Human embryonic stem cell-derived mesenchymal stem cells improved premature ovarian failure
Khadijeh Bahrehbar,Fereshteh Esfandiari,Seyedeh-Nafiseh Hassani,Hossein Baharvand,Department of Stem Cells and Developmental Biology,Cell Science Research Center,Royan Institute for Stem Cell Biology and Technology,Tehran 1665659911,Iran
Khadijeh Bahrehbar,Hossein Baharvand,Department of Developmental Biology,University of Science and Culture,Tehran 1665659911,Iran
Mojtaba Rezazadeh Valojerdi,Rouhollah Fathi,Department of Embryology,Reproductive Biomedicine Research Center,Royan Institute for Reproductive Biomedicine,Tehran 1665659911,Iran
Mojtaba Rezazadeh Valojerdi,Department of Anatomy,Faculty of Medical Science,Tarbiat Modares University,Tehran 1665659911,Iran
Abstract
Key words:Premature ovarian failure;Human embryonic stem cells;Chemotherapy drugs;Mesenchymal stem cell;Bone marrow;Apoptosis
INTRODUCTION
Premature ovarian failure (POF) disease has similar characteristics such as hypoestrogenism,elevated gonadotropin levels and infertility in animal models and in human.Some women also have symptoms such as hot flashes,night sweats,vaginal dryness,chronic anxiety,sadness and depression[1].POF affects 1%-3% of women <40 years of age[2].Hot flashes,depression,anxiety,osteoporosis and sexual dysfunction are the consequences of this disease[3,4].Although the cause of POF is often idiopathic,possible causes include autoimmune disorders,smoking,toxic chemicals,drugs and genetic defects[5-7].Chemotherapeutics such as cyclophosphamide (Cy) and busulfan(Bu) are the most gonadotoxic agents that lead to POF in the majority of patients[8].Currently,ovarian protection methods,oocyte or ovarian tissue cryopreservation and embryo freezing are strategies used for fertility preservation in women diagnosed with cancer.However,these methods have serious disadvantages such as the risk of reintroducing the cancer cells,delays in cancer treatment and low success rate.Therefore,it is necessary to develop advanced therapies for women with POF[9].
Emerging evidence suggests that mesenchymal stem cells (MSCs) derived from bone marrow (BM) and other adult tissues (adipose,skin,amniotic membrane,placenta) and menstrual blood could restore ovarian function in animal models of POF[10-14].A meta-analysis from 16 preclinical studies of animal models was conducted to assess the efficacy of stem cell transplantation.The results indicated that MSC therapy significantly improved ovarian function in cases with POF[15].In two case studies,MSC transplantation also improved POF[15-16].However,despite the promising results,the numbers of harvested MSCs and theirin vitroexpansion was a challenge[17].Moreover,obtaining MSCs from bone marrow requires suitable donors and invasive procedures.The number of bone marrow-derived mesenchymal stem cells (BM-MSCs) is very limited,which greatly restricts use of BM-MSCs for clinical application[18].The immunomodulating feature of MSCs seems to be different between species[19].Human MSCs decrease the secretion of interferon gamma,interleukin 12 and tumor necrosis factor alpha and increase interleukin 10 secretion[20-22].Moreover,human MSC-mediated inhibition of the T cell response could not be reversed by nitric oxide synthase inhibitor compared with mice MSCs[23].Integrin β1 expression is important for mice MSCs migration,while C-X-C chemokine receptor type 4 expression is involved for human MSC migration to sites of tissue injury[24-25].It has been demonstrated that 92% of MSC protein expression is similar in humans and mice[26].MSCs represent only a small proportion of the cells in bone marrow,and their proliferation and differentiation capacity correlates inversely with age[20].
In addition to adult tissue specific MSCs,human embryonic stem cells (ES-MSCs)are an alternative source of MSCs because of their similar phenotypic characteristics that make them attractive candidates for regenerative cellular therapy[27-28].Recently,it has been reported that ES-MSCs have higher capabilities for cell proliferation and suppression of leukocyte growth compared to MSCs from other sources[29-30].ES-MSCs exhibited more potent anti-inflammatory properties than BM-MSCs[17,27,31-33].The therapeutic potential of ES-MSCs has been reported in numerous animal models.When compared with BM-MSCs,these cells showed a significantly greater improvement in models of thioacetamide-induced chronic liver injury and experimental autoimmune encephalitis[30,34].This evidence indicates that ES-MSCs may serve as better sources for clinical applications.
Human ES-MSCs can overcome the obstacles seen with harvesting MSCs from adult tissues,including lack of appropriate donors,limited numbers of cells obtained during the harvesting process,restrictedin vitroexpansion capacity and the invasiveness of the procedures.Thus,we hypothesized that ES-MSCs might restore ovarian structure and function through the paracrine mechanisms of cytokines in a POF model.To address this issue,we used a POF mouse model to evaluate the potential for transplanted ES-MSCs to restore fertility.
MATERIALS AND METHODS
Derivation of MSCs from human ES cells and BM
We isolated and cultured ES-MSCs according to our previously published protocols[17,33].Briefly,we obtained MSCs from human ES cells by culturing these cells in basic fibroblast growth factor-free ES medium to enable embryoid body formation.The resultant embryoid bodies were plated in gelatin-coated plates and cultured in MSC medium.Spontaneous differentiation of the embryoid bodies resulted in an outgrowth of ES-MSCs.These cells were further passaged to obtain a homogenous population with spindle-shaped morphology.Passage-2 human BM-MSCs were prepared from Royan Stem Cell Bank (Tehran,Iran) and cultured in low-glucose Dulbecco’s Modified Eagle Medium (Life Technologies,United States) supplemented with 10% fetal bovine serum (FBS,Life Technologies,United States) for further expansion.The medium was changed every 3 d.
Cell proliferation analysis
We cultured 1 × 106cells/cm2in T25 cm2tissue culture flasks (TPP,Germany) to assess their proliferative ability.The population doubling time was calculated according to the following formula:
Population doubling time = duration × log (2)/log (final concentration) – log (initial concentration)
Karyotype analysis
The cells were treated with 0.66 mmol/L thymidine (Sigma-Aldrich) and incubated at 37 °C for 16 h.After the cells were washed with phosphate buffered saline (PBS),they were left for 5 h and then treated with 0.15 mg/mL colcemid (Invitrogen) for 30 min.Then,the cells were exposed to 0.075 mol/L potassium chloride (Merck) and allowed to incubate at 37 °C for 16 min.After the cells were centrifuged,we removed the supernatant and resuspended the pellet in Carnoy's fixative (3:1 ratio of methanol:glacial acetic acid).The cells were dropped onto precleaned,chilled slides and standard G-band staining was performed for chromosome visualization.We screened at least 20 well-spread metaphase cells of which 10 were evaluated for chromosomal rearrangements.
Flow cytometry analysis
We sought to determine the immunophenotypes of the cultured ES-MSCs and BMMSCs.Surface-marker expression was analyzed by flow cytometry using the following antibodies:Fluorescein isothiocyanate-conjugated human monoclonal antibodies against protein tyrosine phosphatase receptor type C and cluster of differentiation(CD) 90 (CD90);and phycoerythrin-conjugated human monoclonal antibodies against homing cell adhesion molecule,CD73,endoglin,CD11b and CD34.For flow cytometric analysis,the adherent cells were detached by using 0.25% trypsinethylenediaminetetraacetic acid,neutralized by FBS-containing culture medium and disaggregated into single cells by pipetting.The cells were incubated with antibodies for 30 min at 4 °C,washed twice with PBS,resuspended in 0.5 mL PBS and immediately analyzed by fluorescence-activated cell sorting Calibur flow cytometer(Becton Dickinson,United States).Analyses were performed on three independent biological samples.Data were analyzed using the FlowJo software (version 7.6.1).Supplementary Table1 lists the antibodies used in this study.
Multilineage differentiation
Osteogenic,adipogenic and chondrogenic differentiation were verified by alizarin red,oil red O,and alcian blue staining,respectively to confirm the multipotent properties of the ES-MSCs and BM-MSCs.For osteogenesis,the cells were seeded onto 6-well plates at a density of 1 × 105cells/cm2.After 24 h,the medium was replaced by osteogenic differentiation medium,alpha minimum essential medium (Life Technologies,United States) supplemented with 10% FBS (Gibco,United States),0.1 mmol/L dexamethasone (Sigma-Aldrich,United States),10 mmol/L βglycerophosphate (Sigma-Aldrich,United States) and 50 mmol/L ascorbic acid(Sigma-Aldrich,United States) for 2 wk.To induce adipogenesis,the cells were incubated with adipogenic differentiation medium in alpha minimum essential medium supplemented with 10% FBS,10 mg/mL insulin (Sigma-Aldrich,United States),1 mmol/L dexamethasone (Sigma-Aldrich,United States),0.5 mmol/L isobutyl-methylxanthine (Sigma-Aldrich,United States) and 100 mmol/L indomethacin (Sigma-Aldrich,United States) for 3 wk.For chondrogenic differentiation,2.5 × 105cells were collected in a 15 mL tube and centrifuged at 350 g for 5 min.The cell pellet was subsequently cultured for 3 wk using chondrogenic induction medium (chondrogenesis differentiation kit,Gibco,United States) according to the manufacturer’s instructions.Then,the pellets were fixed in 4%paraformaldehyde (Sigma Aldrich,United States) for 30 min,dehydrated in ethanol,cleared in xylene and embedded in paraffin.The paraffin-embedded cells were sectioned into 6 µm sections by using a microtome.The sections were stained with alcian blue.
Measurement of cytokine secretion
We analyzed cytokines secreted by the MSCs.Both ES-MSCs and BM-MSCs were cultured in dishes at densities of 2 × 105cells/cm2each.After a 24 h culture in serumfree media,the culture media was collected and centrifuged at 2000gfor 5 min.The amount of cytokine expression was measured using a vascular endothelial growth factor (VEGF) Human ELISA kit (Invitrogen,United States),insulin-like growth factor 2 (IGF-2) Human ELISA kit (R&D Systems,United States) and hepatocyte growth factor (HGF) Human ELISA kit (R&D Systems,United States).
Experimental animals
All animal experiments were approved by the Institutional Ethical Committee of Royan Institute.Adult female C57BL/6 mice (6-8 wk old) were used in our study.The mice were housed under a 14-10 h light-dark cycle and had free access to food and water.
Estrous cyclicity
Vaginal smears were obtained daily.The four stages of the estrous cycle were determined as follows:Proestrus (100% intact live epithelial cells);estrus (100%cornified epithelial cells);metestrus (about 50% cornified epithelial cells and about 50% leukocytes);and diestrus (80%-100% leukocytes).The mouse estrous cycle lasts for approximately 4 d and includes the proestrus,estrus,metestrus and diestrus stages.Animals with at least two consecutive normal 4-d vaginal estrous cycles were included in the experiments.In order to validate reproductive function,we assessed the animals over 10 consecutive days of the experiment.The number of estrous cycles were checked at 8:00 am daily with a vaginal smear assay starting at 10 d after the animals were injected with Cy and Bu and 4 wk after transplantation of ES-MSCs or BM-MSCs.
Establishment of the POF model
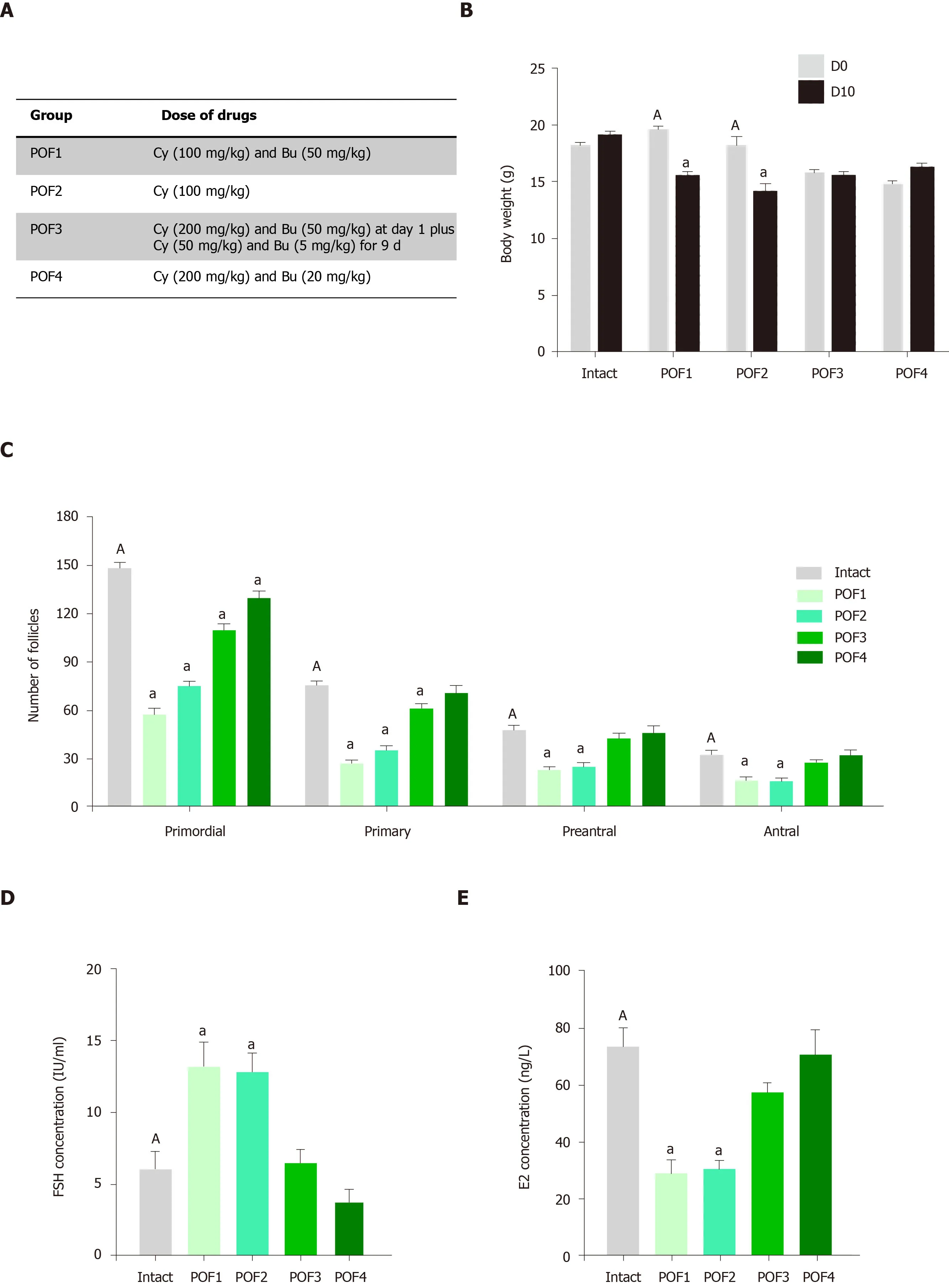
In this study,we used chemotherapy to create the mouse model because chemotherapy is one of the major causes of POF[35].Various reports of POF models generated in mice used from 8-30 mg/kg of Bu plus 50-200 mg/kg of Cy[36-40].or only Cy[41-44].However,none of the previous studies showed any significant decrease in follicle numbers during the developmental stages.Therefore,we assessed different doses of these drugs to create a POF model in our laboratory setting.Female mice were randomly divided into four treatment groups and one intact group.The treatment groups received intraperitoneal injections of different doses of Cy(Endoxan™,Germany) and Bu (Sigma-Aldrich,United States) as follows:Group 1(POF1):50 mg/kg Bu and 100 mg/kg Cy;group 2 (POF2):100 mg/kg Cy for 10 consecutive days;group 3 (POF3):200 mg/kg Cy and 50 mg/kg Bu on the 1stday followed by 50 mg/kg Cy and 5 mg/kg Bu for 9 consecutive days;and group 4(POF4):A single injection of 20 mg/kg Bu and 200 mg/kg Cy.In order to confirm successful establishment of POF in the mouse model,we checked their body weights,estrous cyclicity,concentrations of follicle-stimulating hormone (FSH) and estradiol(E2) hormones and follicle counts.In addition,for further confirmation of POF,we also used the terminal deoxynucleotidyl transferase mediated 2-deoxyuridine 5-triphosphate nick end labeling (TUNEL) assay and real-time PCR assessments,and the mice were allowed to mate 10 d after the injections.
Cell transplantation
Once the POF model was established,we randomly divided the mice into three groups.Vehicle POF mice received medium but no cell transplantation.In the ES-MSC group,POF mice were injected with 1 × 106ES-MSCs.In the BM-MSC group,POF mice were injected intravenously with 1 × 106BM-MSCs in 0.1 mL Dulbecco’s Modified Eagle Medium.In order to evaluate the effects of the transplanted ES-MSCs and BM-MSCs,we assessed the body weights,estrous cyclicity,concentrations of FSH and E2 hormones and follicle counts in the POF mice at 4 wk after the transplantations.In addition,the mice were allowed to mate.The TUNEL assay,Western blot,immunohistochemistry and real-time PCR assessments were also performed.
Hormone assay
Blood samples were obtained from hearts of the anesthetized mice to determine serum levels of E2 and FSH.The blood samples were incubated at room temperature for 1 h,and supernatant was collected after centrifugation at 3000 rpm for 20 min.Hormone levels were determined by ELISA kits (Biotech,Shanghai,China).
Detection of apoptosis by the TUNEL assay
Cell apoptosis in the ovarian tissue was detected by the TUNEL assay.Briefly,5 μm ovarian sections were washed twice in PBS for 5 min after deparaffinization.These sections were permeabilized by incubation in 0.1% Triton X-100 solution and 0.1%sodium citrate for 8 min.Then,the TUNEL assay was performed with anin situcell death detection kit (Roche,Germany) according to the manufacturer’s instructions.Counterstaining with DAPI (Sigma-Aldrich) was used to visualize the nuclei.We observed the cells under a fluorescence microscope (Olympus,Japan) for the presence of apoptosis (green fluorescent color).
Hematoxylin and eosin staining and data quantification
The ovaries were removed and fixed in 4% paraformaldehyde (Sigma-Aldrich) for at least 24 h.The fixed ovaries were dehydrated,embedded in paraffin,serially sectioned into 6 μm sections and mounted on glass microscope slides.Routine hematoxylin and eosin staining was performed for histologic examination under a light microscope.
Follicle counting
Primordial,primary,secondary and antral follicles were counted in each of the five sections based on the method reported by Tilly[45].Only the follicle with a nucleus was counted to avoid duplicate counting of a follicle.The follicles were classified as:Primordial (oocyte surrounded by a single layer of squamous granulosa cells);primary(intact enlarged oocyte with a visible nucleus and one layer of cuboidal granulosa cells);secondary (two or three layers of cuboidal granulosa cells without an antral space);early antral (emerging antral spaces);and preovulatory (the largest follicular types with a defined cumulus granulosa cell layer).Supplementary Tables 3 and 4 present the data for follicle counting both after chemotherapy and cell transplantation.The significance of the changes in follicle numbers in the different study groups were analyzed by two-way analysis of variance.
Gene expression analysis
Quantitative reverse transcriptase–polymerase chain reaction (qRT-PCR) was performed for the apoptosis genes,anti-apoptosis gene [B-cell lymphoma 2 (Bcl2)],apoptosis gene [cysteine-aspartic proteases 3 (caspase 3)],angiogenesis gene (Vegf),proliferation gene (Igf-2),granulosa marker anti-Müllerian hormone (Amh) and oocyte marker [growth/differentiation factor 9 (Gdf9)] in the intact group,POF group and both cell transplantation groups.Total RNA was isolated and purified with TRIzol reagent (Invitrogen) according to the manufacturer’s protocol followed by cDNA synthesis with a cDNA synthesis kit (Fermentas).qRT-PCR reactions were performed using SYBR Green Master Mix (Applied Biosystems) and a real-time PCR system(Corbett Life Science;Rotor-Gene 6000 instrument).The samples were collected from three independent biological replicates.Supplementary Table2 lists the primer sequences used for qRT-PCR.
Western blot analysis
The protein expression of caspase 3 in the ovaries was measured by Western blot.The mice were anesthetized,and we removed their ovaries.The proteins from the ovaries were isolated by the Q Proteome Mammalian Protein Prep kit (Merck,Germany).The total protein concentrations were measured using a standard BCA protein assay kit.The protein from each group was separated on 12% SDS-PAGE and transferred onto PVDF membranes.The blots were then incubated in blocking buffer [2% (w/v) skim milk powder in TBST] for 1 h at room temperature.Then,the membranes were incubated overnight at 4 °C with the primary antibody,anti-caspase 3 (1:2000[46]).The membranes were washed three times with TBST and incubated at room temperature for 1 h with anti-rabbit secondary antibody conjugated to horseradish peroxidase(1:1000).
Immunohistochemistry
Expression of the granulosa cell marker (Amh) was detected by immunohistochemical staining.The ovaries were fixed in formalin and sectioned into 5 μm sections.The sections were incubated at 60 °C for 1 h,deparaffinized in xylene and rehydrated in a graded ethanol series.Then,antigen retrieval was performed by heating the sections in citrate buffer in an oven for 30 min.The sections were washed in H2O2for 30 min to eliminate endogenous peroxidase activity and blocked with goat serum for 1 h at room temperature.After that,the sections were incubated overnight at 4 °C with the primary antibody,anti-Amh.After three washes with PBS for 10 min each time,the secondary antibody,streptavidin,and DAB were used for immunostaining according to the protocol from an immunostaining kit (Merck,Germany).Finally,the sections were counterstained with hematoxylin,dehydrated and mounted.
Mating trial
The mating trial was initiated 10 d after the mice were injected with Cy or 4 wk after transplantation of the ES-MSCs or BM-MSCs and continued for six weeks.Female mice were housed in the same cages with male mice for natural mating.The presence of a copulatory plug indicated successful mating.Males were randomly rotated among the cages after each pregnancy and the numbers of offspring per litter were recorded.
Statistical analysis
All experiments were conducted in at least three independent repeats.All data are shown as mean ± standard error of the mean.One-way analysis of variance was used to determine significant differences among groups with Tukey’s post-hoc test.Viability was analyzed by thettest.P<0.05 were considered significant.
RESULTS
Derivation and characterization of ES-MSCs and BM-MSCs
Figure1A shows the procedure used to derive MSCs from the ES cells.MSCs derived from both human ES and BM formed a homogeneous cell population with spindleshaped morphology and a normal karyotype during long-term culture(Figure1B and 1C).The MSCs successfully differentiated into osteogenic,adipogenic and chondrogenic lineages (Figure1D).Flow cytometry analysis confirmed the expression of MSC-specific markers homing cell adhesion molecule,CD73,CD90 and endoglin by both human ES-MSCs and BM-MSCs;there were no detectable levels of the hematopoietic and endothelial cell markers (CD11b,CD34 and protein tyrosine phosphatase receptor type C) (Figure1E and 1F,Supplementary Figure1).The population doubling time assay showed significant increases in ES-MSC proliferation compared to BM-MSCs (Figure1G;P<0.05).
Establishment of a mouse model of chemotherapy-induced POF
We examined various concentrations of four different combinations of two chemotherapy drugs,Cy and Bu,in order to establish a POF model that showed the significant decreases in follicle numbers for all of the developmental stages(Figure2A).In the intact group,the mice had regular 4-d estrous cycles;however,irregular estrous cycles were observed in the POF1,POF2,POF3 and POF4 mice.On day 5 of the treatment,the POF1,POF2 and POF3 mice were eating less and moved slowly (data not shown).The ovaries of the mice in the intact group were more reddish in color,whereas the ovaries of the mice that survived in the POF groups were pale.All of the animals were weighed before and after modeling,and we found significantly reduced body weights in the POF1 and POF2 groups (Figure2B,Supplementary Figure2A;P<0.05).Furthermore,the size of ovaries in mice treated with the chemotherapy drugs in the POF1,POF2,POF3 and POF4 groups were smaller than ovaries from the intact mice (Supplementary Figure2B).
We performed hematoxylin and eosin staining to evaluate the structures of the ovaries following chemotherapy.Quantification of the follicles showed significant decreases in all of the developmental stages in the POF1 and POF2 groups (Figure2C;Supplementary Figure3;P<0.05),while the POF3 and POF4 groups did not show significant decreases in the number of follicles in the various developmental stages.
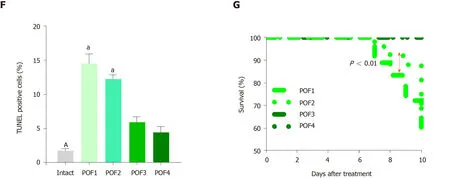
Hormonal analysis demonstrated significant increases in serum levels of FSH and significant decreases in E2 levels in the POF1 and POF2 groups (Figure2D and 2E;P<0.05).TUNEL assay results to evaluate apoptosis in the ovaries following chemotherapy (Supplementary Figure4A) showed a significantly increased percentage of TUNEL-positive cells in the POF1 and POF2 groups compared to the intact group (Figure2F;P<0.05).Next,we sought to determine the optimum POF model by evaluating the survival rate of the mice and the pregnancy rate following chemotherapy.We found significantly higher survival rates in the POF1 group compared to the POF2 group (Figure2G;P<0.01).However,none of the POF mice became pregnant (Supplementary Figure4B).Therefore,we selected the POF2 model as the most appropriate model for induction of POF.
ES-MSCs and BM-MSCs improved the POF model
We explored the possibility that the MSCs could improve the POF mouse model.There were more regular estrous cycles following transplantation of both human ESMSCs and BM-MSCs compared to the vehicle group.Moreover,the ovaries of the mice had an increased red color and were larger in size following transplantation of both ES-MSCs and BM-MSCs in comparison with the vehicle group,but they were less than the intact ovaries (Supplementary Figure5).Body weight significantly increased 4 wk after transplantation of both ES-MSCs and/or BM-MSCs compared to the vehicle group (Figure3A;P<0.05).The survival rate significantly increased following transplantation of ES-MSCs and/or BM-MSCs (more than 60%) compared to the vehicle group (20%) (Figure3B;P<0.01).
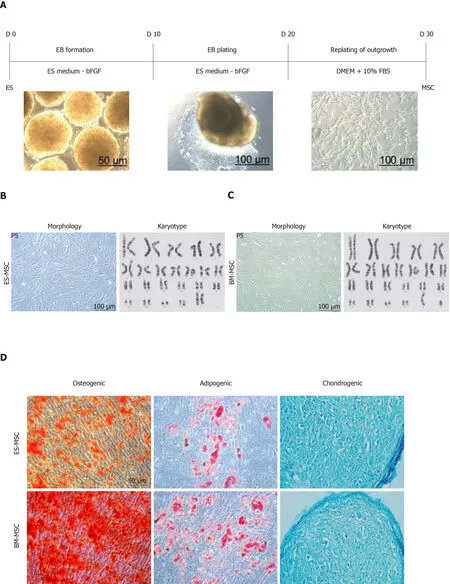
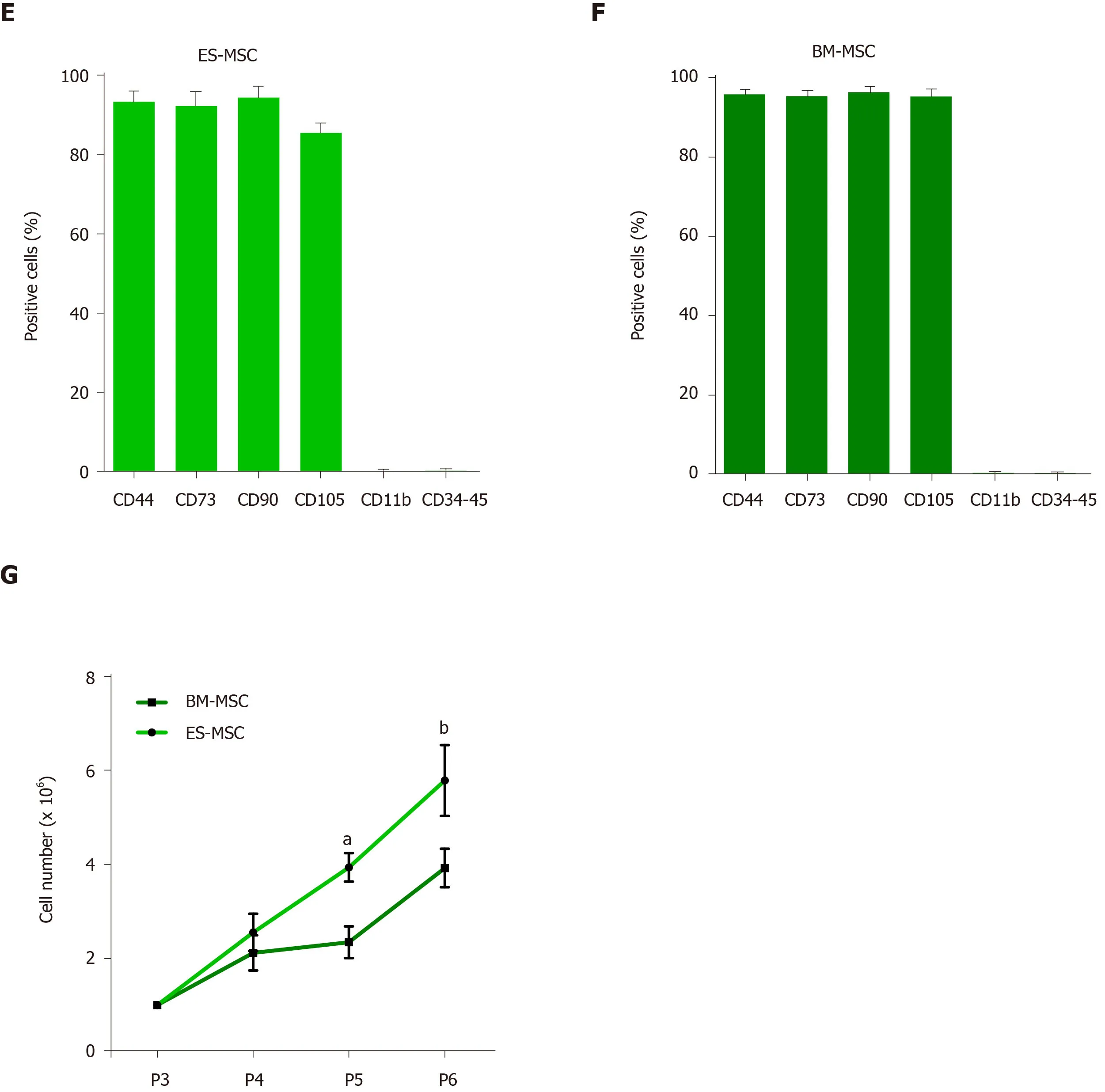
Figure1 Derivation and identification of human embryonic stem cell-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells.A:Schematic presentation of the procedure used to derive human mesenchymal stem cells (MSCs) from embryonic stem (ES) cells.Colonies of ES cells were enzymatically detached and cultured for 10 d in suspension to form embryoid bodies,which were then plated onto gelatin-coated tissue culture plates.After 10 d,outgrowths of the cells that sprouted from embryoid bodies were mechanically isolated by a cell scraper and subsequently expanded in mesenchymal stem cell culture medium;B and C:Morphology and karyotype of ES-MSCs and BM-MSCs.Passage-5 ES-MSCs and BM-MSCs showed a fibroblastic morphology and normal karyotype;D:Alizarin red staining after 14 d of culture in osteogenic medium indicated the osteogenic differentiation potential of ES-MSCs and BM-MSCs (P4).Oil red staining after 21 d of culture in adipogenic medium showed the adipogenic differentiation potential of ES-MSCs and BM-MSCs (P4).Alcian blue staining after 21 d of culture in chondrogenic medium showed chondrogenic differentiation potential of ES-MSCs and BM-MSCs;E,F:Flow cytometric analysis indicated that cultured ES-MSCs and BM-MSCs expressed CD44,CD90,CD73 and endoglin (CD105),but not hematopoietic lineage markers CD11b,CD34 and protein tyrosine phosphatase receptor type C (CD45);G:ES-MSCs proliferated more rapidly than BM-MSCs.Results are expressed as mean ± standard error,aP <0.05,bP <0.01;n = 3-5.ES-MSCs:Embryonic stem cell-derived mesenchymal stem cells;BM-MSCs:Bone marrow-derived mesenchymal stem cells;EBs:Embryoid bodies;bFGF:Basic fibroblast growth factor;P:Passage.
Notably,we observed significant increases in the number of follicles at all stages of development following transplantation of both ES-MSCs and BM-MSCs compared with the vehicle group (Figure3C;P<0.05).Transplantation of both ES-MSCs and BM-MSCs significantly decreased the FSH levels and increased the E2 levels compared with the vehicle group (Figure3D and 3E;P<0.05).
The results of the TUNEL assay confirmed significant decreases in apoptosis in ovaries that received the cell transplantations (Figure4A and 4B,Supplementary Figure6;P<0.05).qRT-PCR was conducted in order to gain further insight into theeffect of transplantation on the ovaries.The results showed significant downregulation of the apoptosis gene,caspase 3,while the anti-apoptotic gene,Bcl2,was significantly upregulated following cell transplantation compared with the vehicle group.In particular,the level of the angiogenesis gene (Vegf),proliferation gene(Igf-2)and granulosa marker(Amh)significantly increased following cell transplantation.In contrast,we observed no significant differences in the oocyte marker,Gdf9,following transplantation (Figure4C;P<0.05).
Cleaved- caspase 3 acts as a functional enzyme[47,48];therefore,to further validate these results,we performed Western blot assessment of cleaved-caspase 3 protein expression.Our results showed a significant increase in the cleaved-caspase 3 protein expression level in ovaries from the vehicle group compared with the control group,whereas the cleaved-caspase 3 protein expression level decreased significantly in the ovaries after transplantation of ES-MSCs and/or BM-MSCs compared with the vehicle group (Figure4D and 4E;P<0.05 ).Previous studies suggested that MSCs secrete cytokines that are important for anti-apoptosis,angiogenesis,anti-inflammation,antifibrosis and immunoregulation,which would improve the microenvironment for promoting regeneration of injured tissues in numerous diseases[49-53].In order to investigate the mechanism that underlies the function of these MSCs,we also analyzed VEGF,IGF-2 and HGF levels in ES-MSCs and BM-MSCs condition media by using ELISA.The results showed that in a similar manner ES-MSCs and BM-MSCs secreted VEGF,IGF-2 and HGFin vitro(Figure4F).However,there were only a few GFPlabelled cells after 4 wk in the ovaries (data not shown).
Immunohistochemistry staining for Amh to confirm the changes in the granulosa cells showed decreased Amh expression in ovaries from the vehicle group compared to the intact group and increased Amh expression in ovaries from both the ES-MSCs and BM-MSCs transplantation groups compared with the vehicle group (Figure5A).
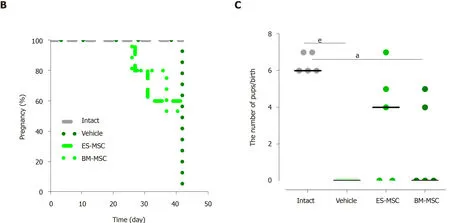
We assessed the ability of mice that received the transplantations to conceive and give birth to offspring.The successful mating rate was investigated over 6 wk,and the presence of a copulatory plug indicated successful mating.The mice that received transplantations of both ES-MSCs (3 out of 5 mice) and/or BM-MSCs (2 out of 5 mice)became pregnant and produced live offspring,9 pups in mice transplanted with BMMSCs and 16 pups in mice that received ES-MSCs.None of the vehicle mice became pregnant.These results showed that ovarian functions in mice with POF were partially restored by transplantation with either ES-MSCs or BM-MSCs (Figure5B and 5C;P<0.05).

Figure3 Effects of human embryonic stem cell-derived mesenchymal stem cells and bone marrow-derived mesenchymal stem cells transplantation in mice with premature ovarian failure.A:Transplantation of embryonic stem cell-derived mesenchymal stem cells (ES-MSCs) and/or bone marrow-derived mesenchymal stem cells (BM-MSCs) improved body weights in mice with premature ovarian failure after 4 wk;B:Survival rate 4 wk after ES-MSCs and/or BM-MSCs transplantation.Survival rate significantly increased in both the ES-MSCs and/or BM-MSCs transplanted mice (more than 60%) compared with the vehicle group (20%);C:The follicle number increased after transplantation.The number of follicles at all stages of development in both cell transplanted groups was significantly higher than that of the vehicle mice,while it was lower than the intact mice;D,E:Both cell transplantations rescued hormone secretion in premature ovarian failure mice.Serum follicle stimulating hormone levels decreased significantly in both cell transplanted groups compared to the vehicle group.The serum estradiol level significantly recovered after both cell transplantations compared to the vehicle group.All data are presented as mean ± standard error.Small letters (a,c) indicate the significance (P <0.05) compared to groups labeled by similar capital letters (A,C);aP <0.05 significance of experimental groups vs the intact group;cP<0.05 significance of ES-MSC and BM-MSC groups vs the vehicle group;n= 3-5.ES-M Cs:Embryonic stem cell-derived mesenchymal stem cells;BM-MSCs:Bone marrow-derived mesenchymal stem cells;POF:Premature ovarian failure;FSH:Follicle stimulating hormone;E2:Estradiol.
DISCUSSION
Understanding the pathogenesis of POF plays an important role in the development of effective therapeutic options for this disease.Therefore,elucidation of the mechanism for POF development is critical for the clinical treatment of POF disease[54].The estrous cycle of female mice is similar to that of humans,although the estrous cycle of mice is shorter than that of humans[55].
In this study,we initially established a mouse POF model by administration of Cy and Bu as the most effective chemotherapeutic drugs.The results indicated that Cy plus Bu in our established model (POF2) significantly decreased the number of follicles at various stages of development and significantly decreased ovarian size and body weight.In line with previous studies,chemotherapy increased primordial follicle recruitment,which led to significant decreases in the number of follicles at different developmental stages[35,56].Apoptotic cells significantly increased in our established POF model,which was consistent with previous findings where chemotherapeutic drugs destroyed highly proliferating cells by activation of apoptosis[57].We observed increased FSH levels and decreased E2 levels,which supported results of studies that showed similar patterns of hormonal changes in POF[15].Previous studies demonstrated that chemotherapeutic drugs can cause POF in various species such as mouse,rat,rabbit and human[16,58-61].Our results were consistent with previous reports as we showed a decrease in the number of follicles,decreased serum E2 levels,increased serum FSH levels and infertility.
MSCs features depend on both the tissue source from which they were obtained and the species.Previous studies indicated that MSCs obtained from various species and sources differ in their biological characteristics such as surface marker expression,proliferative capacity,multilineage differentiation potential and immunomodulation feature[62,63].In this study,we investigated biological properties of ES-MSCs and BMMSCs.We have found that ES-MSCs and BM-MSCs both expressed homing cell adhesion molecule,CD73,CD90 and endoglin,but they showed no expression of CD34,protein tyrosine phosphatase receptor type C and CD 11b,which is consistent with a previous study[31].We indicated that ES-MSCs showed enhanced proliferation capacity compared to the BM-MSCs.On the fourth and fifth passages,there were significant differences between ES-MSCs and BM-MSCs.Previous studies have similarly reported that ES-MSCs are more proliferative compared to BM-MSCs[31,33].
In addition,we demonstrated that multilineage differentiation potential of BMMSCs was greater than ES-MSCs.This finding was consistent with previous studies[31,33].MSCs from different species and sources produce different cytokines.Our results were consistent with previous studies that cytokines secreted from MSCs could influence cell proliferation,differentiation,survival and tissue repair[64,65].We observed no significant difference between ES-MSCs and BM-MSCs secreted cytokines in culture medium.
We transplanted human ES-MSCs into a mouse animal model and showed their capability in restoring ovarian function in POF.In support of transplantation of human derived MSCs to another species,previous studies have demonstrated that the transplantation of MSCs derived from various human tissues including menstrual blood,umbilical cord and amniotic fluid into animal models of POF restore ovarian function[66].
In this study,we transplanted ES-MSCs into a mouse model of POF to investigate the role of these cells and mechanisms of action for improvement of POF.Our results indicated that both ES-MSCs and BM-MSCs showed a similar trend for improvement of POF in this animal model.ES-MSCs improved ovarian structure and function in these mice as evidenced by the increased number of follicles,decreased granulosa cell apoptosis and restored FSH and E2 to near normal levels.E2 is mainly secreted bygranulosa cells,which inhibit FSH secretion.Increased FSH levels could accelerate recruitment of follicles and deplete the follicular pool[14,67,68].Increased apoptosis in the POF group might result in deceased E2 and FSH levels.Transplantation of ES-MSCs inhibited granulosa cell apoptosis and increased E2 secretion,which led to decreases in FSH;therefore,the decreased level of FSH in the ES-MSCs transplantation group resulted in an increased number of follicles.
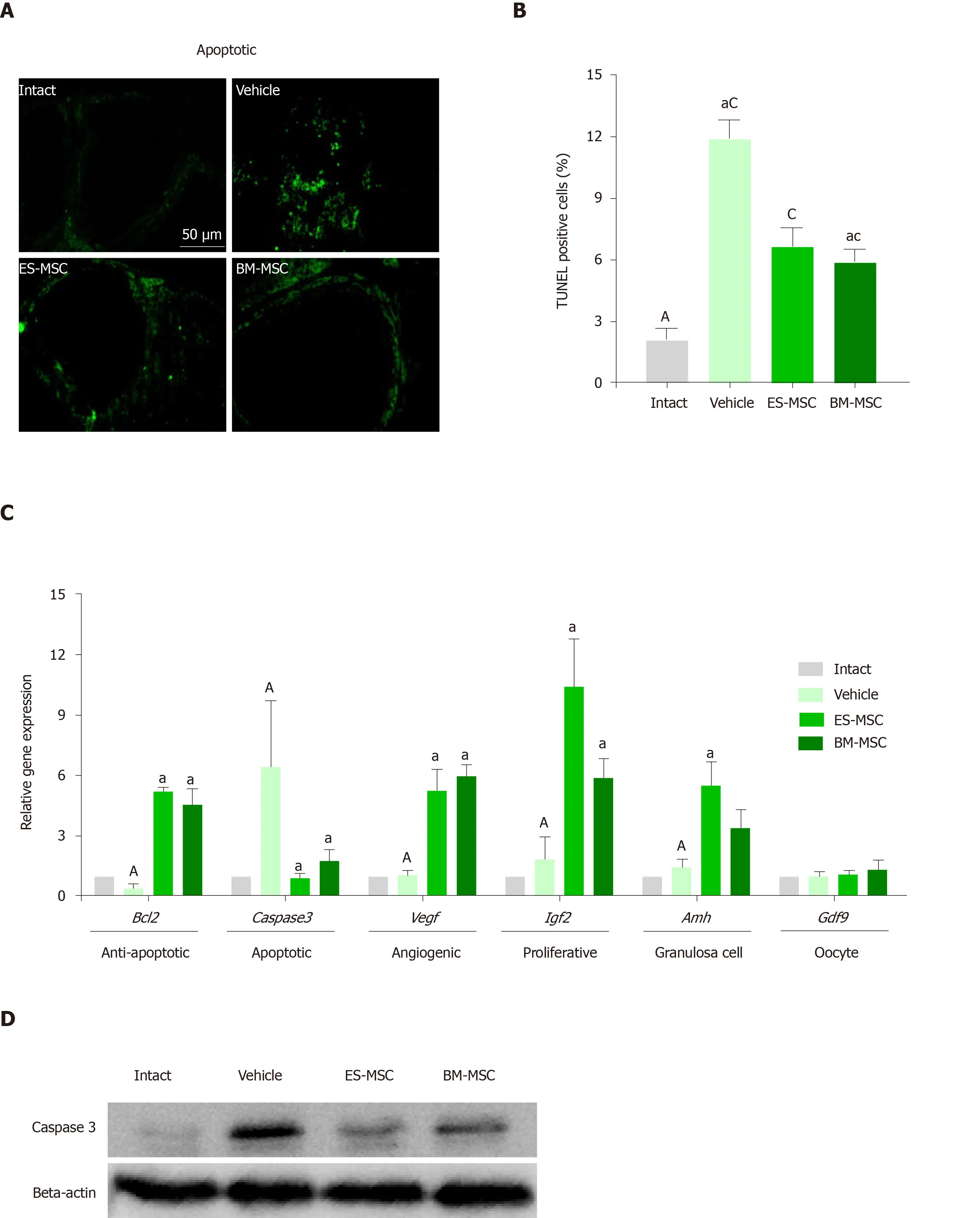

Gene expression and Western blot results reinforced our hypothesis.There were significant increases inAmhexpression (granulosa cell marker) and no difference inGdf9expression (oocyte marker).Expression ofBcl2was upregulated in both of the MSC transplantation groups compared with the POF group,whereascaspase 3expression was downregulated in both of these groups compared with the POF group.
Immunohistochemistry results agreed with the real-time PCR results and indicated that MSCs could increase Amh expression compared with the POF mouse group.Folliculogenesis is mainly affected by interactions between the oocyte and granulosa cells[69].Amh is expressed by granulosa cells and plays an important role in follicle growth[70,71].We observed decreased apoptosis of granulosa cells after transplantation of the MSCs;therefore,ES-MSCs maintained the follicular niche by inhibiting apoptosis of granulosa cells.Previous findings of granulosa cell function in supporting oocytes also confirmed our results[72].Therefore,granulosa cells support oocytes during development from the primordial state to maturation.
We observed that the ES-MSCs secreted VEGF,IGF-2 and HGFin vitro.VEGF,IGF-2 and HGF have an important role in inhibiting granulosa cell apoptosis,stimulating granulosa cell proliferation,inducing angiogenesis and follicle growth.VEGF promotes granulosa and endothelial cell proliferation.IGF-2 and HGF play an important role in suppressing apoptosis of granulosa cells that promote follicle maturation[73-77].We observed decreased mRNA and protein expression of caspase 3 in both of the MSC transplantation groups compared with the POF group.The caspasefamily members are important regulators of apoptosis.Caspase 3 is an effector caspase;its activation results in the final phase of cellular death[78].Our study results suggested that ES-MSCs may have decreased caspase 3 expression in the ES-MSC transplantation group by releasing VEGF,HGF and IGF-2;therefore,these cytokines may inhibit apoptosis in granulosa cells by upregulation ofBcl2and downregulation ofcaspase 3in the ovaries of POF mice.Therefore,there was increased expression of the granulosa cell marker (Amh) after transplantation of the ES-MSCs.Our results might indicate that cytokines secreted by ES-MSCs reduce granulosa cell apoptosis and increase follicles by increasing E2 secretion.

We observed no significant difference inGdf9expression in both MSC transplantation groups compared with POF.Gdf9 is an adult oocyte-specific marker[79,80],and this finding might indicate that transplantation of ES-MSCs restored ovarian function in POF miceviaan indirect effect due to cytokine secretion rather than direct differentiation to oocytes.
These findings suggested that a possible mechanism by ES-MSCs and BM-MSCs restored the injured ovary by cytokine suppression of granulosa cell apoptosis and increased follicular growth.In line with our findings,the results from previous studies suggest that MSCs have an effect on restoring ovarian function by the paracrine mechanism of cytokines[81-85],which plays an important role in increased granulosa cell resistance to chemotherapeutic drugs and improves the ovarian microenvironment and follicle growth[58].
In conclusion,our results indicated that human ES-MSCs could restore ovarian structure and function in chemotherapy-induced POF mice and improve fertility.Transplantation of ES-MSCs improved the disturbed endocrine secretion system,reduced apoptosis rate in the ovaries,and improved folliculogenesis possibly through a paracrine effect and ovarian cell survival.Therefore,ES-MSCs could be a promising source for stem cell therapy in individuals with POF.
ARTICLE HIGHLIGHTS
Research background
Premature ovarian failure (POF) is characterized by amenorrhea,hypoestrogenemia,high gonadotropins and infertility in women under 40-years-old.Previous reports demonstrated that various tissue-specific stem cells could restore ovarian function and folliculogenesis in chemotherapy-induced POF mice.
Research motivation
Human embryonic stem cell-derived MSC (ES-MSC) have advantages,such as higher proliferation,more potent anti-inflammatory properties and lack of obstacles of harvesting tissue-specific MSCs that make them attractive candidates for restoring fertility in patients with POF.
Research objectives
The aim of this study was to evaluate the therapeutic efficacy of ES-MSCs in a model of chemotherapy-induced POF.
Research methods
In this study,we initially established a mouse POF model by administration of cyclophosphamide and busulfan,then we transplanted ES-MSCs and bone marrowderived MSC (BM-MSC) into a mouse model of POF to investigate the role of these cells and mechanisms of action for improvement of POF.
Research results
The POF model established by the 100 mg/kg dose of cyclophosphamide showed significant decreases in body weight,follicle count and estradiol level but had an increased follicle-stimulating hormone level.ES-MSC and/or BM-MSC transplantation significantly improved body weight,follicle count,hormone secretion,survival rate and reproductive function in POF mice.Gene expression and Western blot analysis,terminal deoxynucleotidyl transferase mediated 2-deoxyuridine 5-triphosphate nick end labelling assay and immunohistochemistry indicated that the ES-MSCs or BMMSCs reduced apoptosis in the follicles and restored fertility in chemotherapy-induced POF mice.The results of this study indicated that the effects of ES-MSCs and BMMSCs in restoring ovarian function appear via the paracrine mechanisms of cytokines.
Research conclusions
Our findings demonstrated that human ES-MSCs,similar to BM-MSCs,improved ovarian function and restored fertility in a mouse POF model.
Research perspectives
Our present study results suggest that human ES-MSCs could be a promising source for stem cell therapy in individuals with POF.
 World Journal of Stem Cells2020年8期
World Journal of Stem Cells2020年8期
- World Journal of Stem Cells的其它文章
- Hunting down the dominating subclone of cancer stem cells as a potential new therapeutic target in multiple myeloma:An artificial intelligence perspective
- Role of mesenchymal stem cell derived extracellular vesicles in autoimmunity:A systematic review
- Assessment of tobacco heating system 2.4 on osteogenic differentiation of mesenchymal stem cells and primary human osteoblasts compared to conventional cigarettes
- Mesenchymal stem cell-derived exosomes:Toward cell-free therapeutic strategies in regenerative medicine
- Autophagy in fate determination of mesenchymal stem cells and bone remodeling
- Human embryonic stem cells as an in vitro model for studying developmental origins of type 2 diabetes
