased the antioxidant status of the RBCs and decreased oxidative stress[80].In addition,the anti-diabetic effect on the insulin signalling pathway in the skeletal muscle of STZ-induced rats was fully elucidated.It was found that phosphorylated(p)-AKT and p-glycogen synthase(pGS)expression was increased and that the activation of the insulin signalling pathway was enhanced by OA[81-83].The protective effect of OA is also associated with therapeutic memory,as evidenced by the maintenance of reduced glycaemic levels in mice 4 wk after the termination of OA treatment.This therapeutic memory was associated with FOXO-1 acetylation[84].Additionally,HDACs 4 and 5 and G6Pase expressions were suppressed while histone acetyltransferase 1 expression was increased,suggesting that enzymes involved in epigenetics may have a role in sustained glycaemic control in T2DM,particularly with OA treatment[84-86].The antidiabetic action of OA is mediated in part through the reduction of ghrelin expression,reduced food intake[87].Furthermore,OA prevents and ameliorates the insulin resistance induced by Aroclor 1254 treatment in mice.It notably suppressed the Aroclor 1254-induced increase in ROS,oxidative agents,and NADPH oxidase 4(NOX-4)expression while upregulating the decreased expressions of glutamatecysteine ligase catalytic subunit(GC-LC),glutamate-cysteine ligase modifier(GC-LM)GPX-1,SOD-1 and SOD-2[88].These effects were suggested to be mediated by an increase in PPAR-γ signalling through the upregulation of hepatocyte nuclear factor 1b[88].These results strongly indicate the prophylactic effect of OA on insulin resistance and related metabolic dysfunctions(Table 5)[80,81,84,87-101].
OA derivatives also exhibit significant anti-diabetic effects.12,13 DihydroOA methyl ester(DKS26)reduced the plasma levels of glucose,glycosylated serum protein,ALT and AST.DKS26 also alleviated the glucose tolerance and plasma lipid profiles while raising plasma insulin levels and glucagon like peptide 1(GLP-1)release,which was accompanied by increased levels of cAMP and phosphorylated PKA.Thus,DKS26 is a hypoglycaemic therapeutic that augments the release and expression of GLP-1 mediated by the activation of the cAMP/PKA signalling cascade[89,102].Similarly,the natural OA derivative CHS isolated from the root bark ofAralia taibaiensisexerted an anti-diabetic effect by decreasing blood glucose,triglyceride,free fatty acid and LDL-cholesterol levels in STZ/nicotinamide-induced T2DM rats by activating AMPK[90].One new OA derivative,2a,3b,23a,29a tetrahydroxyolean-12(13)-en-28-oic acid,purified fromMalva parviflorademonstrated a similar anti-diabetic effect on a T2DM mice model[91].Furthermore,a series of synthetic OA derivatives showed inhibitory activity on protein tyrosine phosphatase 1B,which is known to be involved in insulin resistance[103,104].
Table 3 In vivo neuroprotective effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives(2014-2020)
The long-term neonatal intake of OA significantly increased AMPK,adiponectin and GLUT-4 expression while decreasing TNF-α and IL-6 in rats that were fed a high fructose diet,suggesting a potential treatment for the long-term prevention of metabolic diseases such as T2DM and obesity[92-94].Additionally,a nanoformulation of OA ecaciously mitigated the increased levels of NO and MDA and serum CAT and SOD activities in rats fed a high fat and fructose diet[95].Thus,OA is a remarkable prophylactic agent for the long term prevention of diabetes.
In addition to animal models,pre-diabetic human patients were randomised to receive OA-enriched olive oil(equivalent dose,30 mg OA/d)[intervention group(IG)] or the same oil not enriched with OA[control group(CG)] and followed for the incidence of new-onset of T2DM.The results showed that in total,38 new T2DM onset events occurred,31 in the CG and 17 in the IG.Therefore,the intake of OA-enriched olive oil reduced the risk of developing T2DM in pre-diabetic patients,suggesting that OA can be used as a functional food and therapeutic for the prevention of T2DM[92-101].

Figure 4 Molecular mechanism of the action of oleanolic acid and its derivatives on the nervous system.OA:Oleanolic acid;ALT:Alanine aminotransferase;AST:Aspartate aminotransferase;IL:Interleukin;TNF-α:Tumour necrosis factor-α;GSH:Glutathione;STAT3:Signal transducer and activator of transcription 3.
Anti-osteoporotic effects
Osteoporosis is a persistent skeletal disorder characterised by bone microarchitectural deterioration[105].It has become a significant health issue within the elderly population and has led to a considerable socioeconomic burden in society.Scientists are working to develop new therapeutics to treat the development of the disease,and natural products become widespread worldwide[106].
OA is shown to be an anti-osteoporotic natural product,as it increases bone density and remodelling by regulating calcium and vitamin D metabolisms(Table 6 and Figure 7)[107-116].Rats fed OA-enriched diets had improved bone characteristics,higher serum concentrations of 1,25(OH)2D3and less endogenous calcium excretion than did the control group resulting in higher calcium mass[108].Furthermore,the density and microarchitectural characteristics of the bones were significantly improved,1,25(OH)2D3was increased,the renal expression of CYP27B1 and increased,and urinary of Ca2+excretion was increased in mature C57BL/6 ovariectomised(OVX)mice[107].In addition,OA significantly induced the mRNA and protein expression of renal CYP27B1 while suppressing CYP24A1 in human proximal tubule HKC-8 cells,suggesting that its effects were associated with calcium and vitamin D metabolism.Additionally,OA acetate promoted the development and reshaping of bones by properly modulating osteoblast,osteoclast and inflammatory activities with TGF-β regulatory measures in an experimental periodontitis model in mice[109].
As demonstrated by the reversal of biochemical markers and bone density of the lumbar and femur,the OA defends against the osteoporosis caused by prednisone[110].In a glucocorticoid-induced model of rat osteoporosis,a total of 25 possible biomarkers were identified,and OA had a regulatory effect on 17 of these biomarkers associated with some important metabolic pathways,for instance,linoleic acid,valine and isoleucine metabolism,phenylalanine,tyrosine,tryptophan,cysteine and methionine biosynthesis[110].
OA also suppressed the osteoclastogenesis at the early stage and possibly at the late stages in bone marrow macrophages(BMMs),suggesting as a prophylactic and therapeutic agent for bone loss in postmenopausal women[111,112].Mechanical studies revealed that the key parameters inhibited by OA were the c-FOS and nuclear factor of activated T-cells c1(NFAT-c1),bothin vitroRANKL-pretreated BMMs andin vivoin OPG-knockout mice[111].In fact,reproducible results demonstrated that OA inhibited the functions of the osteoclastic genes,including tartrate-resistant acid phosphatase,cathepsin K,and matrix metalloproteinase 9,in the late stage of osteoclastogenesis[111,113].Interestingly,the inhibition of RANKL- induced osteoclastic differentiation in BMMs with the OA acetate(OAA)derived fromVigna angulariswithout cytotoxicity was also reported[114].RANKL-induced osteoclastogenesis was blocked by OAA through PLCγ2-Ca2+-NFAT-c1 signalling[113,114].The findings suggest that OA is a potential drug candidate for the management of postmenopausal osteoporosis and bone loss[107-116].

Table 4 In vivo hepatoprotective effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives(2014-2020)
Anti-cancer effects
Cancer is surpassing cardiovascular diseases as the leading cause of death worldwide[117].Thus,the search for the compounds that selectively kill cancer cells with a mild or no influence on healthy cells is still in progress.In this sense,OA and its derivatives have been observed to exert many anti-cancer actions on various types of tumours.Their molecular mechanisms of these substances are diverse,such as inhibiting the proliferation of cancer cells,preventing cancer cell migration and invasion,restraining angiogenesis,and inducing autophagy and apoptosis.Although a very large number ofin vitrostudies have been carried out showing the inhibition of carcinogenesis,only a fewin vivostudies have confirmed that OA and its derivatives are promising anti-cancer agents(Table 7)[118-125].Researchers introduced various R groups,particularly at the C3and C28positions,to increase the anti-cancer potential of OA[11,126,127].Angiogenesis is one of the hallmarks of cancer and is targeted by OA[128-130].Angiogenesis is an essential means of cancer progression,and OA treatment significantly reduced the intratumoural microvessel density(MVD)in CRC mice and inhibited tumour growth[131-133].The anti-metastatic impact of novel synthetic OA derivatives might have resulted from the downregulation of the VEGF/pFAK/pJNK/pERK/NF-κB cascade[132].Therefore,OA inhibited the proliferation of highly invasive cells and acted as a chemopreventive agent in cancer[8,11,118-126,134-137].

Figure 5 Hepatoprotective effects of oleanolic acid and its natural and synthetic derivatives.OA:Oleanolic acid;IRI:Ischemia-reperfusion injury;NAFLD:Nonalcoholic fatty liver disease;HCC:Hepatocellular carcinoma;HBV:Hepatitis B virus.
Other effects
Although studies have mainly focused on anti-inflammatory,neuroprotective,antiosteoporosis,anti-diabetes effects,OA and its derivatives are reported to possess broad biological activities such as antibacterial,antioxidant,anti-hyperlipidaemic,nephroprotective,cardiovascular protection,anti-infertility,and anti-obesity(Table 8)[29,116,138-178].
Since OA plays an important role in defending against pathogens in plants,it is expected to possess antimicrobial,antiviral,antifungal and antiparasitic activity against a wide range of pathogens.The antibacterial behaviour of OA and its derivatives was tested in specific bacterial strains[179].Further mechanistic investigations suggested that the antiparasitic effect of OA might have resulted from its intearction with the sterol 14-α-demethylase(CYP51),a therapeutic target for leishmaniasis,which impairs the oxidant capacity of the parasite[138,139].Importantly,OA also has the ability to improve parasitemia and anaemia through infection as an effective antimalarian agent[140].The use of an OA-pectin patch removed malaria parasites and improved abnormal HCT values.In comparison,the analysis proved that the levels of IL-6,IL-10 and TNF-a were decreased by day 12.The results indicate that the OA-pectin patch released therapeutic OA doses to alleviate the cytokine release and to ameliorate anaemia caused by malaria.Transdermally administered OA can thus be a potent therapeutic agent for malaria and anaemia treatment[140].OA and its derivatives are reported to exhibit pathogenic antiviral activities against HIV,hepatitis,porcine epidemic diarrhoea virus and influenza virus[141,180-183].OA was shown to be a strong regulator of influenza haemagglutinin(HA).The conjugation of glucose with OA revealed that the HA inhibitory activity of OA was significantly increased with no obvious cytotoxic impact on the MDCK cells[180].Similarly,another OA derivative exhibited anti- HBsAg,anti-HBeAg,and anti-hepatitis B virus antigens secretion activity in HepG2.2.15 cells with inhibitory effect on the viral replication rate superior to that of lamivudine[141,182].
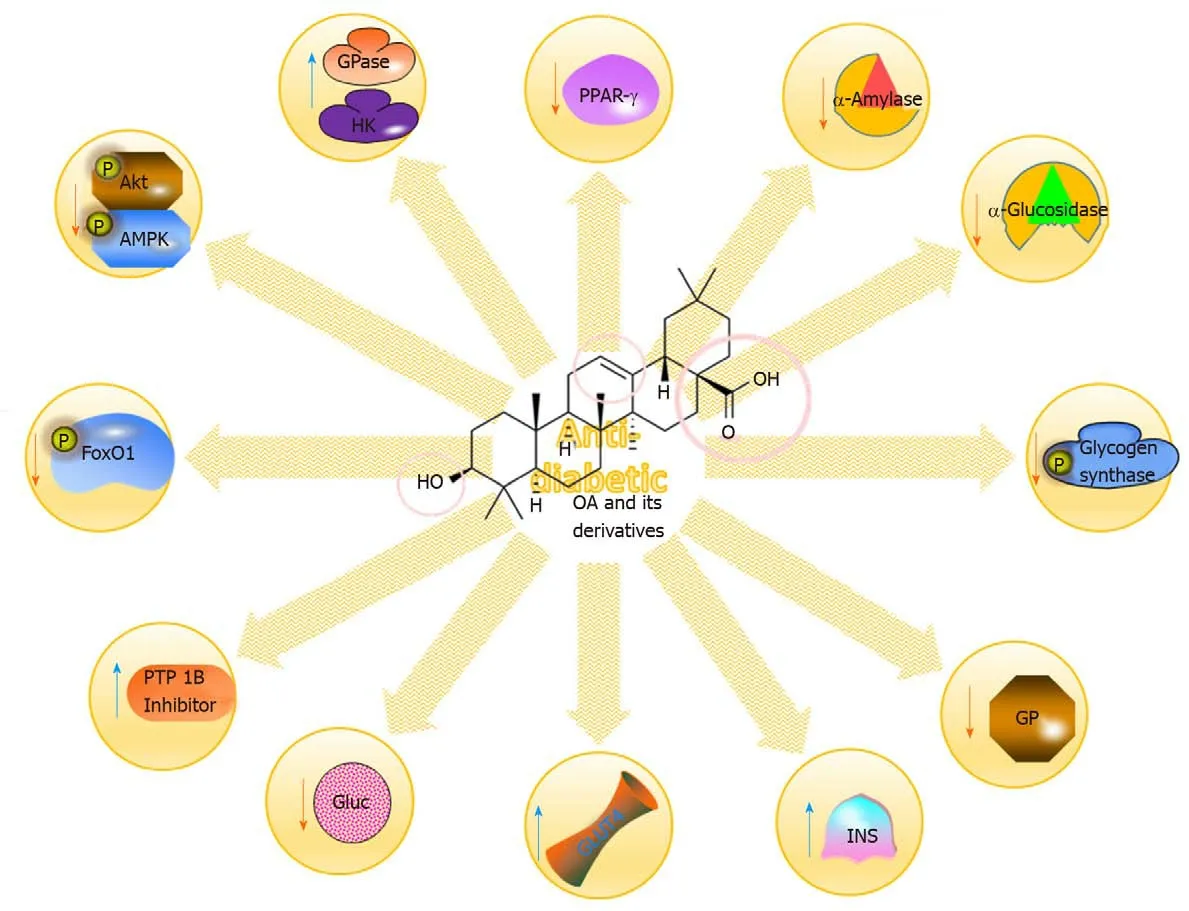
Figure 6 Some of the molecular mechanisms for the anti-diabetic impacts of oleanolic acid and its derivatives.OA:Oleanolic acid;PPAR:Peroxisome proliferator-activated receptor;Akt:Serine/threonine kinase;AMPK:AMP-activated protein kinase.
As oxidative stress under different chronic conditions is considered to be involved in the pathogenic processes,the antioxidant impacts of OA have been investigated.For instance,a decreased intracellular oxidative stress in acute myocardial infarction(MI)was partly due to the protective function of OA[184].OA has been reported to be a potential therapeutic for oxidative stress by inhibiting NO and activating NRF2-ARE signalling pathway[185].It has also been found that OA exerts an anti-allergic effect in allergic diseases such as allergic conjunctivitis and asthma,that is modulated through the GATA-3 and RORγt pathways and through T-cell proliferation[142,143].OA can,therefore,provide a modern prophylactic approach for allergic diseases and potential treatments.
Since cardiovascular diseases are among the leading causes of mortality and morbidity worldwide,the prophylactic and therapeutic effects of OA on cardiovascular disease have been observed.OA and OA derivative therapy also mitigated the high-fat diet mediated atherosclerosis in quail and ox-LDL provoked cytotoxicity in HUVECs by modulating LOX-1,through a decrease in NADPH oxidase and an increase in HO-1 and NRF2 expression[144,145,186].A detailed study used three different animal models,including rabbits that mimicked atherosclerosis,C57BL/6J mice and low-density lipoprotein receptor knockout(LDLR-/-)mice,were applied to study the effect of OA on atherosclerosis[146].All the models revealed that OA retarded the development of atherosclerosis by influencing serum lipid levels,lipid accumulation in the liver and intimal thickening of the artery,which involve genes in lipid metabolism:PPAR-γ,AdipoR1,and AdipoR2.Similarly,the protective effects of OA and its derivatives on diabetes-induced cardiomyopathy andcardiomyocytes injuries were reported to involve anti-oxidative and antiinflammatory mechanisms,PPARγ,and NLRP3 inflammasome signalling pathways[147,148,187,188].Furthermore,the antihypertensive effects of OA synthetic derivatives are attributed to a decrease in vascular resistance with no negative inotropic effect on the heart[149].OA could ameliorate hyperlipidaemia in animal models by modulating CACNA1B,FCN,STEAP3,AMPH,and NR6A expression levels[150].In addition,OA significantly decreased the hepatic expression levels of peroxisome proliferator-activated receptor-g coactivator-1b and the serum levels of triglycerides,total cholesterol,and LDL cholesterol[151].Additionally,a semisynthetic OA derivative at C3position was designed and synthesised to demonstrate farnesoid X receptor modulatory activity in regulating HDL and LDL levels and was found to be more effective[189].

Table 5 In vivo antidiabetic effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives(2014-2020)

OA:Oleanolic acid;OA-Xn:Natural derivatives of oleanolic acid;OA-Xs:Synthetic derivatives of oleanolic acid;STZ:Streptozotocin;HAT-1:Histone acetyltransferase 1;FFA:Free fatty acid;CVDs:Cardiovascular diseases;PGC-1b:Peroxisome proliferator-activated receptor-g coactivator-1b;NRF-1:Nuclear factor erythroid-2-related factor 1;HNF:Hepatocyte nuclear factor;ALT:Alanine aminotransferase;AST:Aspartate aminotransferase;IL:Interleukin;TNF-α:Tumor necrosis factor-α;PPAR:Peroxisome proliferator-activated receptor;T2DM:Type 2 diabetes;MetS:metabolic syndrome;GSH:Glutathione.
OA was demonstrated to increase the fertility of mice involving reversible contraception in male mice by increasing the permeability of the germinal epitheliumviareconstitution of the paracellular junctions between adjacent Sertoli cells[152].In addition,OA efficiently restored testicular function by alleviating germ cell DNA damage and apoptosis through the inactivation of the NF-κB,P53 and P38 cascades and differentiating mouse ESCs into germ cells[153,154].
The nephroprotective activity of OA against oxidative stress-induced renal inflammation,renal fibrosis,drug-induced nephropathy and renal injuries was revealed within vivostudies[155,156,190,191].The beneficial effects of OA on renal fibrosis include reducing renal oxidative stress,increasing the nuclear translocation of NRF2,and mediating EMT in renal tubular epithelium[155,190].Similarly,the activation of NRF2/HO-1 signalling with CDDO-Me treatment in chronic cyclosporine-induced kidney injury and renal ischemia-reperfusion injury revealed beneficial effects[191,157].Furthermore,an acetylate OA derivative reduced RORγT development and prevented SLE pathogenesis in lupus nephritis caused by pristane,suggesting the possible use of OA as an SLE therapy[158].These results support the nephroprotective,antibacterial,antioxidant,anti-hyperlipidaemic,cardiovascular protection,anti-infertility,and antiobesity effects of OA and its derivatives[138-191].
Adverse effects
Increasingly,the adverse effects of the application of herbs used as an ACT are of global concern.In this sense,the paradoxical toxic effects of OA at higher doses and during long-term use have been suggested,as evidenced by liver injury characterised by cholestasis[5].Not only OA but also other OA derivatives,in particular CDDO-Im and CDDO-Me,exhibit this paradoxical hepatotoxicity.Because of these adverse effects,phase-3 clinical trials with CDDO-Me were terminated[192].Although the toxic potential of OA and OA-type triterpenoids was first observed in primary rat hepatocyte cultures,the major concern comes fromin vivostudies[192-194].Although OA is relatively non-toxic,it was shown that repeated oral OA administration produced cholestatic liver injuries in mice,illustrating the hepatotoxic potential of a presumed hepatoprotective compound[1,195,196].
In addition,interactions with phase I and phase II drug-metabolising enzymes such as cytochrome P450(CYP450)and UDP-glucuronosyl- transferases(UGTs)or with the transcriptional inducers of these enzymes might cause adverse reactions.It has been demonstrated that OA alters pregnane X receptor and constitutive androstane receptor promoter activities,which regulate the catalytic activities of CYP3A4 and CYP2B6[197].Additionally,the week inhibition of CYP3A4,UGT1A3 and UGT1A4 and solute carrier transporters activities were reported[198-200].Therefore,information elucidating the drug-drug/drug-herb interactions associated with OA and its derivatives is essential to prevent these adverse reactions.

Table 6 In vivo anti-osteoporotic and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives(2014-2020)
CONCLUSION
This review has presented multiple confirmations of the attenuation and amelioration of various diseases by applying either OA derived from plants or its synthetic and natural derivatives fromin vivoinvestigations.OA and its derivatives have demonstrated diverse molecular mechanisms of action.However,it should be emphasised that there are no confirmations of that OA itself is a candidate for clinical trials since significant efforts have been made to synthesise OA derivatives with less toxic,more potent and bioavailable forms.Nevertheless,there is a reasonable amount of literature,as this literature fully explored in this review.OA and its derivatives have crucial prophylactic and therapeutic potential as an alternative and complementary therapies for diseases including ulcerative colitis,diabetes,cardiovascular diseases.

Table 8 In vivo miscellaneous effects and related mechanisms of action of oleanolic acid and its natural and synthetic derivatives(2014-2020)

OA 25 mg/kg·d,5 wk [146]Low-density lipoprotein receptor knockout(LDLR -/- )mice Review Atherosclerotic AdipoR1,PPAR-γ AdipoR2,TC,LDLC Myocardial injury Cardioprotection,hyperglycemiainduced myocardial injury CASP-3/9,BAX,pERK1/2,HOMER-1α,ERK1/2,SIRT1 BCL-2,ROS OA-Xn 12.5-50 µmol/L [147]Carotid artery injury Proteccts diabetes induced artery injury body weights,serum NO endothelin 1,IL-1β,IL-6 ,IL-18,NLRP-3,CASP-1 OA 100 mg/kg·d,6 wk [148]Vascular injury Hypotensive physiological data physiological data OA,OA-Xn 0.1-100 µmol/L [149]Hiperlipidemia Anti-hiperlipidemic 17 genes(microarray),CACNA-1B TC,TG,HDLC,4 genes OA 3 tablets/d ,4 wk [150]Hiperlipidemia Anti-hiperlipidemic likely via regulation of the miR-98-5p/PGC-1b axi TC,TG,LDL,PGC-1b OA 20 mg/kg,4 wk [151]Fertility Recovered fertility increasing the permeability of the germinal epithelium OA 30 mg/kg [152]Fertility Infertility treatment OCT-4,GDF-9,STRA-8,MVH,ZP-2,ZP-3,ITG-α6,TP-2,SCP-3,ZP-1,ITG-β1 OA 3 µg/mL [153]Fertility/Reproductive function Rejuvenates testicular function BCL-2 pNF-κB,IL-1β ,COX-2 TNF-α,H2AX,pP53,BAX,P38 OA 5-25 mg/kg·d,24 wk[154]Renal fibrosis Attenuates renal fibrosis NRF-2,HO,NQO-1,BAX,HSP-70 BCL-2,OA N.R.[155]Nephropathy Prevent diabetic nephropathy sINS,SOD,adiponectin TG,BUN,Cr,TGF-β,SMAD1/2 OA 100 mg/kg·d,20 wk [156]Renal IRI anti-Renal IRI SOD,GPX,TT,eNOS,NRF-2,PPAR-γ,DDAHs Cre,NGAL,TOS,NO,ADMA,NF-κB,ET-1 OA-Xs 20 mg/kg,5 h before IR[157]Nephritis Lupus/SLE Inhibition of Th17 dierentiation Th17,IL-17A,serum dsDNA,ROR-γt OA-Xs 0-10 µmol/L,50 mg/kg[158]MRSA Anti-microbial Microbe concentration OA-Xs 10-30 µg/mL [159]Circadian clock Mediates circadian clock CLOCK,ELO-VL3,TUBB-2A CLDN-1,BMA-1 AMY-2A5,USP-2,PER-3,THRSP OA 0.01% diet [160]Cisplatin induced nephrotoxicity Prevent neprotoxicity MAP-1A/AB,LC1 CASP-3/9,PARP cleavage,ATG-5,ERK1/2,STAT3,NF-κB OA 10-40 mg/kg [161]Dermatitis/TPAtreated mouse ears Inhibit dermatitis MPO,COX-2,iNOS,TNF-a,IL-1β,pP65 OA-Xn 2,5 or 10 µmol/L [162]Diabetes induced cardiomyopathy Prevent diabetic induced cardiomyopathy via Nrf2 HO-1,SOD,NRF-2,Glycogen,MDA,p-GS OA 80 mg/kg·2 d,14 d [163]Diabetic mesangial cell injury Diabetic renal fibrosis PI3K/AKT/mTOR Autophagy,PTEN,OA 10 µmol/L [164]Gut atrophy/piglet model Prevent gut atrophy TGR-5,FXR OA 50 mg/kg·d,14 d [165]Immune suppression Immune suppressive,anti-RA IL-10 collagen specific sIgG,CD4+ INF-γ,IL-17α,IL-2-/4/6/1β,TNF-α,GM-CSF,MCP-1 ,MMP-1/3 OA-Xs 1-10 mg/kg 18 times between 28 and 53 d after the initial immunisation[166]Immune suppression/gluco corticoid resistance Protecting DEX induced GC impairment Apoptosis,GR binding GR-α OA+I 100 mg/kd·d,21 d [167]Longevity DAF-16,SOD-3,HSP-16.2 CTL-1 OA 0-600 µmol/L·2 d [168]Metal(MeHg)toxicity Mitigate low-dose MeHg toxicity.accumulation of metals in organs OA-Xs 40 µg/kg [169]
IL:Interleukin;TNF-α:Tumor necrosis factor-α;OA:Oleanolic acid;OA-Xn:Natural derivatives of oleanolic acid;OA-Xs:Synthetic derivatives of oleanolic acid;LDH:Lactic dehydrogenase;ERK:Extracellular-signal-regulated kinase;IRI:Ischemia-reperfusion injury;NRF-2:Nuclear factor erythroid-2-related factor 2;JNK:cJUN NH2-terminal kinase;FXR:Farnesoid X receptor;MMP:Matrix metalloproteinase;PGC-1b:Peroxisome proliferator-activated receptorg coactivator-1b;PPAR:Peroxisome proliferator-activated receptor;NF-κB:Nuclear factor-κB;STAT3:Signal transducer and activator of transcription 3;GSH:Glutathione.
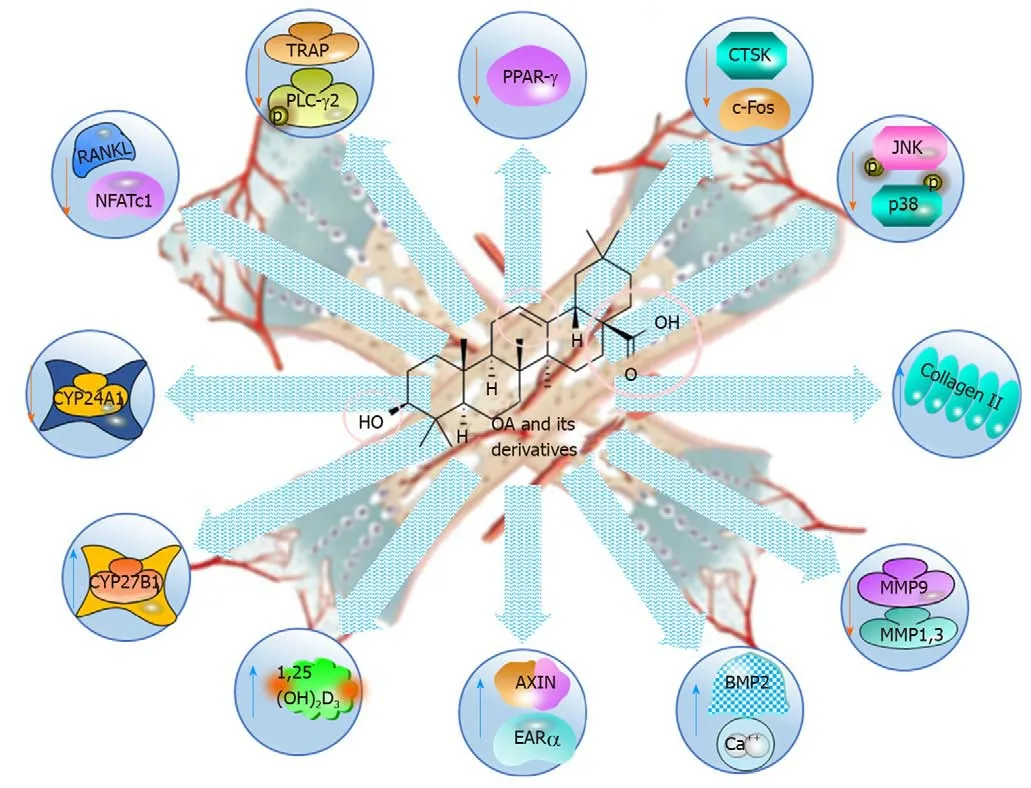
Figure 7 Anti-osteoporotic and bone protective effects of oleanolic acid and its derivatives,illustrating the molecular mechanisms.OA:Oleanolic acid;PPAR:Peroxisome proliferator-activated receptor;CTSK:Cathepsin K;JNK:cJUN NH2-terminal kinase;MMP:Matrix metalloproteinase;NFAT-c1:Nuclear factor of activated T-cells c1;TRAP:Tartrate-resistant acid phosphatase.
 World Journal of Clinical Cases2020年10期
World Journal of Clinical Cases2020年10期
- World Journal of Clinical Cases的其它文章
- French Spine Surgery Society guidelines for management of spinal surgeries during COVID-19 pandemic
- Macrophage regulation of graft-vs-host disease
- Antiphospholipid syndrome and its role in pediatric cerebrovascular diseases:A literature review
- Remotely monitored telerehabilitation for cardiac patients:A review of the current situation
- Keystone design perforator island flap in facial defect reconstruction
- Cross electro-nape-acupuncture ameliorates cerebral hemorrhageinduced brain damage by inhibiting necroptosis
