Parabiosis modeling: protocol, application and perspectives
Cui Yang, Zhi-Lan Liu, Jun Wang, Xian-Le Bu, Yan-Jiang Wang,*, Yang Xiang
1 Institute of Neurology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu,Sichuan 610072, China
2 Chinese Academy of Sciences Sichuan Translational Medicine Research Hospital, Chengdu, Sichuan 610072, China
3 Department of Neurology, General Hospital of Western Theater Command, Chengdu, Sichuan 610083, China
4 Department of Neurology and Center for Clinical Neuroscience, Daping Hospital, Third Military Medical University, Chongqing 400012,China
5 Department of Neurology, Sichuan Provincial People’s Hospital, University of Electronic Science and Technology of China, Chengdu,Sichuan 610072, China
ABSTRACT Parabiosis is a surgical method of animal modeling with a long history. It has been widely used in medical research, particularly in the fields of aging,stem cells, neuroscience, and immunity in the past two decades. The protocols for parabiosis have been improved many times and are now widely accepted.However, researchers need to consider many details, from surgical operation to perioperative management, to reduce mortality and maintain the parabiosis union. Although parabiosis has certain inevitable limitations, it still has broad application prospects as an irreplaceable animal model in the medical research field.
Keywords: Parabiosis; Application; Protocols;Surgical technique; Perioperative management
INTRODUCTION
Parabiosis refers to the condition in which two entire living animals are connected and share a single circulatory system.Parabiosis can occur naturally due to abnormal development of embryos in monozygotic twins, leading to conjoined individuals, also known as Siamese twins in humans (Eggel &Wyss-Coray, 2014). Experimental parabiosis, however, is achieved by surgical operation.
The experimental technique for establishing parabiosis in animals was first introduced in the 1860s by French biologist Paul Bert, who demonstrated the development of a viable cross-circulatory system and formation of new capillaries in two rats surgically attached to each other (Bert, 1864). Since then, parabiosis has become an essential technique in medical research.
In this review, we briefly discuss the application of parabiosis in the field of medical research. We also discuss the development and improvement of parabiosis protocols,highlighting practical details and key points. Finally, we discuss the limitations, challenges, and prospects of parabiosis models.
APPLICATION OF PARABIOSIS IN MEDICAL RESEARCH
Parabiosis was initially used for hormone, cancer, and transplantation research. It also played a critical role in the discovery of leptin and atrial natriuretic factor (ANF)(Chimoskey et al., 1984; Coleman, 2010; Zhang et al., 1994).Various parabiosis animal models have also been used to illustrate the pathogenesis of tumor-induced anorexia (Garzia et al., 2018; Mordes & Rossini, 1981) and the mechanism of transplantation tolerance (Davison, 1966; Nisbet, 1968).
Within the past two decades, parabiosis has been widely employed for research on aging and stem cells, with heterochronic parabiosis playing an essential role. For example, Conboy et al. (2005) found that age-related decline in progenitor cell activity can be modulated by systemic factors that change with age. Soon afterwards, the use of such models helped in the discovery of the systemic mechanisms of aging including the Wnt signaling pathway (Brack et al., 2007)and “pro-aging” factors such as chemokine (C-C motif) ligand(CCL)-11 (Villeda et al., 2011). Several studies also identified growth differentiation factor (GDF)-11 as a circulating rejuvenating factor (Katsimpardi et al., 2014; Loffredo et al.,2013; Sinha et al., 2014). Recently, several age-related mechanisms leading to the accumulation of senescent cells have been identified by quantitative analysis of cytodynamics(Karin & Alon, 2020). Heterochronic parabionts have also been shown to differentially respond to extensive cellular senescence in multiple tissues (Yousefzadeh et al., 2020).Specifically, circulating anti-geronic factors from heterochronic parabionts exert rejuvenating effects on aging vasculature(Kiss et al., 2020).
A series of breakthroughs in neuroscience, including the identification of myelomonocytic cells with distinct roles in neuroinflammation (Ajami et al., 2011, 2007), the origin and differentiation of macrophages in the central nervous system(Goldmann et al., 2016), and the pathogenic role of the circulating mutant Huntingtin protein (Rieux et al., 2020), have been achieved with the assistance of parabiotic models. Using a heterochronic parabiotic mouse model, the Wyss-Coray research team proved that young blood plasma can ameliorate cognitive impairment and Alzheimer’s disease(AD)-type pathology (Middeldorp et al., 2016). Furthermore,we preliminarily revealed the relationship between the peripheral system and major pathogenic substances in the brain, such as amyloid β (Aβ) and tau protein, using isochronic parabiotic mouse models (Bu et al., 2018; Wang et al., 2018;Xiang et al., 2015).
Parabiotic models have also contributed to immunological research, including the discovery of the regulating role of Pselectin in the recruitment of adult thymic progenitors (Rossi et al., 2005), the origin of dendritic cells in peripheral lymphoid organs (Liu et al., 2007), the development of thymic B cells from progenitors within the thymus (Perera et al., 2013), the identification of a multi-organ web of tissue-resident memory T cells that functionally adapt to their environment to stop viral spread within an organism (Kadoki et al., 2017), the selfrenewal of certain cardiac macrophages with negligible blood monocyte input at a steady state (Dick et al., 2019), the mechanism of interleukin (IL)-17 constraining natural killer cell activity (Wang et al., 2019), and the features of resident and migrating CD8+ T cells in lymphoid tissues during chronic lymphocytic choriomeningitis infection (Im et al., 2020). In addition, other important findings, including in the treatment of osteopetrosis (Walker, 1973) and limb regeneration after amputation (Rinkevich et al., 2011), have been made with the assistance of parabiosis models.
DEVELOPMENT OF PARABIOSIS PROTOCOLS
In general, parabiotic models used in medical research are generated by surgical techniques that have evolved over time but remain to be improved and optimized. Currently, the most common approach is connecting the body flanks of paired mice or rats.
One key goal of parabiosis is guaranteeing the nonseparation of paired animals for a certain time. Almost all significant technological advances throughout the history of parabiosis can be attributed to this issue. In the late 1950s,Hervey et al. (1959) systematically improved the surgical protocols as follows. First, the scapulae are sutured together after the surface muscles are removed and the bones are exposed. Second, the femurs are tied together after the bones are separated from the surrounding muscles. Third, the thorax and abdominal muscles, but not the peritoneal muscles or peritoneal cavity, are connected by shallow sutures to prevent the formation of pockets between the bodies of paired animals. Parabiotic animals joined by Hervey’s protocol exhibit good blood exchange, and because of the internal support derived from connected and fused bones, parabiosis can be maintained for a long time and partners are not easily separated (Harris & Martin, 1984). Researchers have also anastomosed the peritoneal cavities of paired partners based on Hervey’s protocol, i.e., the “peritoneal method” (Villeda et al., 2011, 2014). This protocol also has good circulatory exchange characterized by metabolite exchange from one animal to another, even into the brain parenchyma (Castellano et al., 2016).
Nevertheless, the duration of parabiosis does not fully meet the requirements of research, particularly in the field of neurodegeneration. A long survival period is required in many studies that use animal models of neurodegeneration to recapitulate the chronic process of disease in humans. Thus,some strategies for maintaining parabiosis based on our experience are worth noting.
Currently, the use of inbred or littermate mice as paired animals for parabiotic models is common. This is because the incidence of immune incompatibility, i.e., parabiotic intoxication, is high in paired outbred mice due to the continuous exchange of cells and fluids between partners(Finerty & Panos, 1951). However, not all protocols used in previous publications have emphasized the importance of maintaining and feeding paired mice together in one cage for a period before surgical parabiosis. In our experience, this step can elevate the familiarity of the paired mice and promote their adaptation. More importantly, it allows us to identify and exclude mice that are overactive and aggressive and will likely fight with and potentially kill their partners, as parabiosis with these mice cannot be maintained for a long period of time.
Linear incisions are not sufficient for the long-term maintenance of parabiosis. Even when the scapulae are anastomosed and the femurs are tied together, the linear incisions in the thoracoabdominal skin tend to selfanastomose, leading to the formation of a gap or hole between the flanked bodies, ultimately resulting in the separation of the paired animals. Thus, two corresponding skin incisions approximately 0.8-1.0 cm wide should be made and anastomosed to reduce the possibility of anastomosis between the upper and lower edges of the incision of the same mouse. Moreover, subcutaneous tissue alignment and interrupted sutures connecting the chest and abdomen wall should be used to prevent or limit self-anastomosis.
To date, suturing is the most common technique for incision anastomosis. In our experience, the two critical points are at the two ends of the incisions, which we name the head-end and tail-end, respectively. Incisions are most likely to be loosened or ripped at both points during the perioperative period because they bear the strongest drag force as the parabiotic mice move, bite, and fight. In addition to pursestring sutures, metal clips are useful for reinforcing the connection. Moreover, close observation of the two points is required in the days after operation, and early interventions,including iodophor disinfection and supplementary sutures,should be considered if the sutures are damaged.
In heterochronic parabiotic models, young mice are more likely to die once the scapulae and femurs are fixed due to significant differences in body length and weight. Thus, in practice, we connect the scapulae and anastomose the incisions that extend close to the joint between the tail and torso instead of the back of the thigh, but we do not tie the femurs. Additionally, due to differences in body weight, the skin incision needs to be oriented slightly outwards in young mice and oriented slightly downwards in old mice to ensure that the mice can stand independently, freely, and steadily on the ground on all eight limbs and avoid the situation of “a mouse carrying a mouse” after the incisions are sutured.Researchers have observed that the mortality of young mice in parabiotic models is high and propose that this could be due to parabiotic intoxication or parabiotic disharmony (Eggel &Wyss-Coray, 2014). However, the location of surgical incisions and sutures could also be of importance to the survival of young partners in heterochronic parabiosis models.
Postoperatively, bedding material should not be too thick to allow the parabiotic mice to maintain a normal position at the bottom of the cage and to prevent the animals from squeezing each other. Furthermore, avoiding wood chip bedding can prevent the relatively active mouse (dominant partner) from repeatedly making holes in the bedding and thus protect the relatively quiet mouse (subordinate partner) from excessive external injuries and death during this process. This is also why it is necessary to ensure that the mice can stand independently, freely, and steadily on the ground on all eight limbs, as it prevents the subordinate partner from dying due to excessive passive movements.
The feeding mode should also be changed accordingly after surgical parabiosis. It is worth noting that the subordinate partner often loses the ability to eat or drink freely due to the activities of the dominant partner. In general, the animals need a long period of time to adapt to each other and habituate to eating and drinking in coordination. Thus, it is not recommended that food be placed on the top of the cage as usual; instead, a thin layer of ground food should be spread on the bottom of the cage so that both parabiotic mice can obtain food anytime and anywhere. Similarly, in addition to the regular supply of drinking water at the top of the cage, a small bowl of water should be placed at the bottom of the cage to increase the probability of successful water drinking. Hydrogel is also a good option, especially during the perioperative period. Water and food can be manually administered if necessary, even though it is a challenge to hold both mice simultaneously.
PROTOCOLS FOR ESTABLISHING ISOCHRONIC PARABIOSIS
Here, we introduce a protocol for the operative and perioperative management of isochronic parabiotic mice as representative experimental animals.
Preoperative management
1. Prior to the surgical operation, suitable pairs of mice should be selected. Currently, inbred or litter-born mice are the most widely used experimental animals as the incidence of parabiotic disharmony or parabiotic toxicity is significantly lower in these animals than in outbred mice.
2. One pair of mice should be fed in a single cage under conventional feeding conditions. It is highly recommended that each pair of mice be kept together in one cage for at least one week before surgical operation. During this time, all mice should be closely observed, and those that are hyperactive, aggressive, prone to fighting, or likely to mortally wound their partner should be excluded from the parabiotic experiment.
3. It is essential for surgeons to be quite familiar with the operative procedures, especially critical steps such as the design and formation of corresponding skin incisions,exposure of the scapulae, alignment of the tissue, and suture of the subcutaneous tissue and skin.
4. It is strongly recommended that beginners receive specific training under the guidance of experienced surgeons for a period before independently performing these surgical operations. In general, it takes approximately half an hour to one hour from incision to suturing owing to the delicacy of the operation.
5. The cooperation of two surgeons is highly recommended,especially for scapular anastomosis and skin suturing,which both significantly influence total surgical operation time.
Operative procedures
Anesthesia
1. Anesthesia is critical to the success of the surgical operation. An animal anesthesia machine and inhalation anesthetics should be used whenever possible. For inhalation anesthetics, a separate nose cone should be used for each partner mouse to allow adjustment of the depth of anesthesia.
2. Intraperitoneal injection of anesthetic is not recommended because it is difficult to determine whether both paired mice are appropriately anesthetized with relaxed muscles at the same time. Asynchronism of anesthesia is not conducive to designing and creating corresponding flank incisions and makes it difficult to control abdominal pressure, leading to severe intestinal bulges during abdominal cavity anastomosis. More importantly, the recovery time of the paired mice after intraperitoneal anesthesia is often different, resulting in the high likelihood of secondary trauma to the mouse that recovers last due to the activity of the mouse that recovers first postoperatively.
Surgical preparation
1. Standard aseptic surgical procedures are used, as described in previous research (Conboy et al., 2013).
2. A heating pad is necessary given the potential length of the surgical operation.
3. In general, this surgical operation does not need overly precise surgical instruments and equipment, and regular surgical instruments for mice can meet the requirements.Frequently used surgical instruments are shown in Figure 1A.
4. The dorsal positions of the paired mice are adjusted after anesthesia, which can be used as a reference for dorsal skin incision formation. The ventral positions of the paired mice are adjusted after anesthesia, which can be used as a reference for ventral skin incision formation(Figure 1B-D).
5. Following previous study (Conboy et al., 2013), after confirming the state of anesthesia and muscle relaxation,the skin of the paired mice is prepared at the corresponding regions, which extend from the front to the back of the ear, down to the midline of the abdomen, up to the midline of the back, and back to the root of the tail.All hair is removed using an electric shaver in the above regions where incisions will be made. These regions are then cleaned using a hair dryer (Figure 1E, F).
6. Next, the relative positions of the mice are re-adjusted carefully (Figure 1E, F).
7. Conventional iodine tincture and 75% medicinal alcohol are used to disinfect the above regions (Figure 1G).
Surgical procedures
1. For any one of the paired mice, after the operative region is dry, the limbs and tails of the paired mice are fixed with nondrying adhesive tape, respectively (Figure 2A). The skin and subcutaneous tissues of the corresponding region (from the back of the ear to the back of the thigh)are cut with a pointed surgical blade (Figure 2B).
2. Ophthalmic scissors are used to cut the skin vertically along both sides of the above incision to form a 0.8-1.0 cm wide surgical incision, exposing the chest and abdominal walls (Figure 2B-D).
3. Using the tip of the ophthalmic forceps, the corresponding scapula is carefully separated and raised above the shoulder muscles (Figure 2E).
4. The muscles attached to the scapula bone surface are gently removed with the handle of the ophthalmic forceps and the pointed surgical blade until slight bleeding occurs(Figure 2F-H).
5. After a sterile cotton ball is applied for one or two minutes to stop the bleeding, the ophthalmic forceps are used to separate the corresponding thigh muscles and expose the femur (Figure 2I).
6. The muscles covering the femur are gently removed with a surgical blade (Figure 2I).

Figure 1 Surgical preparation of parabiosis mouse model
7. Both mice must undergo the same surgical procedures at corresponding sites (Figure 2J).
8. The mouths and noses of both mice are placed in nose cones, respectively. The relative positions of the paired mice are adjusted again to align the scapulae and femurs well on both sides. Next, the tails are fixed with nondrying adhesive tape (Figure 3A).
9. The two ends of the skin incisions (head-end and tailend) are threaded using 4-0 nylon without sutures,respectively (Figure 3A).
10. The surfaces of both scapulae are overlapped, and one or two interrupted sutures are made using 4-0 nylon(Figure 3B-H).
11. The corresponding femoral bone surfaces are overlapped, and 4-0 nylon is used to bind them together(Figure 3I-L).
12. The corresponding femoral muscle tips of the paired mice are sutured using one or two interrupted sutures(Figure 3M).
13. All deep surgical knots are then confirmed to be stable and fixed.
14. The alignment of the dorsal skin incisions, scapula sutures, and femoral sutures are re-examined(Figure 3N).
15. The relative positions of the mice are adjusted, and skin alignment is determined before skin incision suturing(Figure 4A).
16. The dorsal skin incisions are sutured using interrupted sutures from the tail-end to head-end (Figure 4B-E).
17. The adhesive tape used to fix the mice is removed quickly, and the mice are turned over. Their mouths and noses are placed in nose cones, and their tails are fixed again. Alignment of the ventral skin incisions is checked(Figure 5A).
18. After confirming no active bleeding or foreign body residue, one or two sutures are made at corresponding sites of the thigh muscles, abdominal walls, and chest walls, respectively (Figure 5B-D).
19. The ventral skin incisions are sutured using interrupted sutures from the head-end to tail-end (Figure 6A-F).
20. The two most important skin incision sutures, i.e., headend and tail-end of the incisions, are made (Figure 6E, F).Because these points bear the strongest drag force as the parabiotic mice move, bite, and fight, these sutures are more likely to be loosened or ripped during the perioperative period. Thus, a purse-string suture is recommended, and metal clips are very helpful for reinforcing the connection.
21. After suturing, it is crucial to ensure that there are no suture omissions at any incision site and to sterilize the sutured and operative regions with 75% medicinal alcohol(Figure 7A-D).
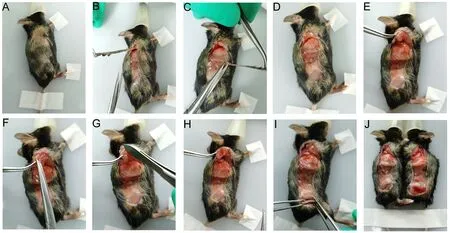
Figure 2 Formation of surgical incisions and management of scapulae and femurs (left mouse taken as example)
Postoperative management
1. After surgery, the mice are placed in a warm box to maintain their body temperature and receive analgesic/anti-inflammatory treatment (acetylsalicylic acid 5 mg/kg)for two weeks.
2. After recovery, the mice are transferred to common cages, and each pair of parabiotic mice are fed in a single cage, with 150 μl of piperacillin tazobactam sodium injected intraperitoneally for seven consecutive days to prevent infection.
3. Mouse activity is observed every day, and bedding, food,and drinking water are provided as discussed in the main text.

Figure 3 Suturing scapulae and securing femurs together
PERSPECTIVES
Currently, the use of parabiotic models still faces several challenges. Parabiosis alters the living habits of the animals involved. Bullying of the subordinate partner by the dominant partner is inevitable, even in isochronic parabiosis. In our experience, body weight equality is more important than age equality in preventing bullying. Thus, the impact of changes in the behavior and habits of parabiotic animals on the biological results needs to be considered. Of note, the well-being and life span of paired mice do not seem to be affected by parabiosis,underlining again that parabiosis is very well tolerated in rodents and has minimal impact on life expectancy (Eggel &Wyss-Coray, 2014).
The use of comprehensive assessments, such as psychobehavioral tests, is a challenge for parabiotic animals due to the severe trauma of surgical separation. Even though some researchers have been able to separate parabiotic mice in a few biological experiments (Kamran et al., 2013), it is extremely difficult to perform traditional psycho-behavioral tests on parabiotic mice, indicating that it may be important to develop behavioral tests that can be specifically applied to parabiotic animals.
Moreover, a recent animal study revealed that dilution of blood with saline containing 5% albumin has an anti-aging effect (Mehdipour et al., 2020). In heterochronic parabiosis,old animals usually have a higher body weight and a larger blood volume than their young partners. Thus, in addition to previously implicated molecules and related pathways,researchers need to evaluate the effects of blood volume itself on aging when interpreting age-related biological effects of parabiosis.

Figure 4 Suturing dorsal skin incisions
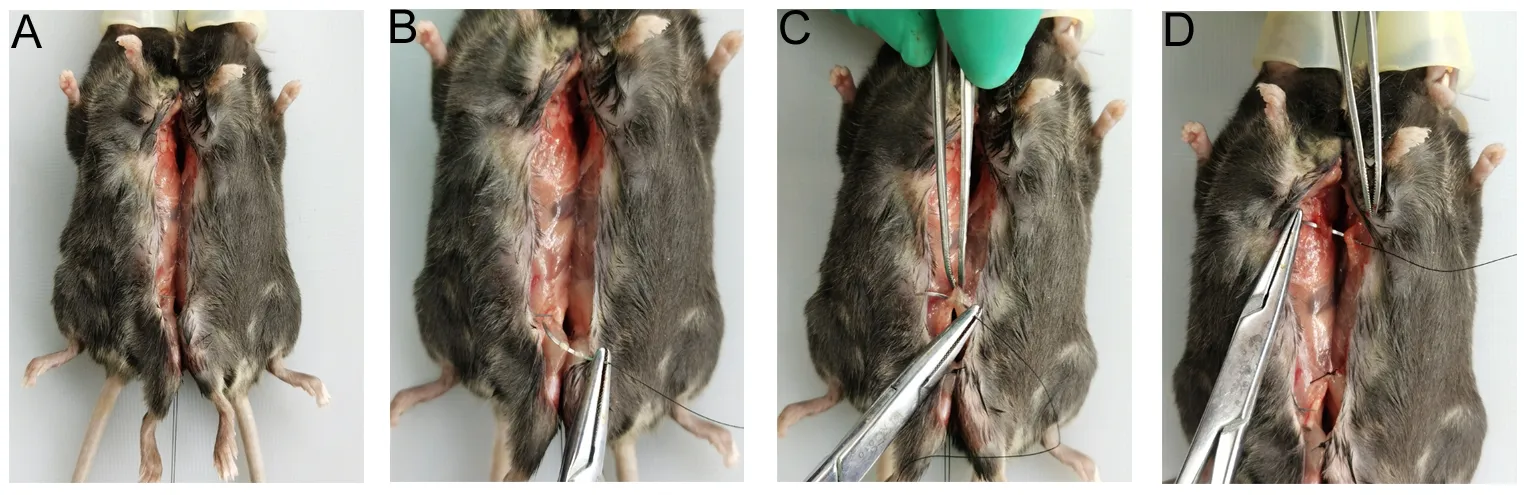
Figure 5 Surgical treatment of ventral structures

Figure 6 Suturing ventral skin incisions
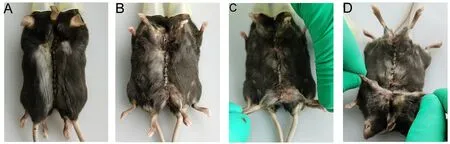
Figure 7 Re-checking all sutured incisions
In summary, the parabiotic technique has been developed for more than 150 years. Its use has experienced ups and downs during this period, but the technique has returned to the spotlight of medical research in the past two decades.Surgical and management procedures for establishing parabiotic models have been increasingly optimized. As a traditional animal research technique, it will play an increasingly important role in future medical research.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
C.Y., Z.L.L., J.W., X.L.B., Y.J.W., and Y.X. conceived the review. C.Y., Z.L.L., J.W., X.L.B., and Y.X. prepared the manuscript. All authors contributed to the discussions. All authors read and approved the final version of the manuscript.
- Zoological Research的其它文章
- Species bias and spillover effects in scientific research on Carnivora in China
- Particulate matter exposure exacerbates susceptibility to SARS-CoV-2 infection in humanized ACE2 mice
- Potential aquatic environmental risks of trifloxystrobin:Enhancement of virus susceptibility in zebrafish through initiation of autophagy
- Northern pig-tailed macaques (Macaca leonina)infected with SARS-CoV-2 show rapid viral clearance and persistent immune response
- A review of the Cypriniform tribe Yunnanilini Prokofiev,2010 from China, with an emphasis on five genera based on morphologies and complete mitochondrial genomes of some species
- Molecular mechanisms of intermuscular bone development in fish: a review

