Cataract-causing allele in CRYAA (Y118D) proceeds through endoplasmic reticulum stress in mouse model
Zhe-Kun Jia, Chen-Xi Fu, Ai-Ling Wang, Ke Yao, Xiang-Jun Chen1,,*
1 Eye Center of the Second Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310009, China
2 Institute of Translational Medicine, Zhejiang University School of Medicine, Hangzhou, Zhejiang 310020, China
ABSTRACT As small heat shock proteins, α-crystallins function as molecular chaperones and inhibit the misfolding and aggregation of β/γ-crystallins. Genetic mutations of CRYAA are associated with protein aggregation and cataract occurrence. One possible process underlying cataract formation is that endoplasmic reticulum stress (ERS) induces the unfolded protein response (UPR), leading to apoptosis. However, the pathogenic mechanism related to this remains unexplored. Here, we successfully constructed a cataract-causing CRYAA (Y118D) mutant mouse model, in which the lenses of the CRYAA-Y118D mutant mice showed severe posterior rupture,abnormal morphological changes, and aberrant arrangement of crystallin fibers. Histological analysis was consistent with the clinical pathological characteristics. We also explored the pathogenic factors involved in cataract development through transcriptome analysis. In addition, based on key pathway analysis, up-regulated genes in CRYAAY118D mutant mice were implicated in the ERS-UPR pathway. This study showed that prolonged activation of the UPR pathway and severe stress response can cause proteotoxic and ERS-induced cell death in CRYAA-Y118D mutant mice.
Keywords: Cataract; αA-crystallin; Unfolded protein response; Endoplasmic reticulum stress
INTRODUCTION
The eye lens is a key refractive medium composed of capsule and crystallin fibers (Banh et al., 2006). The unique shortrange and orderly arrangement of highly concentrated crystallins in the eye lens is important for lens transparency(Delaye et al., 1983). Cataracts, which are the leading cause of blindness worldwide, are produced by protein misfolding and aggregation (Lee et al., 2017). Genetic mutation and environmental stress can affect the protein-folding process,resulting in protein misfolding and, in turn, disruption of lens protein stability, solubility, and interactions (Fu et al., 2021; Li et al., 2020; Xu et al., 2021; Yang et al., 2020b). Crystallins are the most important structural proteins in the lens and contain two superfamilies: i.e., α-crystallins and β/γ-crystallins.Poor crystallin stability results in protein aggregation and precipitation, leading to lens opacity (Moreau et al., 2012).Therefore, exploring the potential pathogenic mechanisms associated with cataracts and the regulatory pathways involved in protein homeostasis in the lens are important for the development of new cataract prevention and treatment strategies.
The small heat shock protein, α-crystallins, which consist of αA- and αB-crystallin, function as molecular chaperones to inhibit the misfolding and aggregation of β/γ-crystallins(Roskamp et al., 2019). In addition, α-crystallin also protects the lens cells against apoptosis (Ahsan et al., 2021). Previous studies have reported that cataract pathogenesis is correlated with aberrant changes in the ratio of crystallin subunits and apoptosis of lens epithelial cells (Li et al., 2008). Furthermore,under environmental stress, α-crystallins act as chaperones to maintain protein stability and solubility and thus transparency of the eye lens (Derham, 1999).CRYAAgene mutant mice show the pathological features of cataracts with nuclear opacity (Graw et al., 2001). In addition, congenital cataracts caused byCRYAAmutation exhibit polymorphisms in different individuals (Yu et al., 2012). Therefore, mouse models with genetic mutation of theCRYAAgene could be applied to explore the pathogenic mechanism underlying cataract formation. Studies have shown that mutations in the β-strand of the conserved αA-crystallin domain (residues 83 to 120)can lead to autosomal dominant cataracts (Huang et al.,2009). Mutation sites in the β-strand have the same physiochemical properties. Furthermore, αA-crystallin position 118 Tyr (Y118) deletion in humans is known to lead to cataracts (Sun et al., 2011). Thus, we studied the effects of αA-crystallin on cataract pathogenesis using a Y118D mouse model.
Endoplasmic reticulum stress (ERS) caused by the excessive accumulation of misfolded proteins plays an important role in cataracts (Zhou et al., 2020) and can trigger the unfolded protein response (UPR) (Ikesugi et al., 2006). In mammals, the UPR pathway is mediated by three ER proteins: i.e., IRE1, PERK, and ATF6 (Bartoszewska et al.,2020). The UPR can alleviate ERS by mediating different signaling pathways at both the transcriptional and translational levels (Uddin et al., 2020). There are two main protein degradation pathways in cells: i.e., autophagy lysosome pathway (ALP) and ubiquitin proteasome system (UPS) (Al Mamun et al., 2020). In addition, pathogenic factors of cataracts can activate the ERS-UPR pathway. Under high levels of ERS, however, the UPR pathway cannot eliminate misfolded proteins, thereby resulting in cellular apoptosis (Lee et al., 2015). Excessive accumulation of unfolded or misfolded proteins can cause ERS-UPR, reactive oxygen species(ROS), and cellular apoptosis in epithelial cells of the lens,ultimately resulting in cataract development (Yang et al.,2015).
αA-crystallin plays a chaperone role in the eye lens and regulates intracellular protein homeostasis by modulating the ERS-UPR pathway (Andley et al., 2016). In this study, we successfully constructed a cataract-causing CRYAA (Y118D)mutant-related mouse model to explore the role of the ERSUPR pathway and other potential pathogenic mechanisms in cataracts. This study should help enrich our understanding of cataract pathogenesis and clarifies the involvement of ERS in the occurrence and development of cataracts by affecting the biological processes of epithelial cells in the lens. Our results suggest that ERS may be a potential biological target for the treatment of cataracts, providing a theoretical and practical basis for the development of new therapeutic drugs and treatments, which has important clinical significance.
MATERIALS AND METHODS
Cataract mouse model
Mouse care and breeding were conducted following animal experimental protocols approved by the Institutional Animal Care and Use Committee (IACUC) of the Laboratory Animal Center, Zhejiang University. Global CRYAA-mutant(αAY118D/Y118D) mice were constructed at GemPharmatech Co.,Ltd. (China) using CRISPR/Cas9 recombination to modifyCRYAA(Shao et al., 2018). The αAY118D/+mutant mice were intercrossed to produce homozygous mice. Genomic DNA was extracted from tails or toes for polymerase chain reaction(PCR) amplification and sequencing to identify mouse genotypes. Phenotypic analysis was performed on homozygous male mice and their lenses were harvested for genome-wide analysis. Mice were humanely sacrificed by carbon dioxide asphyxiation or cervical dislocation. Eyes were harvested and fixed in 4% paraformaldehyde/phosphatebuffered saline (PBS) for paraffin embedding and sectioning or placed in PBS for lens dissection.
Slit-lamp examination
A slit lamp was used to observe lens turbidity and evaluate cataract phenotype. A mixture of 10% phenylephrine hydrochloride and 1% topicaine (Santen Pharmaceutical,China) was used to dilate the pupils. Left eyes of the mice were examined and photographed by an ophthalmologist.Evaluation was performed based on the grading standard of the Ophthalmology Laboratory of Oxford University (Andley,2009).
Histological lens analysis
Fixed eyes were washed several times with PBS. Histological examination was then performed sequentially using the following steps: dehydration-transparency-wax immersionembedding. Trimmed wax blocks were sectioned, and the cornea and retina were simultaneously cut out in a direction parallel to the optic nerve. Sections (3 μm) were then stained using hematoxylin and eosin (H&E).
Lens RNA preparation and transcriptome analysis
Total RNA was extracted from lenses of αAY118D/Y118Dand αA+/+mice using Trizol reagent (#15596018, Invitrogen, USA).Two sets of RNA from six lenses were quantified using spectrophotometry. Samples with an RNA integrity number(RIN)>6.5 were selected for transcriptome sequencing according to the requirements of Novogene (China).Transcriptome data analysis was performed as follows: FPKM(fragments per kilobase per million mapped reads) values of each sample were used for normalization, and then log2 values were calculated using the ratio of the expression levels of the treatment and control groups. Gene Ontology (GO)(http://geneontology.org/) and protein-protein interaction (PPI)analyses were performed for differentially expressed genes(DEGs) with an expression difference fold-change≥2.Expression levels of ERS-UPR marker genes were determined through quantitative PCR (qPCR) using an Applied BiosystemsTMPowerUpTMSYBRTMGreen Master Mix(#A25742, Thermo, USA). Gene expression levels were normalized by β-actin.
Immunoblotting
Lens extracts were centrifuged into water-soluble and -insoluble fractions at 4 °C and 12 000 r/min for 30 min and immunoblotting was performed. Polyclonal antibodies against total α-crystallin (#sc-28 306, Santa Cruz, USA) and the ERSUPR signaling pathway (ATF4, #11815S, CST; GRP78,#3177S, CST; p62, #66184, Protein Tech,USA) were used as primary antibodies. Anti-mouse/rabbit IgG (H+L) antibodies were used as secondary antibodies for an additional hour at room temperature. Image J software was used to analyze the band gray level for calculation.
Data analysis
Data were presented as mean±standard deviation (SD) in graphs generated by GraphPad Prism 8. Statistical analysis was performed by Student’s two-tailedt-test, while one-way analysis of variance (ANOVA) was used when more than two groups were compared. Values ofP<0.05 were considered statistically significant.
RESULTS
Cataract phenotype in αAY118D/Y118D mouse lens
Mutations in the β-strand of the conserved domain of αAcrystallin (residues 83 to 120) can cause autosomal-dominant cataracts (Hejtmancik, 2008). In addition, the αA-Y118del mutation is correlated with congenital cataracts (Sun et al.,2011). As such, we analyzed amino acid sequence conservation between positions 83 to 120 in αA-crystallin. The αA-crystallin sequences of multiple species were retrieved from the UniProt database (http://www.uniprot.org/) and MEGA (http://www.megasoftware.net/) was used for protein sequence conservation analysis. Amino acids at positions 83 to 120 in the αA-crystallin sequence showed significantly high evolutionary conservation among the examined mammals(Figure 1A).
Previous reports have demonstrated that αA-Y118D mutant mice exhibit a cataract phenotype (Xia et al., 2006). Therefore,we explored the pathogenesis of human cataracts using the αA-Y118D mutant mouse model. Based on CRISPR/Cas9 technology, we constructed a αAY118D/Y118Dmutant mouse model (Figure 1B). Specifically, theCRYAAgene was first amplified using primers and sequenced to confirm successful development of the CRYAA-Y118D mutant model (Figure 1C).Mice with knock-in mutations survived and reproduced normally. Intercrossing of αAY118D/+mice produced αAY118D/Y118Dhomozygous offspring and wild-type (WT) mice,while a control group was also established without changes in the lens phenotype. Lenses were observed and analyzed using a slit lamp to determine the effects of αAY118D/Y118D. The anterior segment images in Figure 1D confirmed lens opacity in αAY118D/Y118Dmice compared with WT mice. Lenses harvested from two-month-old αAY118D/Y118Dmice showed completely opaque nuclear cataracts, including cortical fibers,whereas lenses from WT mice showed no abnormalities.These findings indicate that the Y118D mutation in theCRYAAgene can affect the correct folding of crystallins,resulting in nuclear cataracts in mice.
αA-Y118D mutation affects lens histology
To explore the mechanism underlying cataract formation, we analyzed histological changes during lens development in mice with the αA-Y118D protein mutation. Histological changes in the lenses of two-week-old WT and αAY118D/Y118Dmice are shown in Figure 2A. Analysis of the lens cortex in αAY118D/Y118Dmice showed that the lens matrix was arranged in layers, with disordered fiber lamellar arrangement, irregularly shaped anterior fiber cells, and enlarged intercellular spaces.In the lens nucleus, fibers were twisted and damaged,showing a honeycomb structure. Furthermore, excessive proliferation and irregular distribution of epithelia were observed in the equator of the lens, and uniform fiber disintegration and calcium deposition occurred. Histological analysis of WT lenses in the control group showed that the outer membrane was intact, epithelium was neatly arranged,and cell morphology was consistent. Morphology of the lens fiber layer was complete and tightly arranged with normal homogeneity, and the various layers of the lens were closely connected.
Detection of αA-crystallin soluble proteins showed that changes in the lens were partly caused by protein insolubility(Figure 2B). Lenses from WT and αA-Y118D mutant mice were centrifuged. Changes in the ratio of water-soluble protein and precipitate were determined using the supernatant and precipitate of the cortex and nucleus. Results showed that,compared with WT mice, the proportion of precipitate content in total lens protein content was significantly higher in mutant mice.
As a ubiquitin-binding protein encoded bySQSTM1, p62 mediates various biological functions, including intracellular protein renewal (McManus et al., 2012). In some neurodegenerative diseases, abnormal protein aggregates and inclusion bodies formed in cells are often accompanied by p62 (Ichimura et al., 2010). Here, compared with the extremely low expression of p62 found in the WT lenses, the αA-Y118D mutant mouse lenses, especially in the nuclear region, showed significantly increased expression of p62(Figure 2C). Consistent with the phenotypic results, protein insolubility was proportional to the formation of nuclear cataracts. These findings imply that abnormal expression of the αA-crystallin mutant in the lens can cause protein aggregation, thus affecting lens development and protein homeostasis.
Activation of UPR in lens of αA-Y118D mutant mice
Increased insoluble protein content in the mutant mouse lens indicated that aggregated protein was highly expressed and accumulated in the lens. The UPR is a signal transduction mechanism in cells for regulation of misfolded proteins(Malhotra et al., 2007). Activation of the UPR increases the protein-folding ability of cells, however, the apoptosis pathway is activated when cell integrity cannot be restored (Kim et al.,2006). Volcano plots, which are enhanced scatter plots,contain two important boundaries. The first one is significance,whereby theP-value of two groups is obtained by a difference test, and the negative logarithm log10 (P-value) is taken as the ordinate. The second one is abscissa, which displays log2 fold-changes. Thus, based on the obtained volcano plots and screening criteria (fold-change>2,P≤0.05), significant DEGs can be identified for subsequent research. In the current study, transcriptome sequencing and analysis of the lenses identified 825 significant DEGs (>2-fold-change,P≤0.05),including 337 up-regulated genes and 488 down-regulated genes (Figure 3A). Interestingly, GO analysis indicated that the DEGs in the CRYAA-Y118D lens were significantly enriched in two biological processes, i.e., “response to unfolded protein” and “intrinsic apoptotic signaling pathway in response to ERS” (Figure 3B). These findings indicate that the CRYAA-Y118D mutation caused protein structure disorder.Furthermore, the UPR was significantly up-regulated in the lens, resulting in cell apoptosis. Hub genes play important roles in biological processes and regulation of other genes in related pathways. We used the Cytoscape and STRING databases to analyze PPIs with a combined score >0.4.Through the calculation of network structure and weighted connections between nodes using the cytoHubba plug-in algorithm, we extracted the Top 10 key genes (highest degree in the software) and constructed a network graph according to network topology. The ATF4 branch in the UPR signaling pathway was a key gene in the PPI network (Figure 3C). In addition, the expression levels of 127 genes in the KEGG ERS-related pathways were determined, with 12 genes found to be differentially expressed. Up-regulated UPR genes included Trib3>Chac1>Chop>Atf3>Atf4>Cebpb, and downregulated genes included Hspa1b>Hspa1a>Edem3>Sec31b>kLman1l>Cryaa (Figure 3D, E). The PERK/eIF2α/ATF4/Ddit3 signal transduction pathway is a well-studied pathway in ERS(Ma et al., 2016). The UPR caused by continuous highintensity ERS activates the apoptosis pathway through the apoptosis mediator, Ddit3 (Rozpedek et al., 2016).Transcriptome analysis showed that the expression levels of ATF4 and Ddit3 are significantly up-regulated in the cataractous mouse lens compared with levels in the normal group, thus implying ERS response activation. Furthermore,Y118D mutation caused a significant down-regulation in the expression of heat shock proteins, such as Hspa1a and Hspa1b. Heat shock proteins are used as molecular chaperones to promote correct folding of proteins. These findings imply that ERS promotes the apoptosis of lens epithelial cells and the formation of cataracts.
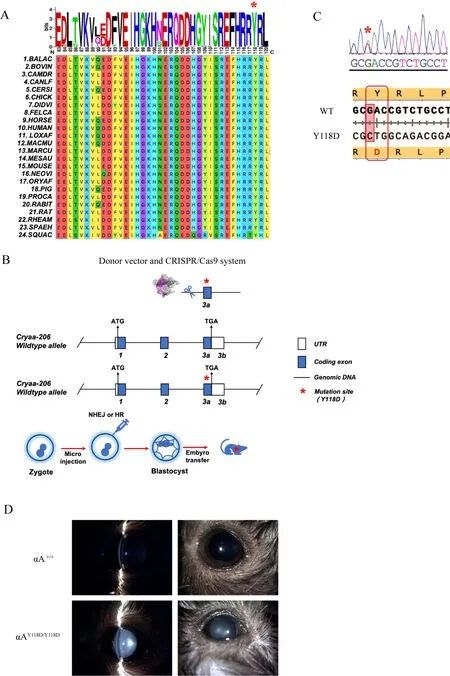
Figure 1 Construction of αAY118D/Y118D mice and evaluation of cataract phenotype

Figure 2 Detection of αAY118D/Y118D mouse lens properties

Figure 3 Bioinformatics analysis of αAY118D/Y118D mouse lens transcriptome
αA-crystallin mutation affects ER function
Transcriptome analysis showed that progressive ERS during lens development in αA-Y118D mutant mice triggers significant transcriptional activation of UPR genes. UPRrelated signaling pathways were further explored through western blotting and real-time qPCR. Results showed that the expression levels of ATF4, GRP78, and p62 increased in the lenses of the αA-Y118D mutant mice (Figure 4A), indicating that gene mutations caused aggregation of crystallins and activation of the ERS signaling pathway. Consistent with the transcriptome analysis, the qPCR results showed that protein aggregation stress in the cataractous lenses activated the PERK-ATF4-Ddit3 pathway. ATF4 and Ddit3 were highly expressed and showed significant differential expression in the nucleus of the cataractous lens compared with levels in WT mice (Figure 4B, C). Although the expression levels of ATF6 and IRE1 in the other two UPR signaling pathways were significantly up-regulated, western blotting indicated that the proteins in the two pathways were not significantly differentially expressed (Supplementary Figure S1).
In summary, the opacity of the crystallins caused by highintensity stress was correlated with the activation and high expression of factors implicated in the ERS PERK-ATF4-Ddit3 pathway.
DISCUSSION
Congenital cataracts are often caused by genetic mutations,resulting in substantial damage to the structure and function of mutant crystallins. One of the most widely studied crystallins is γS-crystallin, which combines properties of β- and γcrystallins. Mutations in γS-crystallin are associated with congenital and progressive juvenile cataracts (Bari et al.,2019b). Compared with WT, mutants exhibit higher aggregation and lower stability (Bari et al., 2019a).Furthermore, denatured γS-crystallins are usually bound by αcrystallin.
αA-crystallin is a member of the small heat shock protein family and plays a molecular chaperone function. αA-crystallin maintains the functions and transparency of the eye lens. A large number ofin vitroexperiments have shown that αAcrystallin can delay the aggregation of thermally or chemically denatured targets (Koteiche et al., 2015). αA-crystallin also plays an important role in lens development and transparency.The R49C mutant of αA-crystallin drives aggregation of γDcrystallin (Wu et al., 2018). Thus, αA-crystallin is an important target in the development of cataract drugs. Makley et al.(2015) reported that 25-dehydrocholesterol can reverse cataracts in mice by targeting αA-crystallin. Mutations in the βstrand of conserved αA-crystallin (residues 83 to 120)domains are known to cause autosomal dominant cataracts.Known mutations in this region include G98R, R116C, R116R,R117H, Y118del, and R119H (Hejtmancik, 2008; Sun et al.,2011; Zhuang et al., 2019). These mutations and the mouse Y118D mutation share common physicochemical properties and are implicated in the mechanism underlying cataract formation (Huang et al., 2009). Therefore, we explored a feasibility study on the pathogenesis of human cataract disease using the αA-Y118D mutation mouse model.
Previous studies have reported that the αA-Y118D mutation impairs the function of lens proteins (Xia et al., 2006).Increases in water-insoluble proteins in the lens can cause a cloudy appearance and the occurrence of dominant nuclear cataracts (Andley et al., 2016; Cheng et al., 2010). Previous studies have reported that αA-crystallin mutations can lead to the appearance of the cataract phenotype and an increase in the expression of certain factors in the ERS-UPR signaling pathway (Frankfater et al., 2020). However, systematic study on ER homeostasis is lacking. In this article, we analyzed ER homeostasis by chip data to identify the possible signaling pathway of αA-Y118D. Mutation of CRYAA-Y118D caused a down-regulation in the overall expression of molecular chaperone proteins, which promoted the misfolding of unfolded proteins. These misfolded proteins were not effectively restored or degraded, resulting in protein aggregation. As αA-crystallin plays a key role in protecting lens transparency, drugs developed to target its key sites may prevent cataract occurrence and development.In vitrostudies have shown that αA-crystallin and chaperone peptides inhibit cell apoptosis caused by oxidative damage (Chothe et al.,2010). Exogenous αA-crystallin and cell-penetrating peptides introduced into cells show similar function to that of endogenous αA-crystallin (Christopher et al., 2014). Moreover,intramuscular injection of exogenous αA-crystallin in mice significantly inhibits the occurrence and development of cataracts (Nahomi et al., 2013). Thus, αA-crystallin is a promising target in the prevention of cataracts; however, its clinical application requires further exploration.
In this study, CRYAA-Y118D mice were established to explore the pathological changes in ERS in hereditary cataracts. The UPR signaling pathway is associated with the pathogenesis of protein aggregation diseases, including Alzheimer’s disease, Parkinson’s disease, and cataracts(Martinez et al., 2019). Here, DEG pathway analysis of the αAY118D lens showed UPR signaling pathway activation, ER protein processing, and pro-apoptotic activation. UPR genes related to ERS include Chac1 and Ddit3, which exert effects through PERK pathway activation and subsequent cell death(Galluzzi et al., 2012). These studies imply that the expression and accumulation of αA-Y118D mutants can trigger high-level ERS and UPR activation, leading to fibroblast death and cataract formation. Several studies have also reported that apoptosis is essential in cataract formation and progression(Tian et al., 2020; Yang et al., 2020a). Our findings showed that significant apoptosis occurred after induction of the ERS response in the αA-Y118D lens (Supplementary Figure S2).Thus, ERS may promote cataract occurrence by inducing cell apoptosis.
ATF4is an important transcription target of the UPR under continuous ERS, providing a key link between ERS and apoptosis (Fusakio et al., 2016).Ddit3encodes a proapoptotic factor. In addition,Chac1is highly expressed in the lens and is a new apoptotic gene of the UPR, playing a downstream role in ATF4-ATF3-Ddit3 (Pendergrass et al.,2005; Mungrue et al., 2009). Here, Ddit3 expression was significantly up-regulated in the αA-Y118D mutant lens,indicating that cataract occurrence may be correlated with programmed cell death. Pro-apoptotic target genes, includingATF4,ATF3,Cebpb, andTrib3, were significantly upregulated. However, the upstream ER sensorperkwas not significantly up-regulated.Cebpbencodes the bZip transcription factor in response to ERS and participates in regulation of Chac1 transcription together withATF4andATF3(Crawford et al., 2015). KEGG analysis also showed that the p53 and HIF-1 signaling pathways play important functions in cataract formation. Phosphorylation and dephosphorylation of p53 modulate protein stability and transcription factor activity (Inoue et al., 2016). HIF-1α, an important regulator of the response to hypoxia, plays a regulatory role in oxidative stress (Li et al., 2019).

Figure 4 Verification of expression of ERS-UPR-related genes
In summary, we explored the role of αA-crystallinin vivousing the αA-Y118D mutant mouse model. This study provides information on the mechanism underlying congenital cataracts caused by αA-crystallin mutation and the pathogenesis of age-related cataracts. Transcriptome analysis of the αA-Y118D lens showed that continuous activation of the UPR ATF4-Ddit3-Chac1 branch caused cell death in the lens,resulting in cataract development (Figure 5). In addition,several other non-UPR-related genes were differentially expressed. Our findings imply that the development of targeted cataract drugs via the lens UPR should consider stress response pathways.

Figure 5 Unfolded protein response (UPR) signaling pathways
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
Z.K.J. acquired and analyzed the data and drafted the manuscript. Z.K.J., C.X.F., and A.L.W. performed the experiments and revised the manuscript. K.Y. and X.J.C.conceived and designed the study, interpreted and analyzed the data, and revised the manuscript. All authors read and approved the final version of the manuscript.
- Zoological Research的其它文章
- Species bias and spillover effects in scientific research on Carnivora in China
- Particulate matter exposure exacerbates susceptibility to SARS-CoV-2 infection in humanized ACE2 mice
- Potential aquatic environmental risks of trifloxystrobin:Enhancement of virus susceptibility in zebrafish through initiation of autophagy
- Northern pig-tailed macaques (Macaca leonina)infected with SARS-CoV-2 show rapid viral clearance and persistent immune response
- A review of the Cypriniform tribe Yunnanilini Prokofiev,2010 from China, with an emphasis on five genera based on morphologies and complete mitochondrial genomes of some species
- Molecular mechanisms of intermuscular bone development in fish: a review

