A new species of Atelopus (Amphibia: Bufonidae) from eastern Panama
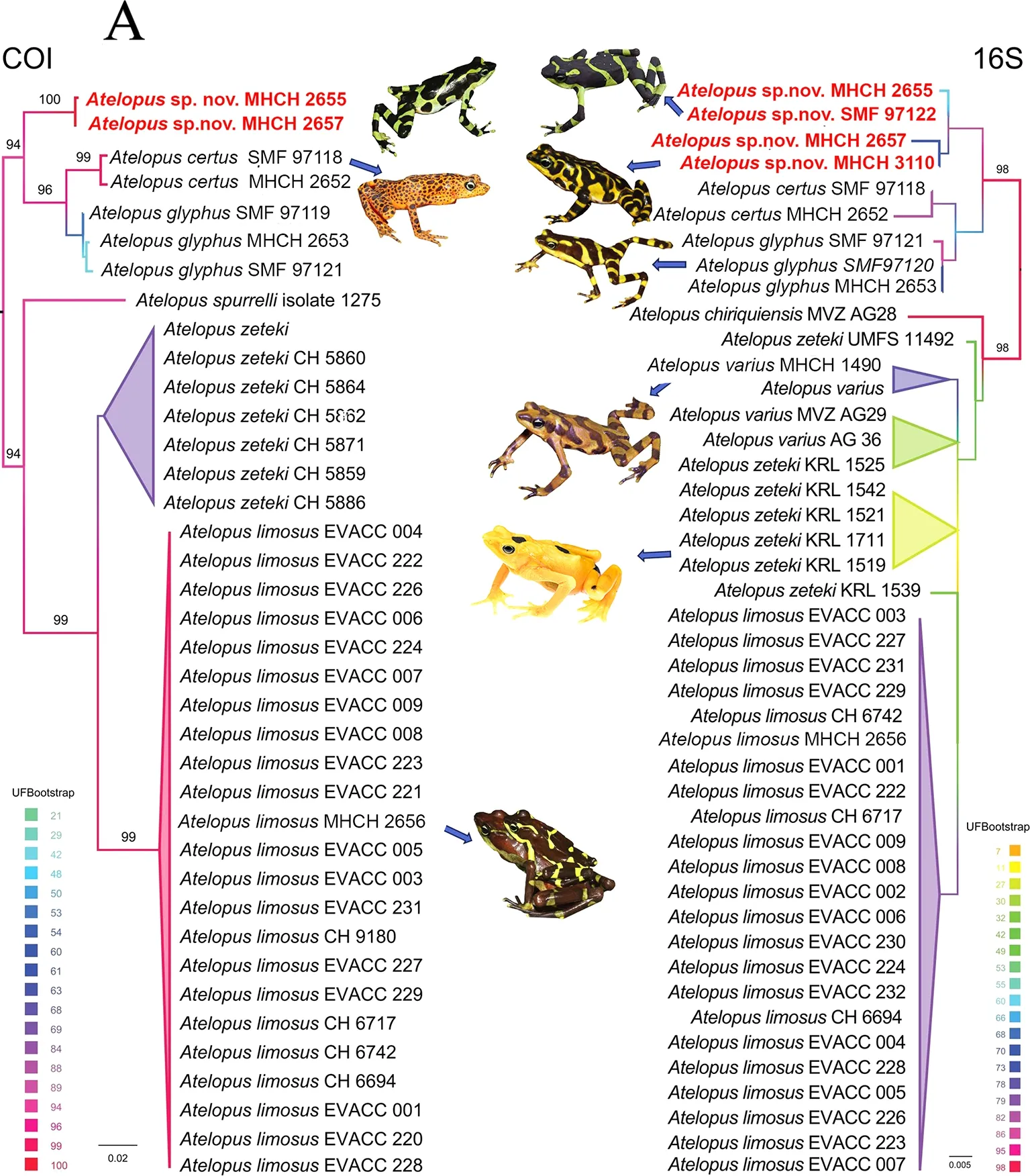
A new species of the genusAtelopus,Atelopus fronterizosp.nov., from eastern Panama is described herein based on molecular, morphological, and bioacoustic evidence. The new species can be distinguished from its congeners occurring in the region by a combination of the following characters: (1)phalangeal reduction in thumb; (2) SVL (females only)(35.1-50.1;n=13), HW/SVL (0.23-0.34;n=59), EYND/HW(0.27-0.39;n=60), TIBL/SVL (0.41-0.56;n=58), and HAL/SVL(0.22-0.28;n=49); (3) dorsal color pattern with green or yellow background and extensive dark olive blotches forming transversal bands or mottling; (4) advertisement call duration 176-235 ms with 19-34 pulses, average pulse rate 131.69 pulses/s, and dominant frequency 2 422.50-2 606.50 Hz. The new species is nested within the Central American clade ofAtelopus. The minimum Kimura-2-parameter (K2P) genetic divergence betweenAtelopus fronterizosp. nov. and its most phylogenetically similar congeners (A.certusandA.glyphus)is >2.6% for 16S and >4.9% forCOI(Table 1). The phylogenetic relationship is strongly supported by ultrafast bootstrap values for the maximum-likelihood trees of both genetic markers (16S, 96;COI, 100, Figure 1A). Bayesian analysis of the concatenated sequences resulted in a tree with similar topology and high posterior probability support (0.99;Supplementary Figure S1). In addition, haplotype networks inferred fromCOIand 16S (Supplementary Figure S2)showed a well-separated clade containing the new species(two forCOI, four for 16S). The number of mutational steps between haplotypes for the new species samples is very low(1-4 in 16S; one inCOI), and the minimum number of mutational steps from the nearest species is nine for 16S(distance toA.certus) and 28 forCOI(distance toA.glyphus).
Harlequin frogs occur from Costa Rica to Bolivia (Lötters,1996; Lötters et al., 2011; Savage, 2002). Nearly a hundred species (AmphibiaWeb, 2020; Frost, 2020) are currently recognized, six of which occur in Panama: i.e.,AtelopuscertusBarbour, 1923;Atelopus chiriquiensisShreve, 1936;Atelopus glyphusDunn, 1931;Atelopus limosusIbáñez R.,Jaramillo, and Solís, 1995;Atelopus varius(Lichtenstein and von Martens, 1856); andAtelopus zetekiDunn, 1933. The geographical distribution of the new species and other PanamanianAtelopusspecies is shown in Figure 1B.
During recent field surveys in the mountain ranges of eastern Panama, we collected fiveAtelopusspecimens, along with recordings of calling males. Subsequent molecular analysis revealed that our samples were clustered together,comprising a clade well separated from all known species.Comparison of our specimens with material in museum collections demonstrated that the species has a long scientific history. It was first collected in 1911 by naturalist Henri Pittier near Puerto Obaldia on the Caribbean coast of Darién(Heckadon-Moreno, 1996). This oldest known specimen,stored in the United States National Museum (catalog No.USNM 48 594), was presented by Dunn (1931) as a paratype ofAtelopus glyphus. Since then, the species has been repeatedly collected and examined (Breder, 1946; Cocroft et al., 1990; Savage, 1972) without any taxonomic consequences. Recently, several authors have identified this taxon asAtelopuscf.limosus(Lewis et al., 2019) orAtelopussp. “Puerto Obaldía-Capurganá” (Ramírez et al., 2020).
Field work was carried out from 2011 to 2017 in the Chucunaque and Tuira Basins of the eastern Panamanian lowlands, and in all principal eastern Panamanian mountain ranges. The collected specimens were euthanized with T61 and fixed with a preservative mixture of 5 mL formalin (40%)and 1 L ethanol 96 (94%), then stored in ethanol (70%). All geographical coordinates were recorded based on the WGS 1984 datum. A map was created using QGIS 2.18 (Las Palmas) with the OpenStreetMap layer (OSM Contributors,2015). Abbreviations for museum collections followed Sabaj(2016).
Morphometric data were taken following Bravo-Valencia &Rivera-Correa (2011). Abbreviations of measurements are as follows: SVL (snout-vent length), TIBL (shank length), FTL(foot length), HL (head length from point behind angle of jaw to tip of snout), HW (head width at widest point), EYDM (eye diameter), EYND (eye to nostril distance), IOID (interorbital internal distance), IND (internarial distance), FAL (length of flexed forearm), HAL (hand length from proximal edge of outer metacarpal tubercle to tip of finger III), and THBL (thumb length from outer metacarpal tubercle to tip of finger I).Measurements of frogs were taken to the nearest 0.1 mm with digital calipers. Webbing formulae followed Myers & Duellman(1982) and Savage & Heyer (1997). Museum and field numbers of voucher specimens are listed in Supplementary Table S1.
The DNA extraction protocols followed Batista et al. (2016).Details on final alignment, maximum-likelihood (ML) analysis,Bayesian analysis, and haplotype network construction are provided in the Supplementary Notes. GenBank accession Nos. of the sequences used (including those submitted by us)are listed in Supplementary Tables S2, S3.
For confirmed candidate species (CCS) and their delimitation, we followed the integrative concept for amphibians of Vieites et al. (2009). For species-level analysis,genetic divergences were calculated using the Kimura 2-parameter (K2P) model (Kimura, 1980) for 16S andCOIseparately in MEGA 6. Default values were retained for all parameters. Statistical analyses of morphometric characters were performed using SPSS 21.0.
Details on call recordings and bioacoustic methodology are described in the Supplementary Notes.
Molecular analysis (mitochondrial DNA (mtDNA) 16S andCOI) ofAtelopusspecimens from the San Blas Mountains(two specimens from Nurra) and Cerro Tacarcuna slopes (one specimen from Pechito Parao near Río Tuquesa and one specimen from Río Púcuro) confirmed that these samples formed a monophyletic lineage and sister clade to the clade containingA.certusandA.glyphusfrom eastern Panama(Figure 1A). Discriminant function analysis (DFA) of morphometric characters ofAtelopusfrom eastern Panama(Supplementary Figure S3) correctly classified the new species, with 80.4% of specimens separated from related species according toa priorigroupings (correct classification for 85.3% ofAtelopus fronterizosp. nov.; 70% ofA.glyphus;50% ofA.certus). The principal morphological variables contributing to the grouping in order of relevance were: (1)HW/SVL, (2) EYND/HW, (3) TIBL/SVL, (4) HAL/SVL; first function: DS=0.23xHW/SVL±0.18xEYND/HW-0.65xTIBL/SVL+4.24xHAL/SVL; second function: DS=-0.22xHW/SVL±0.45xEYND/HW+1.72xTIBL/SVL+5.07xHAL/SVL. When including SVL alone, DFA correctly classified 87% of the specimens. Moreover, the new species can be distinguished from all other species ofAtelopusoccurring in eastern Panama by additional morphological and bioacoustic characters. According to our findings, we describe the species as new to science.

Table 1 K2P distances for mitochondrial fragments of 16S and COl genes of Atelopus species from Panamá and Colombian samples of A spurrelli.
Taxonomic account
Atelopusfronterizosp. nov. (for synonymy see Supplementary Notes)
Holotype:MHCH 3110 (AB 543; Figure 1C-H), from Río Púcuro, Pinogana, Provincia Darién, Panama (N8°1'44.40",W77°22'10.20"; 132 m a.s.l.), collected by Abel Batista on 10 June 2012.
Paratypes:Two adult males, SMF 97122 (AB720) and MHCH 2655 (AB733), both from Nurra, Comarca Wargandi,collected by Milan Veselý and Abel Batista on 2 October 2012(N9°3'36.00", W77°58'44.4"; 339 m a.s.l.); adult male MHCH 2657 (AB830) from Cerro Pechito Parao, Bajo Pequeño, Lajas Blancas, Cémaco, Comarca Embera-Wounaan (N8°28'31.80",W77°31'9.84"; 472 m a.s.l.), collected by Abel Batista and Milan Veselý on 5 November 2012 (Supplementary Figure S4).
Etymology:The species epithet “fronterizo” refers to the area in which the species is distributed, i.e., the border between Panama and Colombia. Panamanian people use “fronterizo”to refer to someone living at the Panamanian border police SENAFRONT (Servicio Nacional de Fronteras) guarding this region, including the habitat of the camouflaged harlequin frog.SENAFRONT has helped increase knowledge of the area’s wildlife by reporting sightings, recording sounds, and photographing observations.
Diagnosis:Medium-sizedAtelopus(average SVL (mm): all 33.7±6.8 (24.2-50.1;n=50); females 43.8±4.1 (35.1-50.1;n=13); males 30.4±2.6 (24.2-34.8;n=37)) characterized by the following combination of characters: (1) body slender,snout protruding, with tip rounded; (2) neural spines weakly visible externally; (3) hindlimbs long, tibiotarsal articulation reaching anterior corner of eye when leg is outstretched forward along body (average in males TIBL/SVL 0.47;n=37);(4) foot shorter than shank (average in males FTL/TIBL 0.81;n=31); (5) tympanic annulus and tympanic membrane absent;(6) dorsal parts of body and limbs smooth to slightly shagreen(visible under stereomicroscope); (7) foot webbing formula I0-0II0-2-III1-3+IV3+-1-V; (8) thumb short (average THBL/HAND 0.32;n=20); (9) plantar and palmar surfaces mostly smooth with subarticular tubercles poorly defined.
Description of holotype:Body slender; neural spines weakly visible externally; head longer than wide (HW/HL=0.94); head length 27% of SVL; snout protruding, with tip rounded, dorsally subelliptical; upper jaw extending beyond lower jaw; nostrils lateral, slightly visible from above; tongue twice as long as wide, anterior two-thirds attached to floor of mouth; canthus rostralis slightly concave from nostril to tip of snout, concave from eye to nostril; nostril closer to tip of snout than to eye;eye diameter slightly larger than distance from eye to nostril;tympanic annulus and tympanum not visible; supratympanic crests slightly visible; parotid glands small and barely visible;vomers without teeth or odontoids; choanae rounded and broadly separated in between. Shank moderately long(TIBL/SVL=0.41); foot shorter than shank (FL/TIBL=0.85);relative length of toes I<II<III<V<IV; foot webbing formula I0-0II0-2-III1-3+IV3+-1-V; outer metatarsal tubercle round,weakly prominent; inner metatarsal tubercle poorly defined,oval, flattened, larger than outer; subarticular tubercles low and rounded. Forelimb long, slender; relative length of fingers I<II<IV<III; hand webbing formula I2-3-II3-4III4-3IV; outer palmar tubercle distinct, rounded, larger than oval inner;subarticular tubercles moderately marked and rounded; thumb moderately short, distance from tip to outer edge of inner palmar tubercle less than length of tubercle (THBL/HAND=0.27). Skin of dorsal surfaces of body and limbs smooth, glandular and weakly rugose in texture (observed under stereomicroscope); ventral surfaces smooth.
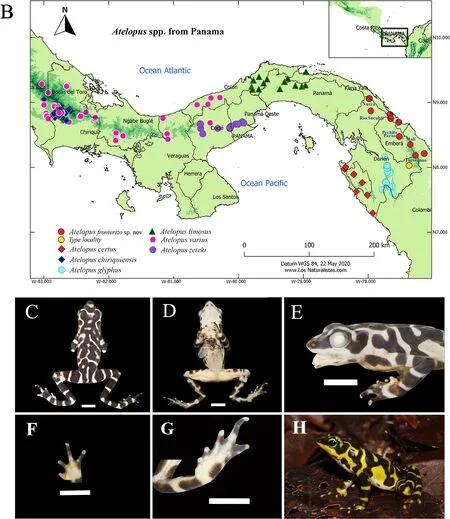
Figure 1 Molecular comparison and geographic distribution of Panamanian species of Atelopus, and main features of holotype of Atelopus fronterizo sp. nov.
Color in life:Dorsum pale yellow with irregular, well-defined,dark brown to almost black blotches, bars, and vermiculation(Figure 1H); upper surfaces of limbs yellow with broad dark olive bands; upper lip yellow, suffused slightly with dark pigmentation; lower lips with small dark olive blotches; throat,chest, and venter creamy white; lower venter and lower surfaces of thighs bright orange; remaining ventral parts of legs yellow with interrupted dark olive transverse bands,extending from dorsal parts; palmar and plantar surfaces orange suffused with dark olive blotches; ventral color of arms white with dark olive transverse bands extending from dorsal surfaces; iris black, with narrow, pale green ring surrounding pupil, reticulated with dark color in external borders.
Color in preservative:Dorsal surfaces dirty white to pale gray, with irregular, well-defined, dark to almost black blotches, bars, and vermiculation; dark bars on exposed areas of limbs and flanks; throat, venter, ventral surfaces of thighs,and palmar and plantar surfaces cream; two tiny, round, dark brown blotches on chest, another on venter (around midlevel)on right side; edges of lower lip with small dark brown blotches; throat with two barely visible diffused dark brown blotches (Figure 1C-G).
Measurements (in mm):Holotype followed by range of males(holotype plus three male paratypes): SVL 29.5 (27.5-34.1);HL 8.5 (8.2-9.3); HW 8.0 (7.7-9.2); IND 2.6 (2.5-3.5); EYND 2.5 (2.3-3.0); EYDM 3.0 (2.5-3.4); TIBL 12.1 (12.1-15.7); FTL 10.3 (9.4-13.8); HAL 6.5 (6.5-8.5); THBL 1.8 (1.8-2.8).
Variation:Measurements and intraspecific variation of known populations are shown in Supplementary Table S4 (males)and Supplementary Table S5 (females). We identified two color morphs, i.e., Tacarcuna and Sucubti morphs. The Tacarcuna morph (Figure 1H) has a dark vermiculation pattern, which agrees well with the holotype description; this population is distributed around Cerro Tacarcuna southward to Colombia (Figure 1B). The Sucubti morph populations occur northwards of Cerro Tacarcuna, with most specimens having broader dark transverse bands on the dorsum compared with the Tacarcuna morph, often with a narrow (sometimes incomplete) light inverted chevron just anterior to the interorbital area, a light chevron across the mid-dorsum, and a light band across the postsacral region, with a definite elongated light area in the suprascapular region; these light blotches share a dorsal ground color, which can be yellow,pale green, or green (Supplementary Figure S5).
Call description: We recorded an Atelopus fronterizo sp.nov.male near Nurra on 3 October 2012, 0949 h, 25.5 °C(locality of paratype MHCH 2 655). The male was calling towards another male sitting about 1 m away. The vocalization produced by the Sucubti morph consists of pulsed calls (buzzlike sounds) emitted at 2 505.94±66.05 Hz (2 422.5-2 605.5;n=8), with a call duration of 209.75±20.25 ms (176-235;n=8),pulses of 28.0±5.48 (19-34,n=7), and pulse rate of 131.69±16.39 pulses/s (97.44-144.68;n=7; Supplementary Table S6). In the recording, we identified three call bouts, with four, five, and eight calls, respectively. A male recorded by C.Myers in 1967 emitted partially pulsed short calls with a downward modulated frequency (Cocroft et al., 1990:Figure 4K-L). That call demonstrated a dominant frequency in the same range as the call recorded by our male but was substantially shorter (~55% of the duration of normal advertisement pulse call recorded in our study). An oscillogram and spectrogram of theAtelopus fronterizosp.nov advertisement call are presented in Supplementary Figure S6.
Distribution and natural history: Atelopus fronterizo sp.nov.occurs in the Darien Mountain range in northeastern Panama and northwestern Colombia (Figure 1B), as well as in the eastern Panamanian montane forests (World Wildlife Fund, 2014) and Chocó-Darién moist forests (Hogan & World Wildlife Fund, 2014). Most specimens were active during the daytime along small streams, although the holotype was found along the moderately sized Púcuro River (Supplementary Figure S7). We encountered three specimens at night at Cerro Pechito Parao (ca. 1-2 km from the Tuquesa River, Bajo Pequeño), sleeping approximately 20-30 cm above the ground in low bushes and far from any stream or river. On the Caribbean side of Nurra, we found 22 individuals during a nighttime search along a 200 m transect following a stream.The next day, we also recorded one calling male and observed one amplectant pair. Several males were observed to use forelimb waving signals during visual interactions with other individuals.
Conservation status:Due to the current declines inAtelopuspopulations caused by the chytrid fungusBatrachochytrium dendrobatidis(Flechas et al., 2017b; La Marca et al., 2005;Lewis et al., 2019), we argue thatAtelopus fronterizosp. nov.should be included in the Critically Endangered category(A3ce), as also suggested by Lewis et al. (2019).Atelopus fronterizosp. nov. faces the same risks of decimation and potential extinction as otherAtelopusspecies that occur in eastern Panama.
Similar species:Atelopus fronterizosp. nov. can be differentiated from other species ofAtelopusoccurring in the Panamanian region and adjacent areas of Colombia by a combination of the following characters: dorsal coloration showing irregular, dark brown bands and blotches on green or yellow background (Figure 1H; Supplementary Figure S5);phalangeal reduction in thumb; larger sized females (SVL);and characteristic advertisement calls. The advertisement call oscillogram ofAtelopus fronterizosp. nov. is easily distinguishable from those of Panamanian congeners by the envelope shape of the call, where the fall time ends abruptly compared to other species in the region (Supplementary Figure S6). From its geographically closest congeners (A.certus,A.glyphus), the new species differs in molecular genetics, with K2P genetic divergences of >2.6% for 16S and>4.9% forCOI(Table 1). Additionally,Atelopus fronterizosp.nov. can be distinguished by the following characters(contrasting features for the new species in parentheses (see Supplementary Tables S4-S6 and Supplementary Figure S8)).Atelopus certus: coloration in life brick red with black spots (vs. pale green or yellow with dark olive irregular vermiculations or transversal bars), head almost as broad as long HL/HW 1.03±0.09 (vs. slightly broader HL/HW 0.94±0.06), shorter shank length TIBL=13.1±0.71 mm (vs.17.1±3.7 mm), longer thumb in proportion to SVL 0.10±0.01(vs. 0.08±0.01); longer call duration 0.30±0.046 ms (vs.0.21±0.02 ms), with higher number of pulses per call 38.5±6.75 (vs. 27.5±5.82), and broader bandwidth 710.4±195.9 Hz (vs. 318.6±254.4 Hz).Atelopus glyphus:smaller in size, females 38.7±3.7 mm,n=10; males 26.5±3.5 mm,n=124 (vs. females 43.8±4.1 mm; males 30.4±2.6 mm); head as broad as long HL/HW 1.01±0.05 (vs. slightly broader HL/HW 0.94±0.06), shorter shank length TIBL 14.9±2.56 mm (vs. 17.07±3.7 mm); longer call duration 0.25±0.013 ms (vs. 0.21±0.02 ms), higher number of pulses per call 35.1±2.77 (vs. 27.5±5.82), and broader bandwidth 623.2±50.1 Hz (vs. 318.6±254.4 Hz).Atelopus limosus: dorsal ground color dark brown, black, or solid olive with variable bright green markings (vs. pale green or yellow with dark olive irregular reticulations or bars), smaller size, females 36.2±0.8 mm,n=5; males 26.3±2.5 mm,n=22 (vs. females 43.8±4.1 mm, males 30.4±2.6 mm), higher call pulse rate (pulses/s)151.6±3.1 (vs. 131.69±16.39), and maximum frequency 2 689±93 Hz (vs. 2 505.94±66.05 Hz).Atelopus spurrelli:smaller size, females 30-34 mm, males 26 mm (Rivera-Correa, 2005) (vs. females 43.8±4.1 mm, males 30.4±2.6 mm).Atelopus varius: dorsal color highly variable, mostly black or chocolate brown with yellow or red bands and blotches (vs. pale yellow or green yellow with dark olive irregular blotches or bands), longer call duration 413-431 ms(vs. 176-235 ms), and more pulses per call 48-50 (vs.19-34).Atelopus zeteki: in life dorsal color uniformly golden yellow, usually with black bands or blotches (vs. pale yellow or green yellow with dark olive irregular reticulations or bands).
Remarks:Many species in the genusAtelopusare convergent in color, shading, and pattern. When coupled with high (but poorly understood) intraspecific variation and cryptic species diversity, this renders species delimitation through morphological characters alone particularly challenging(Coloma et al., 2000; De la Riva et al., 2011; Guayasamin et al., 2010). This likely prevented earlier recognition and description ofAtelopusfronterizosp. nov. by other herpetologists. As outlined above, the identity of this species has raised taxonomic doubts for more than a century, and has been repeatedly confused withA.limosus,A.glyphusandA.spurrelli(Flechas et al., 2017a; Rivera-Correa, 2005;https://www.inaturalist.org/, accessed on 19 January 2021).
Our molecular analysis relied on comparisons of two genetic markers, 16S andCOI, which are consistently used across systematic studies of amphibians (e.g., Acosta-Galvis et al.,2020; Batista et al., 2014a, 2014b, 2014c; Chambers &Hebert, 2016; Jorge et al., 2020; Lötters et al., 2011; Nagy et al., 2012; Vieites et al., 2009). The K2P genetic distance ofAtelopus fronterizosp. nov. to its genetically closest neighbors,A.certusandA glyphus, is slightly smaller than the commonly accepted threshold for these markers in amphibians (i.e., 2.6%/2.6% for 16S and 4.9%/5.2% forCOI).Nevertheless, the morphologically distinct and widely accepted speciesA.glyphusandA.certusshow even lower distances between each other (minimum K2P distance 1.5%in 16S and 2.4% inCOI) and low distances have also been reported between otherAtelopusspecies in Panama (Lewis et al., 2019; Table 1). Other morphologically similar species,which extend, at least partly, to the Darién/Chocó border area between Panama and Colombia (A.limosus,A.spurrelli), are genetically much more distinct (Table 1).
Bioacoustic signals are still poorly studied inAtelopus. Four call types (pulsed calls, pure tone calls, pulsed short calls, and pure tone short calls) are currently recognized within the genus (Lötters et al., 2019). The advertisement call pattern of Atelopus fronterizo sp. nov. resembles that of other Atelopusspecies from Panama in having a pulsed call and pulsed short call (Cocroft et al., 1990; Ibáñez et al., 1995; Jaslow, 1979;Lewis et al., 2019, this study). Pure tone calls and pure tone short calls have not yet been recorded forAtelopus fronterizosp. nov., but the former is present inA.chiriquiensis(Jaslow,1979) and the latter is present inA.zeteki(Cocroft et al.,1990, Lotters et al., 2019). Calls byAtelopus fronterizosp.nov. remain well differentiated from those of otherAtelopusspecies in eastern Panama after temperature correction(Supplementary Table S6; Supplementary Figure S6).
The observedAtelopus fronterizosp. nov. populations were not affected by the chytrid fungus. Likewise, Flechas et al.(2017a) did not findB. dendrobatidisin swab samples from wild populations in Capurganá, Colombia. Although the current situation may be different due to the rapid spread of the pathogen in Panama over the last two decades (Crawford et al., 2010; Lewis et al., 2019; Lips et al., 2006), there is evidence that some species can, at least partially, resistB.dendrobatidiswith the help of anti-B. dendrobatidisactivity from symbiotic skin bacteria. Such effects have been shown for skin bacteria inAtelopus fronterizosp. nov. (Flechas et al.,2017a).
With the immediate need for protection of the vulnerable genusAtelopus(La Marca, 2005; Ramírez et al., 2020; Zippel et al., 2006), we appeal for an urgent conservation plan forAtelopus fronterizosp. nov., including captive breeding programs and subsequent establishment of a founder population (Lewis et al., 2019). Conservation efforts forAtelopus fronterizosp. nov. may serve to protect biodiversity in the borderland jungle and help to secure the future of this and other yet to be recognized species in the Darién Gap.
Nomenclatural acts registration
The electronic version of this article in portable document format represents a published work according to the International Commission on Zoological Nomenclature (ICZN),and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone (see Articles 8.5-8.6 of the Code). This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information can be viewed through any standard web browser by appending the LSID to the prefixhttp://zoobank.org/.
Publication LSID: urn:lsid: zoobank.org:pub:06959E87-EE29-44BD-AA0A-43BE0CCE0183
Atelopus fronterizoLSID:
urn:lsid: zoobank.org:act:0DCD680B-2D5B-4EFF-8831-F6F0 7A0B7F21
SClENTlFlC FlELD SURVEY PERMlSSlON lNFORMATlON
Collection permits 2011 (SC/A-37-11) and 2012 (SC/A-93 633-12) and exportation permits 2012 (SC/A-33-12) and 2013(SEX/A-7-13) were provided by UNARGEN-Ministerio de Ambiente, Panama.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETlNG lNTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRlBUTlONS
M.V. and A.B. designed the study. Both authors equally participated in all aspects of the study, including fieldwork,morphometry, and molecular genetics, and wrote the manuscript. A.B. performed bioacoustic analysis. M.V.finalized molecular genetic outputs (trees and haplotype networks) and submitted sequences to GenBank. M.V. and A.B. revised the manuscript. Both authors read and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
We thank Yorlis Cáceres, Daniel Cáceres, Isaac Pizarro,Gustavo Dogirama, Mario Cuñapa, Anselmo Caicedo, Hugo Martínez, Elacio Méndez, Gilberto Torres, and the people from Bajo Pequeño for field assistance. Michal Motyka and Dominik Kusý kindly helped with molecular data processing; Madian Miranda helped with photography and creating the appendix figures of preserved specimens from the American Museum of Natural History (AMNH). David Kizirian kindly allowed revision of the specimens at the AMNH. We also thank Dennis Voeten,Taylor Cooper, and Joseph Mendelson III for language assistance.

- Zoological Research的其它文章
- Species bias and spillover effects in scientific research on Carnivora in China
- Particulate matter exposure exacerbates susceptibility to SARS-CoV-2 infection in humanized ACE2 mice
- Potential aquatic environmental risks of trifloxystrobin:Enhancement of virus susceptibility in zebrafish through initiation of autophagy
- Northern pig-tailed macaques (Macaca leonina)infected with SARS-CoV-2 show rapid viral clearance and persistent immune response
- A review of the Cypriniform tribe Yunnanilini Prokofiev,2010 from China, with an emphasis on five genera based on morphologies and complete mitochondrial genomes of some species
- Molecular mechanisms of intermuscular bone development in fish: a review

