山竹果皮中异戊烯基双苯吡酮类成分*
汤卓雅,潘月华,张君生,尹胜,唐贵华
(1. 中山大学药学院,广东广州510006;2. 济南大学生物科学与技术学院,山东济南250022)
Alzheimer’s disease (AD) is a chronic neurodegenerative disease characterized by progressive memory loss and cognitive impairment. Oxidative stress caused by the generation of reactive oxygen species (ROS) could lead to neuronal damage inducing cell death, playing an important role in the initiation and progression of AD[1]. Therefore, the development of neuroprotective drugs is considered to be one of the attractive therapeutic strategies for AD patients.In the studies of AD drugs, a growing number of bioactive natural products have been found to be capable of providing neuroprotection against various insults and damage[2]. Flavonoids are a large group of natural polyphenolic compounds with a variety of bioactivities especially anti-inflammatory, anticancer, neuroprotective, and cardiovascular properties[3-5].They are ubiquitous in plants including herbs,fruits,and vegetables.
Garcinia mangostana L. (Clusiaceae) is a highly valuable plant widely cultivated in tropical regions of Africa and Asia[6]. Its fruits are well-known as pleasant-tasting mangosteen in normal diets, and its pericarps, stem and root barks, and leaves are used as folk medicine for treatment of various diseases such as diarrhea, gonorrhea, and skin rashes[7].Xanthones, a subclass of flavonoids, are the most characteristic constituents of G. mangostana, which exhibit anti-inflammatory, antioxidant, cytotoxic,antimicrobial, and antimalarial activities[7]. Recently, several xanthones isolated from mangosteen shown multifunctional activities against AD[8]. In our continuing search for natural multi-targeted agents for AD[9-10], 22 known xanthones including 21 prenylated ones were isolated from the pericarps of G. mangostana. The neuroprotection of these xanthones were evaluated in glutamate-induced HT22 cells, and two compounds exhibited significant neuroprotective activity at a concentration of 1 μmol/L. Herein, the isolation, structural elucidation, and neuroprotection of these xanthones are described.
1 Materials and methods
1.1 Instruments and reagents
NMR spectra were recorded on Bruker AM-400 and AM-500 spectrometers using TMS as an internal standard. MS were recorded on a Finnigan LC QDECAinstrument. A Shimadzu LC-20AT equipped with a SPD-M20A PDA detector was used for HPLC, and a YMC pack ODS-A column (250 mm × 10 mm, S-5 μm, 12 nm) was used for semipreparative HPLC separation. Silica gel (300-400 mesh, Qingdao Haiyang Chemical Co., Ltd.), reversed phase C18(Rp-C18) silica gel (12 nm, S-50 μm, YMC Co. Ltd.), MCI gel (CHP20P, 75-150 μm,Mitsubishi Chemical Industries Ltd.), and SephadexLH-20 (Amersham Biosciences) were used for column chromatography (CC). Glutamate was purchased from Research Biochemicals International(Natick, MA, USA). Trypsin, DMSO, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and fetal bovine serum (FBS) were obtained from Gibco-BRL(Grand Island,NY,USA).
1.2 Plant material
The fruits of G. mangostana were purchased from Guangzhou, Guangdong Province, China, in August 2015. The plant was identified by one of the authors (Guihua Tang), and a voucher specimen(H20150801) was deposited at the School of Pharmaceutical Sciences,Sun Yat-sen University.
1.3 Extraction and isolation
After removing the pulps, the air-dried pericarps of G. mangostana (5.0 kg) were powdered and extracted with 95% EtOH (15 L) for three times at room temperature. The combined 95% EtOH extracts were concentrated in vacuo to a black residue(500 g),which was suspended in water and then partitioned successively with petroleum ether and EtOAc to give two corresponding portions. The EtOAc extract(200 g) was subjected to CC over MCI gel eluting with a gradient of increasing MeOH in H2O (20%–100%) to yield four fractions (A-D). Fr. A was subjected to polyamide CC eluting with a gradient of increasing MeOH in H2O (20%-100%) to gain three fractions (A1-A3). Subsequently, each fraction was subjected to CC over silica gel (CH2Cl2-MeOH,15∶1), Sephadex LH-20 (MeOH), Rp-C18column (MeOH-H2O, 40%-100%), and then further purified by semipreparative HPLC (MeOHH2O, 50%-100%) to yield pure compounds. Compounds 2 (20 mg, tR7.0 min) and 1 (33 mg, tR8.0 min) were obtained from Fr. A1. Fr. A2gave compounds 8 (360 mg), 13 (30 mg), and 7(13 mg). Fr. A3gave compounds 12 (80 mg), 17(36 mg), and 3 (8 mg). Fr. C was subjected to CC over silica gel using petroleum ether-EtOAc(50∶1→0∶1) to gain four fractions (C1-C4). Subsequently, each fraction was subjected to CC over Rp-C18column, silica gel, Sephadex LH-20, and HPLC to yield pure compounds. Compounds 6 (120 mg), 16 (20 mg), and 14 (15 mg) were obtained from Fr. C1. Compound 10 (35 mg) was yielded from Fr. C2. Fr. C3gave compounds 11 (85 mg), 22 (40 mg), and 21 (23 mg). Fr. D was chromatographed on a Rp-C18column (MeOHH2O, 30%-100%) to gain three subfractions (D1-D3). Subsequently, each fraction was subjected to CC over Rp-C18column, silica gel, Sephadex LH-20, and HPLC to yield pure compounds. Fr. D1gave compounds 9 (620 mg), 20 (21 mg), 19 (7.5 mg), 15 (16 mg), and 18 (5 mg). Compounds 4 (40 mg) and 5 (15.4 mg) were yielded from Fr. C2. The purity of all isolated compounds was estimated to be greater than 95%, as determined by1H NMR spectra.
1.4 Bioactivity assay
The HT22 cells were maintained in DMEM supplemented with 10% (V/V) FBS and incubated at 37 ℃under 5% CO2. Compounds 1-22 were tested for their cytotoxicity in HT22 cells and their protective effects on glutamate induced neuronal death in HT22 cells by using MTT Assay. Briefly, cells were seeded in 96-well plates (1 × 104cells/well), and six wells were used for each treatment group. The group treated with 0.1% (v/v) DMSO was the vehicle control. After 24 h incubation, HT22 cells were pretreated with different concentrations of compounds for 30 min before exposure to glutamate (2 mmol/L). Following incubation for 24 h,cell growth was measured at indicated time points by addition of 10 μL of MTT (5 mg/mL) at 37 ℃for 2 h, and DMSO (100 μL) was added to dissolve the formazan crystals. Optical density was measured using a microplate reader (Bio-Tek, USA) at 570 nm, and all data were represented as fold increase relative to the untreated control.
2 Results and discussion
2.1 Structural identification
The air-dried pericarps of G. mangostana were extracted with 95% EtOH. After concentrating the EtOH in vacuo, the black residue was suspended in water and then partitioned with petroleum ether and EtOAc, respectively. The EtOAc fraction was subjected to column chromatography over MCI gel, silica gel, Rp-C18, Sephadex LH-20, and semipreparative HPLC to obtain compounds 1-22.
The structures 1-22 (Fig. 1) were determined by comparison of their spectroscopic data with literature data. All compounds (1-22) were identified as known xanthones. They were 1,3,7-trihydroxyxanthone (1),1,3,6,7-tetrahydroxy-8-prenylxanthone (2), 1,3,5-trihydroxy-4-prenylxanthone(3), 8-deoxygartanin (4), cudraxanthone G(5), gartanin (6), 6-deoxy-γ-mangostin (7),γ-mangostin (8),α-mangostin (9),1,3-dihydroxy-6, 7-dimethoxy-2, 8-diprenylxanthone(10), β-mangostin (11), garcinone D (12),garcinone B (13), mangostenone D (14), 3-Omethylmangostenone D (15), 9-hydroxycalabaxanthone (16), 11-hydroxy-1-isomangostin (17),brasilixanthone B (18), garcimangosxanthone D(19), BR-xanthone A (20), tovophyllin A(21), and 1,3,6-trihydroxy-2,5-bis(3-methylbut-2-enyl) -6′,6′-dimethyl-4′,5′-dihydropyrano[2,3′:7,8] xanthone (22), respectively. Compound 1 is a simple xantone and 2-22 represent the type of prenylated xanthones.
2.1.1 1,3,7-Trihydroxyxanthone(1)
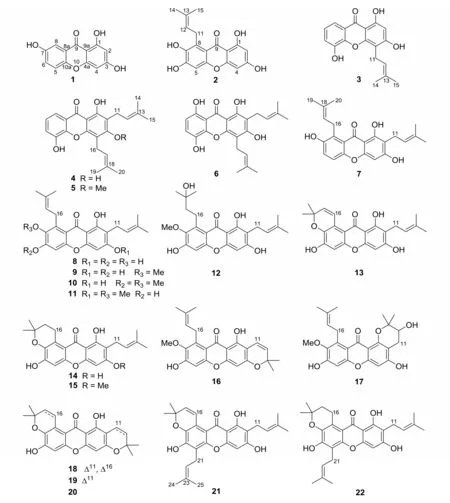
Fig.1 The chemical structures of compounds 1-22
Yellow solid; C13H8O5; Mr= 244.0;1H NMR(400 MHz, Methanol-d4) δ:7.46 (1H, s, H-8), 7.33 (1H, d, J = 8.0 Hz, H-5), 7.22(1H, d, J = 8.0 Hz, H-6), 6.29 (1H, s,H-4), 6.15 (1H, s, H-2);13C NMR (100 MHz, Methanol-d4) δ: 181.7 (C, C-9),167.4 (C, C-3), 164.6 (C, C-1), 159.5(C, C-4a), 155.3 (C, C-7), 151.2 (C,C-10a), 125.2 (CH, C-6), 122.1 (C, C-8a), 119.8 (CH, C-5), 109.4 (CH, C-8), 103.6 (C, C-9a), 99.0 (CH, C-2),94.8 (CH, C-4). The NMR and MS data were in consistent with those reported in the literature[11-12].Thus,compound 1 was determined as 1,3,7-trihydroxyxanthone.
2.1.2 1,3,6,7-Tetrahydroxy-8-prenylxanthone(2) Yellow powder; C18H16O6; Mr= 328.1;1H NMR (400 MHz, Methanol-d4) δ:6.60 (1H,s, H-5), 6.11 (1H, br. s, H-4), 6.03(1H, br. s, H-2), 5.19 (1H, t, J = 6.7 Hz, H-12), 4.02 (2H, d, J = 6.7 Hz, H-11), 1.76 (3H, s, H-14), 1.59 (3H, s,H-15);13C NMR (100 MHz, Methanol-d4) δ:183.4 (C, C-9), 165.5 (C, C-3), 164.6(C, C-1), 158.4 (C, C-4a), 154.1 (C,C-10a), 153.5 (C, C-6), 142.2 (C, C-7), 131.8 (C, C-13), 129.4 (C, C-8),124.7 (CH, C-12), 112.0 (C, C-8a),104.0 (C, C-9a), 101.0 (CH, C-5), 98.5(CH, C-2), 93.8 (CH, C-4), 26.6 (CH2,C-11), 26.1 (CH3, C-15), 18.3 (CH3, C-14). Its NMR and MS data were identical with those reported in the literature[13]. Therefore, it was determined to be 1,3,6,7-tetrahydroxy-8-prenylxanthone.
2.1.3 1,3,5-Trihydroxy-4-prenylxanthone (3)Yellow solid; C18H16O5; Mr= 312.1;1H NMR(400 MHz, Methanol-d4) δ:7.61 (1H, d, J=7.7 Hz, H-8), 7.23 (1H, d, J = 7.7 Hz,H-6), 7.17 (1H, t, J = 7.7 Hz, H-7),6.24 (1H, s, H-2), 5.34 (1H, t, J = 6.7 Hz, H-12), 3.53 (2H, d, J = 6.7 Hz, H-11), 1.85 (3H, s, H-14), 1.66 (3H, s,H-15);13C NMR (100 MHz, Methanol-d4) δ 182.5 (C, C-9), 165.1 (C, C-1), 162.3(C, C-3), 156.0 (C, C-4a), 147.8 (C,C-5), 147.1 (C, C-10a), 132.3 (C, C-13), 124.6 (CH, C-7), 123.6 (CH, C-6), 122.4 (C, C-8a), 121.2 (CH, C-12),116.1 (CH, C-8), 108.5 (C, C-4), 103.8(C, C-9a), 98.6 (CH, C-2), 26.0 (CH3,C-15), 22.4 (CH2, C-11), 18.0 (CH3, C-14). Its NMR and MS data were in accordance with those of 1, 3, 5-trihydroxy-4- (3-methylbut-2-enyl) -9H-xanthen-9-one[14]. Thus, compound 3 was determined as shown and named 1,3,5-trihydroxy-4-prenylxanthone.
2.1.4 8-Deoxygartanin (4) Yellow solid;C23H24O5;Mr=380.2;1H NMR (400 MHz, Methanol-d4) δ:7.57 (1H, dd, J = 7.8 and 1.5 Hz, H-8), 7.17 (1H, dd, J = 7.8 and 1.5 Hz, H-6), 7.11 (1H, t, J = 7.8 Hz, H-7), 5.28 (1H, t, J = 7.1 Hz, H-12),5.20(1H, t, J = 7.0 Hz, H-17), 3.58 (2H, d,J = 7.1 Hz, H-11), 3.35 (2H, d, J = 7.0 Hz, H-16), 1.87 and 1.79 (each 3H, s, H-15 and H-20), 1.67 (6H, s, H-14 and H-19);13C NMR (100 MHz, Methanol-d4) δ:182.5 (C, C-9), 162.3 (C, C-3), 159.3(C, C-1), 153.9 (C, C-4a), 147.7 (C,C-5), 146.9 (C, C-10a), 132.9 and 132.7(each C, C-13 and C-18), 124.4 (CH, C-7), 123.45 and 123.37 (each CH, C-12 and C-17), 122.4 (C, C-8a), 120.9 (CH, C-6),116.2 (CH, C-8), 111.7 (C, C-2), 108.0(C, C-4), 103.9 (C, C-9a), 26.0 and 25.9(each CH3, C-14 and C-19), 22.6 and 22.4(each CH2, C-11 and C-16), 18.1 and 18.0(each CH3, C-15 and C-20). Its NMR and MS data were identical with those reported in the literature[15-16]. Hence, it was defined as 8-deoxygartanin.
2.1.5 Cudraxanthone G (5) Yellow prisms;C24H26O5;Mr=394.2;1H NMR (400 MHz, Methanol-d4) δ:7.63 (1H, dd, J = 7.8 and 1.7 Hz, H-8), 7.24 (1H, dd, J = 7.8 and 1.7 Hz, H-6), 7.18 (1H, t, J = 7.8 Hz, H-7), 5.31 (1H, t, J = 6.9 Hz, H-17), 5.24(1H, t, J = 6.8 Hz, H-12), 3.80 (3H, s,3-OCH3), 3.59 (2H, d, J = 6.9 Hz, H-16), 3.36 (2H, d, J = 6.8 Hz, H-11),1.85 and 1.80 (each 3H, s, H-15 and H-20),1.68 (6H, s, H-14 and H-19);13C NMR(100 MHz, Methanol-d4)δ:183.4(C, C-9),165.1 (C, C-3), 159.8 (C, C-1), 154.2(C, C-4a), 148.1 (C, C-5), 147.2 (C,C-10a),132.6 and 132.3 (each C,C-13 and C-18), 124.8 (CH, C-7), 124.0 and 123.9(each CH, C-12 and C-17), 122.4 (C, C-8a), 121.4 (CH, C-6), 118.1 (C, C-2),116.2 (CH, C-8), 114.9 (C, C-4), 106.8(C, C-9a), 62.4 (CH3, 3-OCH3), 25.94 and 25.87 (each CH3, C-14 and C-19), 23.5 and 23.3 (each CH2, C-11 and C-16), 18.1 and 18.0 (each CH3, C-15 and C-20). The NMR and MS data were in consistent with those reported in the literature[17]. So 5 was elucidated as cudraxanthone G.
2.1.6 Gartanin(6) Yellow needles; C23H24O6;Mr=396.2;1H NMR (400 MHz, Methanol-d4)δ :7.14 (1H, d, J = 8.8 Hz, H-7), 6.52(1H, d, J = 8.8 Hz, H-6), 5.27 (1H, t,J = 6.8 Hz, H-12), 5.20 (1H, t, J = 7.0 Hz, H-17), 3.57 (2H, d, J = 7.0 Hz, H-16), 3.35 (2H, d, J = 6.8 Hz, H-11),1.87 and 1.81 (each 3H, s, H-15 and H-20),1.69 (6H, s, H-14 and H-19);13C NMR(100 MHz, Methanol-d4) δ: 186.0 (C, C-9), 163.2 (C, C-3), 158.9 (C, C-1),154.2 (C, C-8), 154.0 (C, C-4a), 145.6(C, C-10a), 138.6 (C, C-5), 133.0 and 132.7 (each C, C-13 and C-18), 124.2(CH, C-6), 123.3 and 123.2 (each CH, C-12 and C-17), 112.1 (CH, C-7), 109.7 (C,C-2), 108.6 (C, C-8a), 108.3 (C, C-4),102.8 (C, C-9a), 26.0 and 25.9 (each CH3,C-14 and C-19), 22.6 and 22.4 (each CH2, C-11 and C-16), 18.1 and 18.0 (each CH3, C-15 and C-20). Its NMR and MS data were identical with those reported in the literature[16]. Therefore,compound 6 was determined to be gartanin.
2.1.7 6-Deoxy-γ-mangostin(7) Yellow solid;C23H24O5;Mr=380.2;1H NMR (400 MHz, Methanol-d4) δ:7.18 (1H, d, J = 8.9 Hz, H-6),7.11 (1H, d, J = 8.9 Hz, H-5), 6.24(1H, s, H-4), 5.26 (2H, m, H-12 and H-17), 3.30 (4H, m, H-11 and H-16), 1.85 and 1.80 (each 3H, s, H-15 and H-20), 1.68(6H, s, H-14 and H-19);13C NMR (100 MHz, Methanol-d4) δ: 184.4 (C, C-9),164.2 (C, C-3), 161.6 (C, C-1), 156.5(C, C-4a), 152.5 (C, C-7), 152.4 (C,C-10a), 131.73 and 131.71 (each C, C-13 and C-18), 129.5 (C, C-8), 124.8 and 123.9(each CH, C-12 and C-17), 123.5 (CH, C-6), 119.8 (C, C-2), 116.6 (CH, C-5),111.2 (C, C-8a), 104.2 (C, C-9a), 93.1(CH, C-4), 26.5 (CH2, C-16), 26.1 and 26.0 (each CH3, C-14 and C-19), 22.2(CH2, C-11), 18.3 and 17.9 (each CH3, C-15 and C-20). The NMR and MS data of 7 were in consistent with those reported in the literature[18].Thus, this compound was assigned as 6-deoxy-γmangostin.
2.1.8 γ -Mangostin (8) Yellow crystals;C23H24O6;Mr= 396.2;1H NMR (400 MHz, Methanol-d4)δ:6.68 (1H, s, H-5), 6.25 (1H,s, H-4), 5.27 (2H, m, H-12 and H-17),4.13 (2H, d, J = 6.8 Hz, H-16), 3.32(2H, d, J = 6.8 Hz, H-11), 1.86 and 1.80(each 3H, s, H-15 and H-20), 1.68 (6H,s,H-14 and H-19);13C NMR (100 MHz,Methanol-d4)δ:183.5 (C, C-9), 163.3 (C, C-3), 161.5 (C, C-1), 156.3 (C, C-4a),154.2 (C, C-6), 153.7 (C, C-10a), 142.2(C, C-7), 131.7 and 131.6 (each C, C-13 and C-18), 129.3 (C, C-8), 124.9 and 124.0(each CH, C-12 and C-17), 112.0 (C, C-2), 111.1 (C, C-8a), 103.9 (C, C-9a),100.9 (CH, C-5), 92.9 (CH, C-4), 26.6(CH2, C-16), 26.1 and 26.0 (each CH3, C-14 and C-19), 22.2 (CH2, C-11), 18.3 and 17.9(each CH3, C-15 and C-20). Its NMR and MS data were in accordance with those reported in the literature[16]. Therefore, compound 8 was determined to be γ-mangostin.
2.1.9 α -Mangostin (9) Yellow needles;C24H26O6;Mr=410.2;1H NMR (400 MHz, Methanol-d4)δ:6.58 (1H, s, H-5), 6.14 (1H,s, H-4), 5.13-5.26 (2H, m, H-12 and H-17), 3.98 (2H, d, J = 6.3 Hz, H-16),3.72 (3H, s, 7-OCH3), 3.23 (2H, d, J =7.1 Hz, H-11), 1.79 and 1.76 (each 3H, s,H-15 and H-20), 1.64 (6H, s, H-14 and H-19);13C NMR (100 MHz, Methanol-d4) δ:182.9 (C, C-9), 163.3 (C, C-3), 161.4(C, C-1), 157.5 (C, C-10a), 156.5 (C,C-6), 156.0 (C, C-4a), 144.5 (C, C-7),138.3 (C, C-8), 131.55 and 131.51 (each C, C-13 and C-18), 125.1 (CH, C-17),123.9 (CH, C-12), 112.2 (C, C-8a),111.3 (C, C-2), 103.7 (C, C-9a), 102.7(CH, C-5), 93.1 (CH, C-4), 61.3 (CH3,7-OCH3), 27.1 (CH2, C-16), 25.9 (CH3×2, C-14 and C-19), 22.2 (CH2, C-11),18.3 and 17.9 (each CH3, C-15 and C-20). Its NMR and MS data were identical with those reported in the literature[16,19]. Thus, the structure of 9 was defined as α-mangostin.
2.1.10 1,3-Dihydroxy-6,7-dimethoxy-2,8-diprenylxanthone(10)Yellow powder;C25H28O6;Mr=424.2;1H NMR (400 MHz, Acetone-d6) δ 13.73 (1H, s, 1-OH), 6.92 (1H, s, H-5), 6.42 (1H, s, H-4), 5.27 (2H, m,H-12 and H-17), 4.12 (2H, d, J = 6.8 Hz,H-16), 4.02 and 3.77 (each 3H, s, 6-OCH3and 7-OCH3), 3.35 (2H, d, J = 7.2 Hz, H-11), 1.82 and 1.78 (each 3H, s, H-15 and H-20), 1.65 (6H, s, H-14 and H-19);13C NMR (100 MHz, Acetone-d6) δ:182.9 (C,C-9), 163.2 (C, C-3), 161.6 (C, C-1),159.4 (C, C-4a), 156.2 (C, C-10a),155.7 (C, C-6), 145.1 (C, C-7), 137.3(C, C-8), 131.4 and 131.3 (each C, C-13 and 18), 124.8 and 123.5 (each CH, C-12 and C-17), 112.2 (C, C-8a), 111.2 (C, C-2), 103.7 (C, C-9a), 99.6 (CH, C-5),93.2 (CH, C-4), 60.9 and 56.7 (each CH3,6-OCH3and 7-OCH3), 26.7 (CH2, C-16),26.0 and 25.9 (each CH3, C-14 and C-19),22.0 (CH2, C-11), 18.3 and 17.9 (each CH3, C-15 and C-20). The NMR and MS data were in consistent with those reported in the literature[20]. Hence, its structure was determined to be 1,3-dihydroxy-6,7-dimethoxy-2,8-diprenylxanthone.
2.1.11 β -Mangostin (11) Yellow solid;C25H28O6;Mr=424.2;1H NMR (400 MHz, CDCl3) δ:13.44 (1H, s, 1-OH),7.28 (1H,s, H-5), 6.34 (1H, s, H-4),5.21-5.30(2H, m, H-12 and H-17), 4.11 (2H, d,J = 6.1 Hz, H-16), 3.92 and 3.83 (each 3H,s, 3-OCH3and 7-OCH3), 3.37 (2H, d, J =7.1 Hz, H-11),1.85 and 1.82 (each 3H, s,H-15 and H-20), 1.714 and 1.705 (each 3H,s, H-14 and H-19);13C NMR (100 MHz, CDCl3) δ:181.9 (C, C-9), 163.5 (C, C-3),159.7 (C, C-1), 155.7 (C, C-4a), 155.2(C, C-10a), 154.5 (C, C-6), 142.6 (C,C-7), 137.0 (C, C-8), 132.0 and 131.6(each C, C-13 and C-18), 123.2 and 122.3(each CH, C-12 and C-17), 112.3 (C, C-8a), 111.5 (C, C-2), 103.8 (C, C-9a),101.5 (CH, C-5), 88.8 (CH, C-4), 62.0 and 55.8 (each CH3, 3-OCH3and 7-OCH3),26.5 (CH2, C-16), 25.8 (CH3× 2, C-14 and C-19), 21.3 (CH2, C-11), 18.2 and 17.7(each CH3, C-15 and C-20). Its NMR and MS data were identical with those reported in the literature[16,19]. So compound 11 was elucidated as β-mangostin.
2.1.12 Garcinone D (12) Yellow solid;C24H28O7;Mr=428.2;1H NMR (400 MHz, Methanol-d4) δ:6.55 (1H, s, H-5), 6.14 (1H,s, H-4), 5.22 (1H, t, J = 7.4 Hz, H-12), 3.80 (3H, s, 7-OCH3), 3.17-3.31(4H,m,H-11 and H-16),1.77 (3H,s,H-15), 1.71 (2H, m, H-17), 1.66 (3H, s,H-20), 1.33 (6H, s, H-14 and H-19);13C NMR (100 MHz, Methanol-d4) δ:182.8 (C,C-9), 163.3 (C, C-3), 161.3 (C, C-1),157.5 (C, C-6), 156.5 (C, C-10a), 155.9(C, C-4a), 144.4 (C, C-7), 139.4 (C,C-8), 131.5 (C, C-13), 123.9 (CH, C-12), 112.0 (C, C-8a), 111.2 (C, C-2),103.6 (C, C-9a), 102.7 (CH, C-5), 93.2(CH, C-4), 72.1 (C, C-18), 61.5 (CH3,7-OCH3), 45.4 (CH2, C-17), 29.0 (CH3×2, C-19 and C-20), 26.0 (CH3, C-14),23.5 (CH2, C-16), 22.2 (CH2, C-11),17.9 (CH3, C-15). The NMR and MS data of 12 were in consistent with those reported in the literature[16]. Therefore, its structure was defined as garcinone D.
2.1.13 Garcinone B (13) Yellow needles;C23H22O6;Mr=394.1;1H NMR (400 MHz, Methanol-d4) δ:7.99 (1H, d, J = 10.2 Hz, H-16), 6.69 (1H, s, H-5), 6.26 (1H, s,H-4), 5.83 (1H, d, J = 10.2 Hz, H-17),5.24 (1H, t, J = 7.2 Hz, H-12), 3.29(2H, d, J = 7.2 Hz, H-11), 1.80 (3H, s,H-15), 1.68 (3H, s, H-14), 1.48 (6H,s,H-19 and H-20);13C NMR (100 MHz,Methanol-d4)δ:182.1 (C, C-9), 162.4 (C, C-3), 160.0 (C, C-1), 155.0 (C, C-6),153.0 (C, C-10a), 152.7 (C, C-4a),138.0 (C, C-7), 131.9 (CH, C-17),130.3 (C, C-13), 122.5 (C, C-8), 120.8(CH, C-12), 120.0 (CH, C-16), 110.1(C, C-8a), 107.4 (C, C-2), 102.5 (C,C-9a), 102.2 (CH, C-5), 91.9 (CH, C-4), 75.4 (C, C-18), 25.8 (CH3× 2, C-19 and 20), 24.6 (CH3, C-14), 20.8 (CH2, C-11), 16.5 (CH3, C-15). Its NMR and MS data were in accordance with those reported in the literature[21], suggesting that 12 was identical to garcinone B.
2.1.14 Mangostenone D (14) Yellow solid;C23H24O6;Mr=396.2;1H NMR (400 MHz, Methanol-d4) δ:6.64 (1H, s, H-5), 6.24 (1H,s, H-4), 5.25 (1H, t, J = 7.1 Hz, H-12), 3.45 (2H, t, J=6.7 Hz, H-16), 3.29(2H, d, J = 7.1 Hz, H-11), 1.89-1.83(2H, m, H-17), 1.79 (3H, s, H-15),1.68 (3H, s, H-14), 1.38 (6H, s, H-19 and H-20);13C NMR (100 MHz, Methanol-d4)δ: 183.6 (C, C-9), 163.4 (C, C-3),161.4 (C, C-1), 156.3 (C, C-6), 154.4(C, C-10a), 154.3 (C, C-4a), 140.4 (C,C-7), 131.6 (C, C-13), 124.0 (CH, C-12), 122.7 (C, C-8), 111.7 (C, C-8a),111.2 (C, C-2), 103.9 (C, C-9a), 101.7(CH, C-5), 93.1 (CH, C-4), 75.4 (C,C-18), 33.8 (CH2, C-17), 26.6 (CH3× 2,C-19 and C-20), 26.0 (CH3, C-14), 23.7(CH2, C-16), 22.2 (CH2, C-11), 17.9(CH3, C-15). Its NMR and MS data were identical with those reported in the literature[22], indicating that the structure of 14 was the same as mangostenone D.
2.1.15 3-O-Methylmangostenone D(15) Yellow crystals; C24H26O6; Mr=410.2;1H NMR (400 MHz, CDCl3) δ:13.46 (1H, s, 1-OH),6.81 (1H, s, H-5), 6.36 (1H, s, H-4),5.25 (1H, m, H-12), 3.92 (3H, s, 3-OCH3), 3.53 (2H, d, J = 6.8 Hz, H-16),3.37 (2H, d, J = 7.1 Hz, H-11), 1.90(2H, t, J = 6.8 Hz, H-17), 1.81 (3H, s,H-15), 1.70 (3H, s, H-14), 1.41 (6H,s, H-19 and H-20);13C NMR (100 MHz, CDCl3) δ:182.5 (C, C-9), 163.3 (C, C-3),159.6 (C, C-1), 155.4 (C, C-4a), 153.1(C, C-10a), 151.5 (C, C-6), 138.0 (C,C-7), 131.6 (C, C-13), 122.4 (CH, C-12), 121.4 (C, C-8), 111.5 (C, C-8a),111.3 (C, C-2), 104.0 (C, C-9a), 100.3(CH, C-5), 88.8 (CH, C-4), 75.5 (C,C-18), 55.8 (CH3, 3-OCH3), 32.9 (CH2,C-17), 26.5 (CH3× 2, C-19 and C-20), 25.8(CH3, C-14), 22.3 (CH2, C-16), 21.3(CH2, C-11), 17.8 (CH3, C-15). The NMR and MS data were in consistent with those reported in the literature[23]. Thus, the structure of 15 was determined to be 3-O-methylmangostenone D.
2.1.16 9-Hydroxycalabaxanthone (16) Yellow solid; C24H24O6; Mr=408.2;1H NMR (400 MHz, Methanol-d4)δ:6.54 (1H, d, J = 10.0 Hz, H-12), 6.53 (1H, s, H-5), 5.98(1H, s, H-4), 5.53 (1H, d, J = 10.0 Hz,H-11), 5.21 (1H, t, J = 6.3 Hz, H-17),3.95 (2H, d, J = 6.3 Hz, H-16), 3.74(3H, s, 7-OCH3), 1.80 (3H, s, H-20),1.68 (3H, s, H-19), 1.42 (6H, s, H-14 and H-15);13C NMR (100 MHz, Methanol-d4)δ: 182.8 (C, C-9), 160.7 (C, C-3),158.7 (C, C-1), 157.9 (C, C-6), 157.3(C, C-10a), 156.4 (C, C-4a), 144.9 (C,C-7), 138.3 (C, C-8), 131.5 (C, C-18),127.9 (CH, C-12), 125.1 (CH, C-17),116.5 (CH, C-11), 112.0 (C, C-8a),105.2 (C, C-2), 104.3 (C, C-9a), 102.9(CH, C-5), 94.9 (CH, C-4), 78.8 (C,C-13), 61.3 (CH3, 7-OCH3), 28.7 (CH3×2, C-14 and C-15), 27.2 (CH2, C-16),26.0 (CH3, C-19), 18.4 (CH3, C-20). Its NMR and MS data were in accordance with those reported in the literature[16], which indicated that 16 have the same structure as 9-hydroxycalabaxanthone.
2.1.17 11-Hydroxy-1-isomangostin(17) Yellow solid; C24H26O7;Mr=426.2;1H NMR(400 MHz,Methanol-d4) δ:6.65 (1H, s, H-5), 6.31(1H, s, H-4), 5.30 (1H, t, J = 6.2 Hz,H-17), 4.05 (2H, d, J = 6.2 Hz, H-16),3.79 (1H, dd, J = 7.4 and 5.6 Hz, H-12),3.75 (3H, s, 7-OCH3), 2.91 (1H, dd, J =17.0 and 5.6 Hz,H-11a),2.56 (1H,dd,J=17.0 and 7.4 Hz, H-11b), 1.82 (3H, s, H-20), 1.67 (3H, s, H-19), 1.47 (3H, s,H-15), 1.35 (3H, s, H-14);13C NMR (100 MHz, Methanol-d4)δ:178.9(C,C-9), 162.1(C,C-3),158.3 (C,C-4a),156.7 (C,C-1), 156.1 (C, C-6), 155.6 (C, C-10a),144.7 (C, C-7), 138.3 (C, C-8), 131.3(C, C-18), 125.7 (CH, C-17), 114.9(C, C-8a), 107.5 (C, C-9a), 105.3 (C,C-2), 102.3 (CH, C-5), 94.4 (CH, C-4), 79.5 (C, C-13), 69.6 (CH, C-12),61.2 (CH3, 7-OCH3), 27.1 (CH2, C-16),27.0 (CH2, C-11), 26.0 (CH3, C-19),25.6 (CH3, C-14), 20.6 (CH3, C-15),18.3 (CH3, C-20). The NMR and MS data were in consistent with those reported in the literature[24],which suggested that the structure of 17 was identical to 11-hydroxy-1-isomangostin.
2.1.18 Brasilixanthone B(18) Yellow powder;C23H20O6;Mr=392.1;1H NMR (400 MHz,CDCl3)δ:13.61 (1H, s, 1-OH), 8.01 (1H, d,J = 10.2 Hz, H-16), 6.82 (1H, s, H-5),6.72 (1H, d, J = 10.1 Hz, H-11), 6.25(1H, s, H-4), 5.82 (1H, d, J = 10.2 Hz,H-17), 5.57 (1H, d, J = 10.1 Hz, H-12),1.49 (6H, s, H-19 and H-20), 1.47 (6H,s, H-14 and H-15);13C NMR (100 MHz, CDCl3) δ:182.4 (C, C-9), 160.0 (C, C-3),157.8 (C, C-1), 156.5 (C, C-4a), 153.1(C, C-6), 150.9 (C, C-10a), 136.9 (C,C-7), 132.3 (CH, C-17), 127.2 (CH, C-12), 121.0 (CH, C-16), 119.7 (C, C-8), 115.7 (CH, C-11), 108.6 (C, C-8a),104.4 (C, C-2), 103.9 (C, C-9a), 102.4(CH, C-5), 94.3 (CH, C-4), 78.0 (C ×2, C-13 and C-18), 28.3 (CH3× 2, C-14 and C-15), 27.3 (CH3× 2, C-19 and C-20). Its NMR and MS data were identical with those reported in the literature[25]. Thus, compound 18 was assigned to be brasilixanthone B.
2.1.19 Garcimangosxanthone D(19) Yellow powder; C23H22O6;Mr= 394.1;1H NMR (400 MHz,CDCl3) δ: 13.72 (1H, s, 1-OH), 6.80(1H, s, H-5), 6.73 (1H, d, J = 10.0 Hz,H-11), 6.25 (1H, s, H-4), 5.56 (1H,d, J = 10.0 Hz, H-12), 3.49 (2H, t, J =6.8 Hz, H-16), 1.88 (2H, t, J = 6.8 Hz,H-17), 1.46 (6H, s, H-14 and H-15), 1.39(6H, s, H-19 and H-20);13C NMR (100 MHz, CDCl3) δ: 182.6 (C, C-9), 159.6(C, C-3), 157.8 (C, C-1), 156.5 (C, C-4a), 153.1 (C, C-6), 151.7 (C, C-10a),138.0 (C, C-7), 127.1 (CH, C-12),121.3 (C, C-8), 115.7 (CH, C-11),111.3 (C, C-8a), 104.3 (C, C-2), 103.9(C, C-9a), 100.5 (CH, C-5), 94.1 (CH,C-4), 77.8 (C, C-13), 75.6 (C, C-18),32.9 (CH2, C-17), 28.3 (CH3× 2, C-14 and C-15), 26.5 (CH3× 2, C-19 and C-20), 22.3(CH2, C-16). The NMR and MS data were in consistent with those reported in the literature[26]. Therefore, the structure of 19 was determined as garcimangosxanthone D.
2.1.20 BR-xanthone A(20) Yellow needles;C23H24O6;Mr= 396.2;1H NMR (400 MHz, CDCl3)δ:13.73 (1H, s, 1-OH), 6.79 (1H,s, H-5), 6.24 (1H, s, H-4), 3.50 (2H,t, J = 6.7 Hz, H-16), 2.71 (2H, t, J = 6.8 Hz, H-11), 1.88 (2H, t, J = 6.7 Hz, H-17), 1.83 (2H, t, J=6.8 Hz, H-12), 1.39(6H, s, H-19 and H-20), 1.37 (6H, s, H-14 and H-15);13C NMR (100 MHz, CDCl3)δ:182.6 (C, C-9), 160.5 (C, C-1), 160.4(C, C-6), 154.9 (C, C-10a), 153.3 (C,C-3), 151.5 (C, C-4a), 137.8 (C, C-7),121.3 (C, C-8), 111.2 (C, C-2), 103.5(C, C-8a), 103.0 (C, C-9a), 100.5(CH, C-5), 94.0 (CH, C-4), 75.9 (C,
C-13), 75.5 (C, C-18), 32.9 (CH2, C-16), 31.9 (CH2, C-11), 26.8 and 26.5(each CH3× 2, C-14, C-15, C-19, and C-20), 22.4 (CH2, C-17), 16.1 (CH2, C-12). Its NMR and MS data were in accordance with those reported in the literature[27], suggesting that 20 had the same structure as BR-xanthone A.
2.1.21 Tovophyllin A (21) Yellow solid;C28H30O6;Mr=462.2;1H NMR (400 MHz, Acetone-d6) δ: 13.72 (1H, s, 1-OH), 8.05(1H, d, J = 10.2 Hz, H-16), 6.51 (1H,s, H-4), 5.88 (1H, d, J = 10.2 Hz, H-17), 5.47-5.18 (2H, m, H-12 and H-22),3.59 (2H, d, J = 7.4 Hz, H-21), 3.37(2H, d, J = 7.2 Hz, H-11), 1.89 (3H, s,H-25), 1.80 (3H, s, H-15), 1.66 (6H,s, H-14 and H-24), 1.47 (6H, s, H-19 and H-20).13C NMR (100 MHz, Acetone-d6) δ:183.4 (C, C-9), 163.1 (C, C-3), 161.4(C, C-1), 155.9 (C, C-4a), 151.8 (C,C-10a), 150.7 (C, C-6), 138.2 (C, C-7), 132.6 (CH, C-17), 132.4 (C, C-23), 131.4 (C, C-13), 123.5 (CH, C-12), 122.3 (CH, C-22), 121.6 (CH, C-16), 118.2 (C, C-8), 116.2 (C, C-5),111.0 (C, C-2), 108.4 (C, C-8a), 103.7(C, C-9a), 93.3 (CH, C-4), 76.8 (C, C-18), 27.2 (CH3× 2, C-19 and C-20), 25.9(CH3× 2, C-14 and C-24), 23.2 (CH2, C-21), 22.0 (CH2, C-11), 18.1 (CH3, C-25),17.9 (CH3,C-15). The NMR and MS data were in consistent with those reported in the literature[28].Hence,the structure of 21 was defined as tovophyllin A.
2.1.22 1,3,6-Trihydroxy-2,5-bis(3-methylbut-2-enyl)-6′,6′-dimethyl-4′,5′-dihydropyrano[2,3′:7,8]xanthone(22) Yellow solid; C28H32O6; Mr=464.2;1H NMR (400 MHz, Methanol-d4) δ :6.29 (1H, s, H-4), 5.24 (2H, m, H-12 and H-22), 3.52 (2H, d, J = 6.6 Hz, H-21), 3.43 (2H, m, H-16), 3.28 (2H,m, H-11), 1.89 (3H, s, H-25), 1.85(2H, m, H-17), 1.78 (3H, s, H-15),1.66 (6H, s, H-14 and H-24), 1.38 (6H,s,H-19 and H-20);13C NMR (100 MHz,Methanol-d4)δ:184.0 (C, C-9), 163.4 (C, C-3), 161.3 (C, C-1), 156.3 (C, C-4a),152.3 (C, C-10a), 151.7 (C, C-6), 139.8(C, C-7), 132.5 (C, C-13), 131.6 (C,C-23), 124.0 (CH, C-12), 123.1 (CH, C-22), 119.8 (C, C-8), 114.3 (C, C-5),111.5 (C, C-8a), 111.2 (C, C-2), 103.9(C, C-9a), 93.1 (CH, C-4), 75.6 (C, C-18), 34.0 (CH2, C-17), 26.6 (CH3× 2, C-19 and C-20), 26.0 (CH3× 2, C-14 and C-24), 23.5 (CH2, C-16), 23.2 (CH2, C-21), 22.2 (CH2, C-11), 18.2 (CH3, C-25), 17.9 (CH3, C-15). Its NMR and MS data were identical with those reported in the literature[29].Therefore, compound 22 was elucidated to be 1,3,6-trihydroxy-2,5-bis (3-methylbut-2-enyl) -6′,6′-dimethyl-4′,5′-dihydropyrano [2,3′:7,8]xanthone.
2.2 The results of preliminary screening for potential neuroprotective natural products in glutamateinduced HT22 cells
The isolated compounds were evaluated for their ability to protect neuronal damage in the model of glutamate-induced HT22. Initially, the results of cytotoxicity assay showed that most of the compounds had no obvious cytotoxicity to HT22 cells at a concentration of 30 μmol/L. At a sub-concentration of 10 μmol/L, 11 compounds (2, 5-10, 14, 16, 19,and 22) exhibited well protective effects on glutamate-induced cell death in HT22 cells (Fig. 2A).Furthermore, these active compounds were subjected to evaluate their activities at the lowest tested concentration of 1 μmol/L, and compounds 6 and 8 still showed neuroprotection (Fig.2B).
In conclusion, the chemical constituents of G.mangostana were studied, and 22 xanthones including 21 prenylated ones were isolated from the pericarps extracts. Compounds 3 and 15 were isolated from this plant for the first time. The neuroprotective effects of all compounds against glutamate-induced cell death were investigated in murine hippocampal neuronal cell line HT22. Among them, compounds 6 and 8 were the most active natural products. These findings suggested that active xanthones might be promising agents for anti-AD drugs development, and also expanded the potential usage of this medicinal-edible plant.

Fig.2 The neuroprotective effects of the isolated compounds on glutamate-induced cytotoxicity in HT22 cells. Cells were pretreated with or without different compounds at indicated concentrations (A:10 and 3 μmol/L;B:1 μmol/L) for 30 min and then incubated with 2 mmol/L glutamate for 24 h. Cell viability was determined by MTT assay. Glu:glutamate. Data are presented as means±S.D. One-way ANOVA followed by Tukey’s test. ###P <0.001 vs. control group;***P <0.001,**P <0.01,*P <0.05 vs. glutamate-treated group

