Assessment of diaphragmatic function by ultrasonography:Current approach and perspectives
Alain Boussuges,Sarah Rives,Institut de Recherche Biomédicale des Armées,ERRSO,Toulon 83800,France
Alain Boussuges,Center for Cardiovascular and Nutrition Research (C2VN),Aix Marseille Université,INSERM (1260),INRAE (1263),Toulon 83800,France
Julie Finance,Fabienne Brégeon,Service d’Explorations Fonctionnelles Respiratoires,CHU Nord,Assistance Publique des Hôpitaux de Marseille et Aix Marseille Université,IRD,APHM,MEPHI,IHU-Méditerranée Infection,Marseille 13015,France
Abstract
Key words:Ultrasound;Hemidiaphragm;Motion;Thickness;Paralysis;Dysfunction;Two-dimensional mode;M-mode;Speckle tracking imaging;Ultrasound shear wave elastography
INTRODUCTION
Over the last 25 years,numerous studies have supported the advantage of ultrasonography (US) in the assessment of diaphragmatic function.Various ultrasonographic methods,such as measurement of diaphragmatic excursions by twodimensional (BD)[1,2]or M-mode[3,4]and changes in diaphragm thickness during inspiration[5],have been proposed.In this review,we report the investigation methods,the criteria for normal and pathological diaphragmatic function and the perspectives of development in adult patients.
METHODS OF DIAPHRAGMATIC FUNCTION ASSESSMENT
Study of the diaphragmatic excursion by M-mode US
Since the 1970s[6],authors have reported that diaphragmatic motion could be recorded using M-mode or two-dimensional mode (B-mode) ultrasonography.To assess the diaphragmatic motion by M-mode US a 2.5-5 MHz phased array transducer is appropriate.B-mode is used to search for a better position of the probe to obtain a good visualization of the motion of each hemidiaphragm.B-mode is important for selecting the exploration line.Indeed,to measure the larger excursion of the hemidiaphragm,the line of the M-mode should be perpendicular to the posterior part of the hemidiaphragm[3,7].In most patients,a subcostal or a low intercostal probe position is appropriate.The excursion of both hemidiaphragms can be measured using M-mode US.
To record the diaphragmatic motion of the right hemidiaphragm,the liver is used as a window.The probe is placed between the mid-clavicular and the mean axillary lines,below the right costal margin,and directed medially,cephalad and dorsally,so that the ultrasound beam reaches the posterior part of the vault of the right hemidiaphragm perpendicularly.After correct visualization of the right hemidiaphragm by B-mode,M-mode is used to display the motion of the diaphragm along the selected line.The inspiratory and expiratory craniocaudal displacements of the diaphragm (seen as a bright line),lead to a shortening and a lengthening of the probe-diaphragm distance,respectively.
For the left hemidiaphragmatic motion recording,the spleen window is used to obtain a two-dimensional image of the diaphragm.The probe is placed subcostally or on the last coasts between the anterior and the posterior axillary lines,to obtain the best imaging of the left hemidiaphragm.The motion is recorded using M-mode US as previously described for the right side.It has been demonstrated that diaphragm excursion measurement using the M-mode technique was a reproducible method in standing and supine patients[8,9].
Respiratory maneuvers and variables measured
For better assessment of the diaphragmatic function,measurements can be performed during various respiratory maneuvers such as quiet breathing,sniffing and deep breathing[8].At least 3 different recordings should be taken for each maneuver to calculate a mean value.
Quiet breathing:Several variables can be measured from the M-mode quiet breathing graph,during inspiration and expiration (Figure1).To measure the excursion during inspiration,the first caliper is placed at the foot of the slope of the diaphragmatic echoic line and the second calliper is placed at the apex.The diaphragm inspiratory excursion,(in cm) can be divided by the inspiratory time (in second) to obtain the diaphragm inspiratory mean velocity (in cm/s).During expiration,the same measurements can be performed between the beginning and the end of the expiratory slope giving the diaphragm expiratory amplitude,the expiratory time,and the diaphragm expiratory velocity.
Other maneuvers:Diaphragmatic motion can be also recorded during a voluntary,vigorous sniff maneuver.Lastly,it is interesting to assess the excursion during a deep breathing.This maneuver is performed at the end of a quiet expiration and the patient should breathe in as deeply as he possibly can.From the M-mode graph recorded during these two maneuvers,the amplitude and the velocity of excursion are measured from the baseline to the point of maximum height of inspiration on the graphs.
Method difficulties and supplementary procedures
The accuracy of US in the measurement of diaphragmatic motion may be impaired due to difficulties in obtaining the entire excursion during deep breathing.Indeed,during inspiration the descending lung can mask the diaphragm.This phenomenon is particularly frequent on the left side[8,10].To make the recording of the entire diaphragmatic excursion easier,it is possible to displace the probe caudally on the abdomen and to decrease the angle of the probe with the abdominal wall to maintain a direction of ultrasound perpendicular to the hemidiaphragm.This maneuver is effective more frequently on the right side.On the left side,the US window delivered by the spleen is small and,despite this maneuver,recording may remain impossible.Furthermore,it is frequently difficult to obtain an adequate angle of visualization of the left hemidiaphragm.In such circumstances,the accuracy of the measurement is impaired.To overcome these limits,it is advantageous to use complementary procedures.Some authors have reported that recording the entire excursion of both hemidiaphragms was successful more frequently using angle-independent M-mode sonography (AMM) than with standard M-mode[11,12].Furthermore,the difficulties in aligning the M-mode cursor with the axis of diaphragmatic displacement lead to an overestimation (on average + 17%) of the displacement when compared with a correct alignment provided by the AMM[12].Consequently,the use of AMM can be recommended to make recording easier and to decrease the overestimation of the excursion of the two leaves secondary to orientation and translation errors (Figure2).
Normal values
The normal values for the diaphragmatic excursions studied by M-mode US have been previously reported during resting breathing,voluntary sniffing and deep breathing (Table1)[4,8-10,13-16].For the same volume inspired,excursions are physiologically larger in the supine position when compared with sitting or standing positions[17].Consequently,it is important to use the appropriate normal values according to the position of the subject.During quiet breathing the excursions have been measured by most authors as between 10 mm and 25 mm on both sides[7,8,14].
Using M-mode US,sniffing leads to a sharp downstroke of the hemidiaphragm(Figure3).Mean excursion is around 3 cm on both sides[8,18]and lower limit values are calculated at 1.6 cm in women and 1.8 cm in men.The diaphragmatic motion induced by the sniff maneuver can also be studied by tissue Doppler imaging (TDI).Using a cardiac probe,the TDI process is activated and the sample volume is placed perpendicular to the diaphragmatic motion.It has been demonstrated that the TDI velocities were significantly related to sniff nasal pressure.In healthy volunteers,the median normal peak sniff TDI was estimated at 13 cm/s and 12 cm/s for the right and left hemidiaphragm,respectively[18].
Several previous studies have estimated the excursion of both hemidiaphragms during deep breathing.The maximal excursion of the right hemidiaphragm was estimated to be about 6-7 cm.Indeed,the mean excursion measured in volunteers of both sexes was 6 ± 0.7 cm by Cohenet al[19]and 6.8 ± 0.8 cm by Targhettaet al[3].Since these first studies performed in the 1990s,more recent works have demonstrated that several factors such as age,anthropometric data,and gender affected diaphragmatic motion[4,8,9].The mean diaphragmatic excursions of the two hemidiaphragms have been determined for men and women (Table1).Furthermore,in 1995,Houstonet al[20]have reported that in healthy volunteers,the right-to-left ratio of hemidiaphragmatic excursion during deep inspiration was in the range of 0.5-1.6.Consequently,this ratio has been proposed as an index of normal diaphragmatic motion.
Alternative procedures for assessing diaphragmatic motion

Figure1 Diaphragmatic motion recorded by M-mode ultrasonography:measurement of diaphragm excursion,inspiratory time and velocity of contraction.Ordinate in centimeter,abscissa in second.d:Diaphragm excursion;t:Inspiratory time;v:Velocity of contraction.
The accuracy of M-mode US in the evaluation of diaphragmatic displacement may be limited,because of difficulties in obtaining an adequate angle of visualization of the hemidiaphragm.Some authors have,therefore,proposed alternative methods.
Houstonet al[20,21]have measured diaphragmatic motion directly on BD images in a longitudinal plane including the maximal renal bipolar length,and the posterior muscular crus of each hemidiaphragm.The variation of the distance between the diaphragm and the kidney,at the end of expiration and inspiration is used to assess diaphragmatic excursion[22].It is also possible to measure the upward and downward movement of the diaphragm using the lung silhouette method[23,24].The transducer is placed at the lowest part of the lung in the scapular line.The probe orientation is longitudinal to measure the distance between the highest and lowest points of the lung silhouette during inspiration.The advantage of this procedure is that it can be performed on both sides.
Skaarupet al[25]have proposed another method for measuring the displacement of the two leaves.On a BD image,the authors have traced the area between the diaphragm and the borders of the ultrasound image.From the study of 19 healthy volunteers,the authors reported that this measurement could be acquired from a lateral mid-axillary view on both hemidiaphragms.If the ultrasound transducer is kept in a fixed position during the respiratory maneuvers,the changes in area are secondary to the sole diaphragm displacement providing a simple,feasible means of assessing diaphragmatic function.
Other procedures reflecting the diaphragmatic motion have been proposed.Toledoet al[26]have measured the craniocaudal displacement of the left branches of the portal vein.This technique proved to be a valid tool for indirect assessment of right hemidiaphragmatic mobility,because it showed a mean difference between US and radiography of only 0.4 mm.For the left hemidiaphragmatic motion,the same team[27]proposed measuring the craniocaudal displacement of the splenic hilum or the inferior pole of the spleen.Indeed,they found a positive correlation between these two methods and the left hemidiaphragmatic excursion measured by radiography or ultrasonography.
Measurement of the diaphragm thickness
The measurement of diaphragm thickness and inspiratory thicknening has also been proposed for assessing diaphragmatic function.Both hemidiaphragms can be visualized near their zone of attachment where the diaphram abuts to the rib cage(zone of apposition)[28,29].Measurement of diaphragm thickness is performed using a high frequency linear probe (>7 MHz).For better image quality,a high-resolution linear probe (around 12 MHz) is recommended.As an appropriate procedure,the probe should be angled perpendicular to the chest wall.It should be placed below(0.5-2 cm) the phrenico-costal sinus near the anterior or the mid-axillary line at the eighth or ninth intercostal space.In this location,the distance between the skin and the diaphragm is estimated between 0.8 cm and 4.9 cm[30].The depth of the diaphragm is increased in subjects with a high body mass index[30].
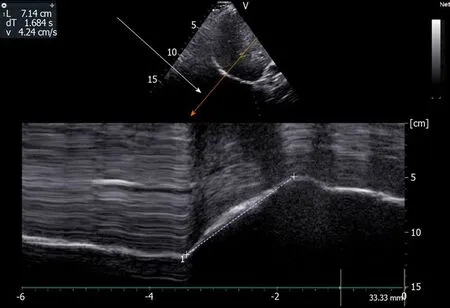
Figure2 Use of angle-independent M-mode sonography (arrow) to obtain a perpendicular approach of the right hemidiaphragm:Measurement of excursion (7.14 cm) during deep breathing.Ordinate in centimeter,abscissa in second.
The diaphragm is identified as a three-layered structure with two parallel echogenic lines,the diaphragmatic pleura and the peritoneal fascia,enclosing the hypoechoic diaphragmatic muscle.A third hyperechoic line is frequently seen in the middle of the non-echogenic layer,considered to be the fibrous layer in the center of the diaphragm.The accuracy of the measurement of diaphragm thickness by ultrasound has been demonstrated by Waitet al[28]and Cohnet al[29]in comparative studies,using anatomical control by necropsy in human cadavers.
The thickness of both hemidiaphragms can be measured directly from the frozen Bmode images (Figure4).Some authors recommended using M-mode tracing (Figure5) to display the changes in thickness during the breathing cycle[31].Most authors have measured the right and left diaphragm thicknesses,as the distance from the middle of the pleural membrane to the middle of the peritoneal membrane[5,13,32-34].Some other procedures have been proposed such as measurements from the inner edges[35]or the outer edges[29]of the pleural and peritoneal membranes.
Previous studies which used B mode imaging of the diaphragm have determined normal diaphragm muscle thickness at the end of a normal expiration [at functional residual capacity (FRC)].In healthy volunteers,the normal values are affected by gender[34]and physical fitness[31]showing diaphragm thickness lower in women and sedentary subjects.Furthermore,it has been reported that diaphragm thickness was greater in the supine or standing position than in the supine position[36].The mean value of thickness has been variously estimated (Table2)[5,13,31,33-35,37-40].The thickness varies depending on which intercostal space is chosen,the hemidiaphragms being thicker at the lower intercostal space[35].
Although it was frequently found in a population of healthy individuals that one hemidiaphragm is thicker than the other,the mean thickness of the two hemidiaphragms appeared not to be significantly different.As a result of the study of a population of 150 healthy subjects,Boonet al[35]have determined that a side-to-side difference in thickness,measured at FRC,greater than 3.3 mm should be considered as abnormal.
Diaphragmatic function has been related to inspiratory thickening.Consequently,for better detection of diaphragmatic dysfunction,it is necessary to measure thickness at both end expiration and end inspiration.The result has been expressed as the thickening ratio (TR),i.e.,thickness at end inspiration divided by thickness at end expiration.Some authors reported the percentage of thickening [or thickening fraction(TF)] calculated as the ratio:Thickness at end inspiration - thickness at end expiration divided by thickness at end expiration.
During quiet breathing,the normal thickness has been estimated from the study of 200 healthy volunteers at 1.9 ± 0.5 mm (from 1.2 mm to 2.79 mm) at end expiration and at 2.6 ± 0.6 mm (from 1.65 mm to 3.7 mm) at end inspiration[41].In this work,the percentage of thickening during quiet breathing ranged from 24.5% to 53.2%.From the study of 150 healthy volunteers,Harperet al[42]reported that the mean thickening from resting expiration to resting inspiration was 20% ± 15.5% on the right side and 23.5% ± 24.4% on the left side.Interestingly,in some healthy subjects (29% of hemidiaphragms investigated) the percentage of thickening was lower than 10%during tidal breathing.Furthermore,in 3 volunteers the increase in thickness was nil on one side[42].
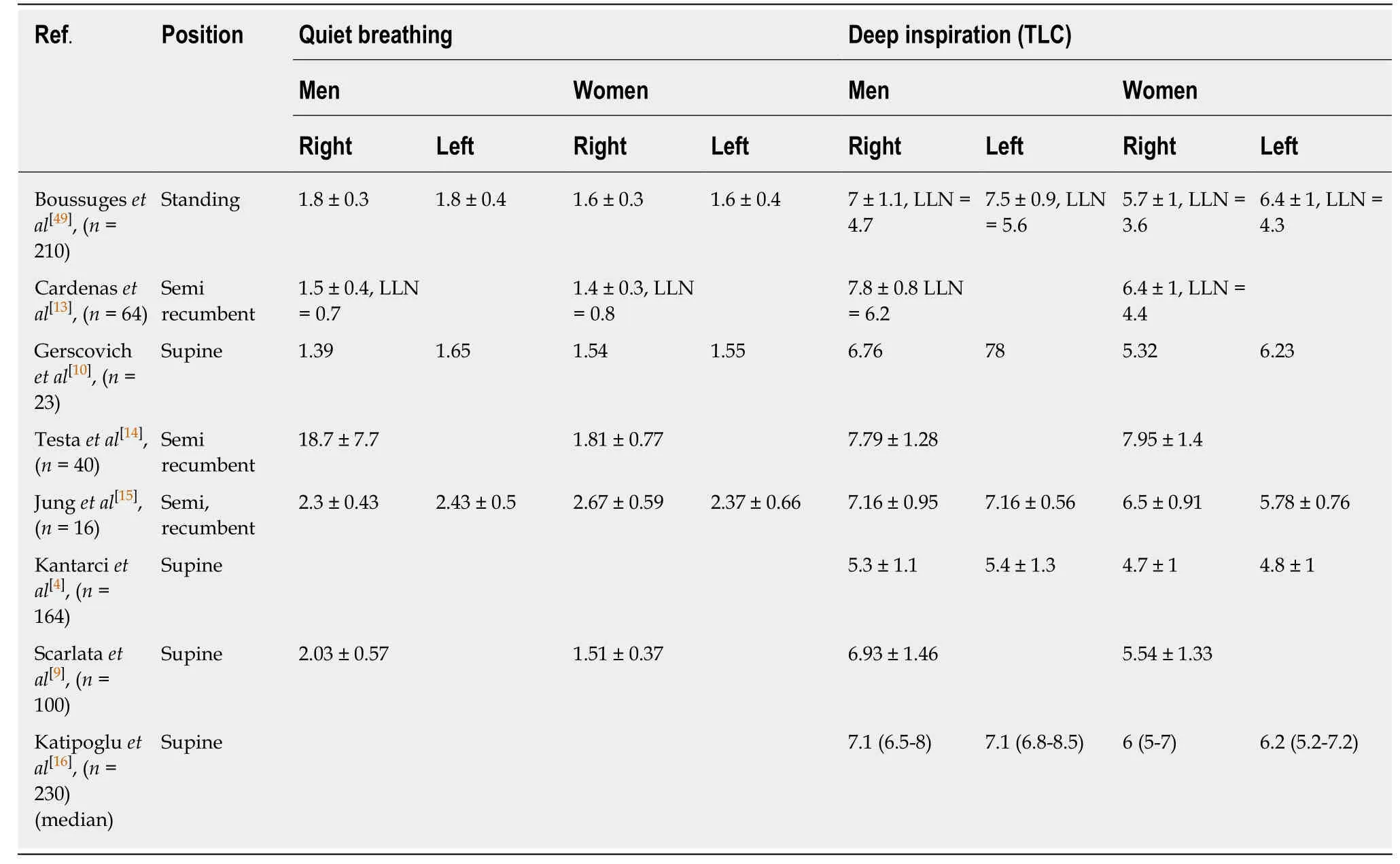
Table1 Normal values in centimeters for the diaphragmatic excursions
Consequently,the percentage of thickening during a deep inspiration is more informative for estimating diaphragmatic function than during quiet breathing.The most frequently used method is the analysis of the thickening from the end of a normal expiration (FRC) to the end of a maximal inspiration [at total lung capacity(TLC)].The normal percentage of thickening has been variously estimated (Table2).Most authors considered a TR greater than 1.2[35]and a TF greater than 20%[33]to be normal.
Most frequently it is easy to measure the changes in thickness at inspiration during quiet breathing[41].On the other hand,it is sometimes difficult to record the thickening at end deep inspiration because the diaphragm can be obscured by the lung coming into the field of the BD image.To attenuate this phenomenon,the probe can be displaced in a lower intercostal space allowing the hemidiaphragm to be visualized.When the recording of the thickness remains impossible at TLC,the percentage of thickening at inspiration during quiet breathing or during a sub-maximal inspiration can be informative.
ABNORMAL FINDINGS
The benefit of diaphragm US has been demonstrated in the detection of diaphragm paralysis or diaphragmatic dysfunction.The method has a high sensitivity (93%) and specificity (100%) for the detection of diaphragmatic dysfunction,induced by neuromuscular disease when compared with the diagnosis of clinicians based on clinical and functional assessments,including pulmonary function testing,chest Xray,fluoroscopy,phrenic nerve conduction study and diaphragm electromyography[43].Table3 reports the main etiologies of diaphragmatic dysfunction.
Hemidiaphragm paralysis
Phrenic nerve injury is the main cause of unilateral diaphragm paralysis.A lesion of the phrenic nerve can be secondary to thoracic and cervical surgery or to medical invasive procedures such as atrial fibrillation ablation[44-46].Furthermore,nerve conduction can be impaired by an inflammatory process or a compression.

Figure3 Diaphragmatic motion recorded by M-mode ultrasonography during voluntary sniffing (excursion =4.51 cm).Ordinate in centimeter,abscissa in second.
Study of diaphragmatic excursion
In patients suffering from unilateral diaphragmatic paralysis,the study of diaphragmatic motion by M-mode US during breathing at rest,recorded a nil or paradoxical excursion (i.e.,a cranial displacement)[47-49].Voluntary sniffing has been used traditionnally in combination with fluoroscopy to detect paradoxical motion in favor of a hemidiaphragm paralysis.During sniffing,the decrease in thoracic pleural pressure induces a passive displacement of the paralyzed hemidiaphragm toward the cranial extremity.This paradoxical movement can be recorded by ultrasonography.The M-mode graph displays a mirror image when compared with the normal finding in healthy subjects (Figures 3 and 6).The fast negative excursion measured around 1cm in magnitude[49].
During deep breathing (Figure7),on the paralyzed side a paradoxical motion can be observed at the beginning of the inspiration followed by a reestablishment of the motion in the cranio-caudal direction[49].This profile is the result of the balance between the decrease in pleural pressure,which leads to a cranial displacement,and the force generated by the intact hemidiaphragm and the inspiratory accessory muscles,which induces a caudal displacement[50].Consequently,the time course of the displacement during deep breathing should be carefully examined and the presence of a terminal caudal displacement should not lead the investigator to conclude that there is normal function of the hemidiaphragm.Furthermore,when a paralyzed hemidiaphragm is suspected,it can be beneficial to study the diaphragmatic displacements in the supine position.Indeed,in this position the abdominal visceral mass increases the diaphragmatic work,and it has been reported that the supine position increased paradoxical movement and decreased the compensatory active expiration by the anterior abdominal wall[51].This may mask the paralysis.
Lastly,in patients suffering from hemidiaphragm paralysis,the measurement of the excursions on the healthy side can be informative.Indeed,large excursions of the normal side are more frequently observed during quiet breathing in relation to a compensatory mechanism[20,49].The diagnosis of bilateral paralysis is especially difficult because the comparative study of both hemidiaphragms is not informative.In such circumstances,the assessment of the inspiratory diaphragm thickening is particularly important.
Measurement of diaphragm thickness
The measurement of the diaphragm thickness in the zone of apposition of the diaphragm to the rib cage,has demonstrated its benefit in the diagnosis of diaphragmatic paralysis[33].A chronically paralyzed diaphragm is atrophic and does not thicken during inspiration.To detect abnormally low thickness,previous studies have determined the lower limit of normality (LLN) for thickness measured after unforced expiration at FRC.This threshold has been variously estimated as 1.2 mm by Cardenaset al[13],Thimmaiahet al[41]and Harperet al[42],from 1.4 mm and 1.7 mm depending on the side and gender by Boonet al[35]and 2 mm by Gottesmanet al[33].Nevertheless,the diaphragm can be thin in some subjects who have a normal functioning diaphragm,suffering from generalized muscle wasting or in small individuals[33].Consequently,a better means of detection for diaphragm paralysis is the analysis of inspiratory diaphragm thickening.Gottesmanet al[33]have reported that the change in diaphragm thickness was between 28% and 96% on the healthy side of patients suffering from hemidiaphragm paralysis.In contrast,on the paralyzed side,the hemidiaphragm did not thicken significantly or even became thinner;the change in thickness was quantified from -35% to +5%.According to these findings,the threshold of 20% is accepted by most authors for the diagnosis of hemidiaphragm paralysis.
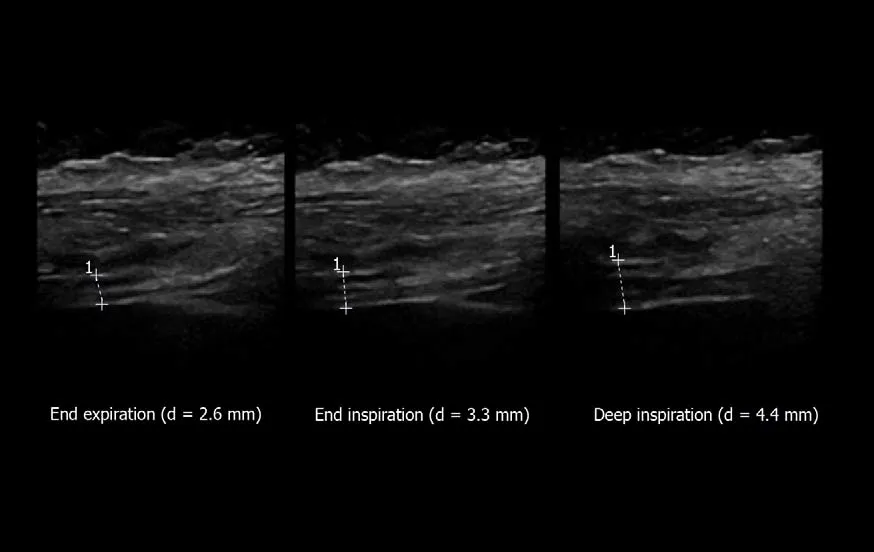
Figure4 Measurement of diaphragm thickness using B-mode ultrasonography:The diaphragm thickening is calculated from the measurement of thickness at both end expiration and end inspiration [here = (4.4-2.6)/2.6= 69%].Ordinate in centimeter,abscissa in second.
Diaphragm US can be used advantageously to assess potential functional recovery in subjects with diaphragm paralysis.Serial examinations should be performed.Indeed,it has been reported that recovery can occur within a few days in some patients after atrial fibrillation catheter ablation,for example[52].Nevertheless,in other subjects,recovery occurs after several months (from 1 mo to 12 mo in the study by Sacheret al[52],with a mean of 15 mo for Summerhillet al[53]).Lastly,persitent paralysis has been recorded in patients with a percentage of thickening equal to zero[53].
Diaphragmatic dysfunction,hypokinesia
To detect diaphragmatic dysfunction without paralysis,some previous studies[8,13,35]have researched the values corresponding to the LLN for diaphragmatic motion and thickness (Tables 1 and 2).It is important to use the LLN according to the gender and the body position of the subjects investigated.When the examination records a value lower than the LLN,diaphragmatic dysfunction is suspected.Consequently,US may be useful as a screening test in patients suffering from various diseases known to impair diaphragmatic function.
Detection of diaphragmatic dysfunction in neuromuscular disease
Ultrasonic methods can be useful to assess the contribution of the impairment in diaphragmatic function to respiratory failure in patients suffering from neurological or muscular diseases such as amyotrophic lateral sclerosis (ALS),Duchenne muscular dystrophy (DMD),myotonic dystrophy or myasthenia gravis[54].
ALS:In ALS patients,it has been demonstrated that the measurement of diaphragm thickness was correlated with the amplitude of the motor response induced by electrical phrenic nerve stimulation[55].The TF and the ratio between diaphragm thickness at the end of tidal volume and diaphragm thickness at maximal inspiration(ΔTmax = end-inspiratory Vt Tdi divided by end-inspiratory TLC Tdi) correlated with respiratory function tests[56].According to the European Federation of Neurological Societies Consensus Conference,a forced vital capacity (FVC) < 50% or a sniff inspiratory nasal pressure < 40 cm H2O are recognized as thresholds for impairment of the pulmonary function test (PFT),indicating the need for ventilator support such as non-invasive ventilation[57].In ALS patients,PFT can be difficult to record,particularly in patients with weakness of the facial muscles.In these circumstances,ΔTmax has been proposed by Fantiniet al[58]to detect diaphragmatic dysfunction and the indication for mechanical ventilation support.Indeed,when ΔTmax is greater than 0.75,it provides 75% sensitivity and 85% specificity in predicting a FVC value lower than 50% of predicted value.
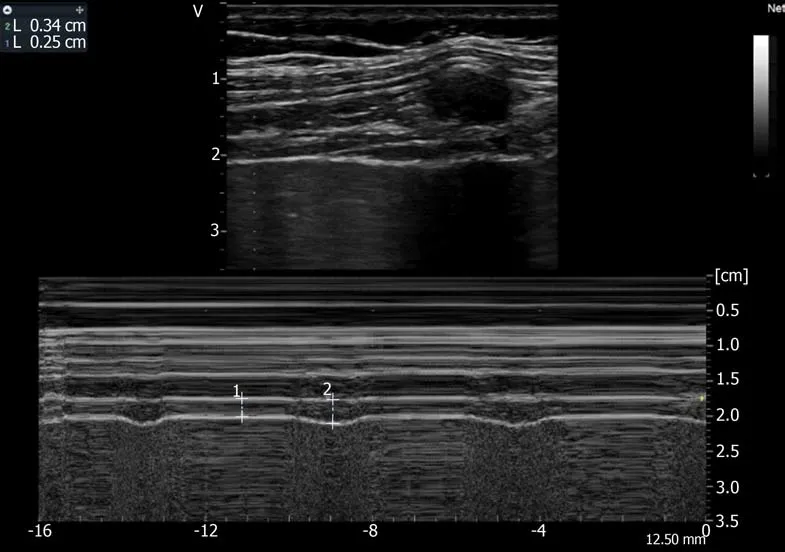
Figure5 Recording of the changes in diaphragm thickness during quiet breathing using M-mode tracing(measurement of thickness at end expiration (1 L = 0.25 cm),at end inspiration (2 L = 0.34 cm).Ordinate in centimeter,abscissa in second.
DMD:De Bruinet al[59]reported a thicker diaphragm in young patients (from 7 to 12 years old) suffering from DMD,when compared with healthy controls.In contrast,the decrease in both diaphragm muscle thickness and TR during inspiration suggested a decrease in diaphragm contractility.These results were attributed to a diaphragmatic pseudo-hypertrophy in young DMD patients.In older patients (>14 years old),recent studies[60,61]reported progressive atrophy of diaphragm muscle associated with a decrease in the TF related to the impairment of the respiratory function tests (decrease in maximal inspiratory pressure with age).In the Fayssoilet al[61]study performed in DMD patients with ages ranging from 21 to 31,the right and left diaphragmatic TFs were assessed as 12.7% and 15.5%,respectively.Furthermore,the right and left diaphragmatic motions were impaired and the excursions were inversely correlated with age.
Stroke patients:It has been reported that stroke patients exhibited significantly reduced diaphragmatic excursion on the hemiplegic side[15,62].Furthermore,Kimet al[39]have shown that the diaphragm was thinner (at end expiration and deep inspiration)on the affected side when compared with the other side.In right hemiplegic patients,Junget al[15]have reported that diaphragmatic excursions could be reduced on both sides.Consequently,the study of diaphragmatic motion after stroke can be of benefit in guiding medical therapy and respiratory physiotherapy and therefore,in preventing pulmonary complications.
Detection of phrenic nerve injury:To assess the contribution of phrenic neuropathy to diaphragmatic dysfunction,Johnsonet al[63]have proposed combining diaphragm US with phrenic nerve conduction studies.The cervical stimulation of the phrenic nerve leads to a diaphragmatic movement of on average 2 cm on both sides in healthy subjects.The magnitude of displacement is correlated with the stimulus intensity.In patients with phrenic neuropathy,the hemidiaphragmatic displacement induced by stimulation is reduced when compared with the healthy side and with the range of normal values.
Respiratory diseases
In chronic obstructive pulmonary disease (COPD) patients,previous studies have reported changes in diaphragmatic function when compared with healthy subjects.The diaphragmatic motion during quiet breathing is higher in patients with severe COPD patients,when compared with healthy controls[64]or with patients suffering from mild COPD[65].An increase in diaphragm thickness has also been reported in patients with severe COPD (GOLD grade C)[65].Nevertheless,the results on this topic are conflicting.Some works have not reported a significant difference in diaphragm thickness between control subjects and COPD patients[38,66,67].In patients with major airtrapping,Bariaet al[38]have shown a higher TR on the left hemidiaphragm,when compared with control subjects,suggesting an increase in the work of breathing in severe COPD patients.
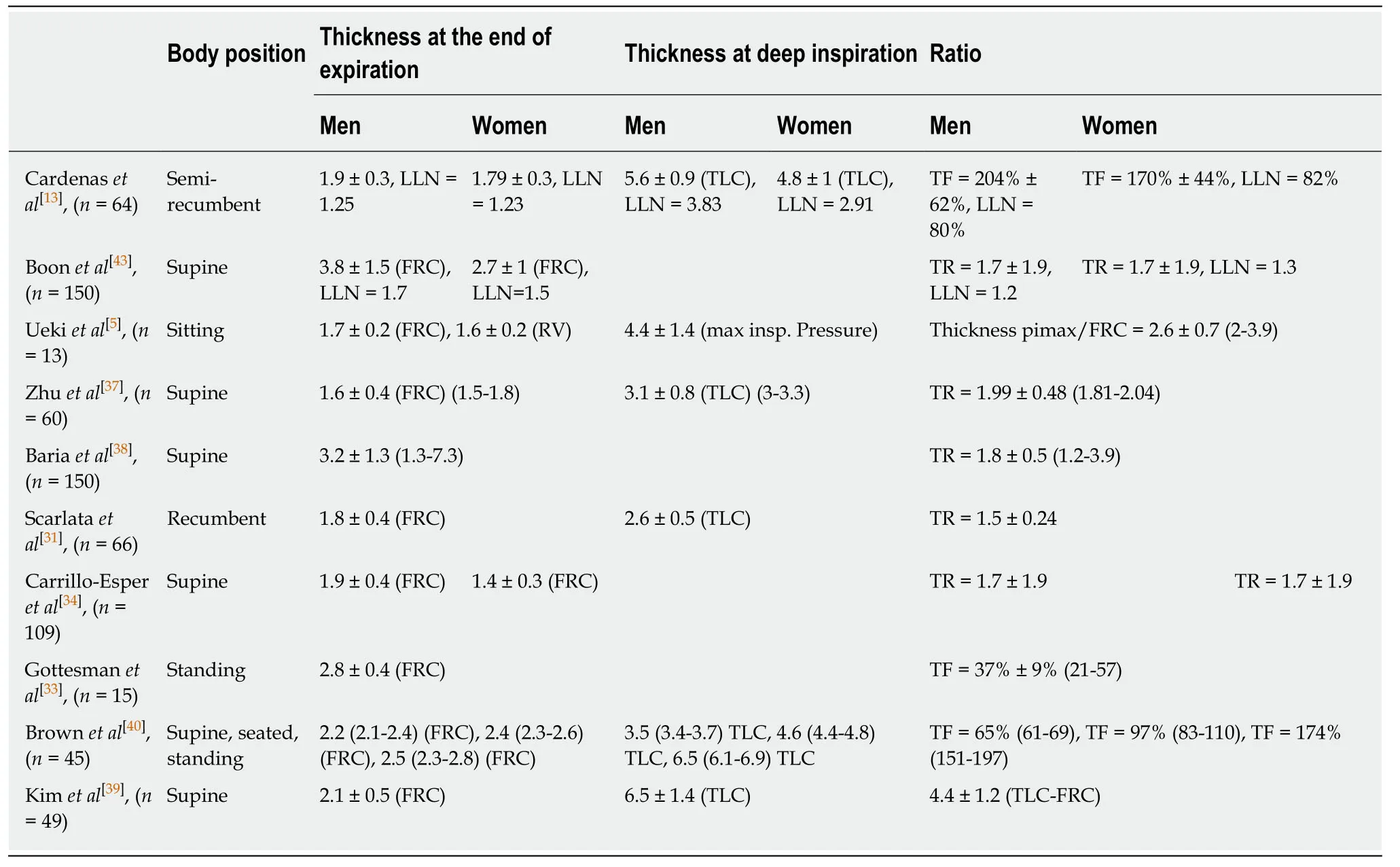
Table2 Average values in millimeters for the right hemidiaphragm thickness
Several studies have reported that in patients with moderate or severe COPD the excursions of the two leaves were decreased during deep inspiration[64,68-70].The diaphragmatic excursion measured during deep inspiration is positively correlated with forced expiratory volume in the first second (FEV1)[24].The reduction in diaphragmatic motion is significantely associated with the parameters that quantify air trapping such as residual volume (RV) and RV/TLC[69]and induces a decrease in exercise tolerance[71].On the other hand,it has recently been reported that the diaphragmatic excursion during deep inspiration increased after a rehabilitation program[64].
New echographic indices related to the severity of airway obstruction have been proposed from the right diaphragmatic motion recorded by M-mode US during an expiratory maximal effort.During a forced open-mouth breath,Zanforlinet al[72]measured the maximum expiratory diaphragmatic excursion (EDEMax) and the diaphragmatic excursion in the first second of expiration (FEDE1).The ratio EDEMax/FEDE1 named by the authors as the M-mode Index of Obstruction (MIO),has shown a linear correlation with the ratio between FEV1 and vital capacity.According to the study by Zanforlinet al[72],a MIO lower than 77 suggests an obstructive spirometric pattern with a 95.5% positive predictive value.Consequently,these above-mentioned studies suggested that diaphragmatic US can be informative for both the severity of COPD and the improvement in respiratory function induced by pulmonary rehabilitation.
It is well recognized that,during sprirometry,breathing through a mouthpiece leads to changes in diaphragmatic motion including an increase in diaphragmatic excursion[7,73]and a decrease in breathing rate.Consequently,when the ultrasonographic examination is performed in combination with spirometry,an alteration in the diaphragmatic kinetics can be expected in comparison with quiet breathing without a mouthpiece.
Furthermore,it can be beneficial to analyze diaphragmatic kinetics recorded by Mmode US in patients suffering from a disease with increased inspiratory resistance.Indeed,Soilemeziet al[73]have studied the changes in the diaphragmatic pattern,recorded by M-mode US,secondary to an increase in inspiratoy resistance(inspiratory flow-resistive load = 50 cm H2O/L/s) in healthy subjects.They reported a decrease in breathing rate and an increase in both diaphragmatic excursion and inspiratory time in comparison with breathing without load.Consequently,diaphragm US should be useful in assessing the impact of an upper airway obstruction such as a tracheal stenosis on respiratory function.

Table3 Principal causes of diaphragmatic dysfunction
Trauma patients
US has demonstrated its great benefit in the initial assessment of trauma patients.FAST (focused assessment with sonography for trauma) examination is commonly used for early diagnosis of several life-threatening injuries by the detection of pericardial and intraperitoneal fluid (assumed to be hemoperitoneum in the context of trauma)[74].The examination can be extended advantageously to detect pleural effusion and pneumothorax (extended FAST)[75].In trauma patients,diaphragmatic rupture and phrenic nerve injury may be difficult to identify and the diagnosis is frequently delayed.It has been reported that ultrasound examination can be informative by the detection of abnormal motion of the hemidiaphragm,or more frequently,a lack of excursion during inspiration[76,77].Furthermore,in the case of diaphragmatic rupture secondary to penetrating or blunt thoracic or abdominal trauma,it is sometimes possible to visualize the diaphragmatic defect and the intraabdominal organs in the chest cavity[78,79].The accuracy of FAST in detecting diaphragmatic rupture has been assessed by Sharifiet al[80],from the study of 24 patients after thoracoabdominal penetrating trauma.When compared with the gold standard method,i.e.laparoscopy,US has good specificity (100%) but low sensitivity(50%).Consequently,a normal result from the FAST examination is not sufficient to eliminate diaphragmatic injury definitively.
In patients suffering from multiple rib fractures,diaphragmatic dysfunction can be secondary to the severity of the pain.In such circumstances,the inability to cough and to breathe deeply expose the patient to a high risk of pneumonia.Using diaphragmatic ultrasonography,it is possible to control the improvement of the diaphragmatic motion secondary to the use of an appropriate analgesia such as paravertebral block[81].
Critically ill patients
The benefit of the assessment of the diaphragmatic function by US in intensive care units has been widely investigated.In patients submitted to mechanical ventilation,an early decrease in diaphragm thickness is common[82,83].Moreover,the diaphragmatic motion has been assessed as a predictive factor for the weaning process.Two parameters have been studied,such as the diaphragmatic excursion and the fraction of thickening recorded during a spontaneous breathing trial[84,85].Some authors have proposed more sophisticated ultrasound indices such as the ratio between respiratory frequency and diaphragmatic displacement[86]or the product of diaphragmatic excursion and inspiratory time[87].Various cut-off values for these parameters have been proposed to predict weaning failure[88].Nevertheless,their predictive values have been questioned[88-90].Indeed,weaning failure is not routinely secondary to diaphragmatic dysfunction.Various etiologies leading to hypoxia may be implicated,such as heart failure,shock,or severe sepsis,for example.In these circumstances,diaphragmatic function can be unimpaired but the increase in the work of breathing can be insufficient to counteract hypoxia.Consequently,a multidisciplinary approach including clinical reasoning and echographic study of lung,heart and diaphragm should be more informative[91,92].

Figure6 Paradoxical motion (-1.26 cm) recorded by M-mode ultrasonography in patient with left hemidiaphragm paralysis.Ordinate in centimeter,abscissa in second.
PERSPECTIVES
Speckle tracking imaging
Two dimensional speckle tracking imaging (STI) is a recent echographic method initially used in cardiology.STI is based on the study of the displacement of the specklesi.e.the ultrasound signals reflected from microstructures in the tissue.The tracking of groups of speckles allows the measurement of the displacement and the deformation (the strain) of the organ studied.Positive strain values indicate passive extension whereas negative values reflect active shortening of a segment related to the initial length.The strain rate is used as the measure of the velocity of deformation.To estimate the potential advantage of STI,some authors have performed an assessment of the diaphragmatic function of healthy subjects using this technic.Ordeet al[93]have reported that the hemidiaphragmatic strain was correlated to the results from conventional methodsi.e.the measurement of excursion or percentage of thickening.Furthermore,the measurement of the left hemidiaphragmatic displacement during deep breathing might be easier with STI than with M-mode US[94].Other advantages of STI when compared with traditional methods have been suggested.M-mode US measures excursion on a single line perpendicular to the diaphragm.In contrast,STI is angle-independent and allows the assessment of the pattern of the regional contraction of the hemidiaphragm.It is therefore possible to divide the hemidiaphragm into three segments,the crura,the dome and the zone of apposition.Using this method,Yeet al[95]have oberved that the shortening (corresponding to negative strain values) of the right hemidiaphragm began in the zone of apposition and then in the crura.In healthy volunteers submitted to an increase in inspiratory load (50% of maximal inspiratory pressure)[96],the right diaphragmatic strain changed from -22% at baseline to -42% during loading.The strain and strain rate were significantly correlated with transdiaphragmatic pressure suggesting that STI can provide good markers for diaphragmatic effort.
Consequently,STI might be a promising tool for investigating diaphragmatic function.Unfortunately,no ultrasonographic machine has been developed to study diaphragmatic strain.The software of the ultrasound machine is designed to track two-dimensional speckle motion in echocardiograms.Consequently,during the STI analysis of the diaphragm,the tracking time is related to a cardiac cycle time period and not to the breathing cycle.To study the maximum deformation of the diaphragm,the analysis of the diaphragmatic motion during the entire inspiratory phase is required.To make the STI analysis of the diaphragmatic function easier,it should be advantageous to record the respiratory signals,i.e.the beginning of inspiration and expiration,on the ultrasound machineviaECG cables using a simple device developped in the laboratory[97].
Assessment of the work of breathing
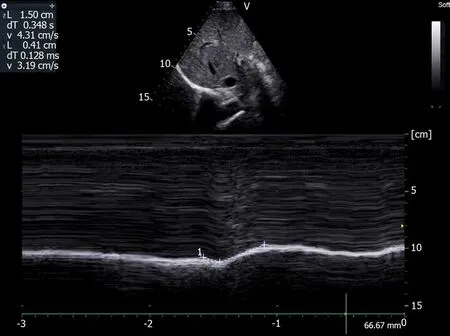
Figure7 Motion recorded during deep breathing in patient suffering from right hemidiaphragm paralysis:Paradoxical motion at the beginning of inspiration (-0.41 cm) terminal excursion in the cranio-caudal direction (+1.5 cm).Ordinate in centimeter,abscissa in second.
The work of breathing is the energy expanded for inspiration and expiration.It can be assessed by the measurement of thoracic pressure using an esophageal balloon allowing the changes in pleural pressure to be estimated.The work of breathing per breath (during each respiratory cycle) is estimated using the product of the change in thoracic pressure and the change in pulmonary volume.In the intensive care unit,it has been demonstrated that an inappropriate increase in the work of breathing during a spontaneous breathing trial was associated with a high risk of weaning failure[98].To estimate the work of breathing non-invasively,some authors have proposed using the thickening of the diaphragm at the zone of apposition,instead of the measurement of the thoracic pressure[99].In critically ill patients,respiratory effort is well estimated by the measurement of the transdiaphragmatic pressure (gastric pressure minus esophageal pressure) and it has been reported that the TF was significantly correlated to the transdiaphragmatic pressure-time product per breath[99].Consequently,important information on the work of breathing can be obtained from the simultaneous measurement of the diaphragmatic motion and the gas volume of the corresponding breathing cycle.
Ultrasound shear wave elastography
Another method that can be used to estimate the diaphragmatic force is ultrasound shear wave elastography.For this method,shear waves are generated inside tissues by means of focused ultrasonic beams and their propagation is followed using ultrasound imaging.The shear wave velocity directly correlates with tissue stiffness and active muscle force[100].Chinoet al[101]have investigated the changes in shear modulus of the diaphragm induced by an increase in inspiratory mouth pressure.They have reported that the diaphragmatic shear modulus increased together with submaximal inspiratory mouth pressure.Furthermore,Bachassonet al[102]have shown that the shear wave modulus of the diaphragm was correlated with transdiaphragmatic pressure.Consequently,shear wave elastography appears to offer a new non-invasive means of assessment of diaphragmatic strength.
CONCLUSION
The procedure for assessment of diaphragmatic function by US is now well defined.The combination of the study of diaphragmatic excursion and percentage of thickening allows hemidiaphragm paralysis to be diagnosed.In circumstances or diseases known to have a potential impact on the phrenic nerve or diaphragm muscle,US can offer a simple means of detecting diaphragmatic dysfunction.Repetitive examinations are beneficial in assessing recovery or progressive impairment.Recent technologies such as STI or ultrasound shear wave elastography and the combination of ultrasound with clinical parameters should provide a better analysis of diaphragmatic function.
 World Journal of Clinical Cases2020年12期
World Journal of Clinical Cases2020年12期
- World Journal of Clinical Cases的其它文章
- Computer navigation-assisted minimally invasive percutaneous screw placement for pelvic fractures
- Research on diagnosis-related group grouping of inpatient medical expenditure in colorectal cancer patients based on a decision tree model
- Evaluation of internal and shell stiffness in the differential diagnosis of breast non-mass lesions by shear wave elastography
- Real-time three-dimensional echocardiography predicts cardiotoxicity induced by postoperative chemotherapy in breast cancer patients
- Lenvatinib for large hepatocellular carcinomas with portal trunk invasion:Two case reports
- Biopsy-proven acute phosphate nephropathy:A case report
