Preparation and characterization of Fe2O3/Bi2WO6 composite and photocatalytic degradation mechanism of microcystin-LR
Li-xiao Ni *,Cun-hao Du Han-qi Wu Yan Li Xiang-lan Li Chu Xu
a Key Laboratory of Integrated Regulation and Resource Development on Shallow Lakes,Ministry of Education,Hohai University,Nanjing 210098,China
b College of Environment,Hohai University,Nanjing 210098,China
Abstract The long-standing popularity of semiconductor photocatalysis,due to its great potential in a variety of applications,has resulted in the creation of numerous semiconductor photocatalysts,and it stimulated the development of various characterization methods.In this study,Fe2O3/Bi2WO6 composite with a flower-like microsphere and hierarchical structure was synthesized with the facile hydrothermal-impregnation method without any surfactants.X-ray diffraction(XRD),scanning electron microscopy(SEM),ultraviolet-visible(UV-Vis)diffuse reflectance spectroscopy,and photoluminescence spectroscopy were used to characterize the structures of the samples.The specific surface area was estimated with the Brunauer-Emmett-Teller(BET)method,and pore size distribution was determined using the Barrett-Joyner-Halenda(BJH)method.The synthesized Fe2O3/Bi2WO6 composite had an average diameter of approximately 4 nm,with smaller specific surface area and larger pore diameter than those of pristine Bi2WO6.The results of XRD and SEManalyses confirmed that the composite was composed of Fe2O3 and Bi2WO6.The absorption edge of Bi2WO6 was at a wavelength of 460 nm.By contrast,the absorption edge of Fe2O3/Bi2WO6 to visible light was redshifted to 520 nm,with narrower bandgap width and stronger visible light response.It was also found that the main active substances in the degradation of microcystin-LR(MC-LR)were hydroxyl radicals(·OH)and electron holes(h+).Consequently,the results further showed that the heterojunction between Fe2O3 and Bi2WO6 can improve the charge transfer rate and effectively separate the photoinduced electrons and holes.Compared with Bi2WO6,Fe2O3/Bi2WO6 had no significant difference in the adsorption capacity of MC-LR and had more efficient photocatalytic degradation activity of MC-LR.The degradation rates of MC-LR by Fe2O3/Bi2WO6 and Bi2WO6 reached 80%and 56%,respectively.The degradation efficiency of MC-LR was affected by the initial pH value,initial Fe2O3/Bi2WO6 concentration,and initial MC-LR concentration.
Keywords:Photocatalysis;Composite photocatalyst;Fe2O3/Bi2WO6;Bi2WO6;MC-LR
1.Introduction
Due to water eutrophication,cyanobacteria multiply and gather in large numbers in a short period of time,and the rupture of cyanobacteria cells will release a variety of algal toxins such as microcystins,which are extremely hazardous pollutant compounds.Microcystins are very stable and cannot be removed through ordinary chemical treatments because of the existence of some unique amino acids and their cyclic structures,which contribute to their resistance to thermal and hydrolytic degradation(Cruz et al.,2011;Massey et al.,2018;Pantelic et al.,2013;Umehara et al.,2017).There are more than 90 microcystin variants,of which,microcystin-LR(MC-LR)is generally acknowledged to be the most toxic.It can damage various organs,such as the liver and nervous system.As a result,the World Health Organization(WHO)has set a provisional guidance value of MC-LR in drinking water at 1.0μg/L(WHO,2017).In the past few decades,many approaches to removing MC-LR have been investigated,including semiconductor photocatalytic oxidation,which has attracted significant attention because of its high oxidation activity and environmental friendliness(Pelaez et al.,2013;Schneider et al.,2019;Shephard et al.,2002;Wang et al.,2015).
At present,most of the widely studied semiconductor photocatalysts are n-type semiconductor compounds with wide band gaps,such as CdS,SnO2,TiO2,ZnO,ZnS,PbS,and MoO3.Of these semiconductors,TiO2,CdS,and ZnO have the highest catalytic activity,but CdS and ZnO are prone to photochemical corrosion and produce Cd2+and Zn2+,which are toxic to organisms and harmful to the environment.TiO2photocatalytic oxidation technology has attracted much attention owing to advantages such as its safety of use,stability,low cost,and ability to thoroughly remove pollutants(Liu et al.,2009).However,TiO2can only respond to approximately 3%of ultraviolet light in the sun,and the photocatalytic degradation of MC-LR by TiO2can only be carried out under ultraviolet light.Therefore,TiO2is difficult to popularize and use.In recent years,Bi2WO6,a typical Aurivillius oxide,has been a promising photocatalyst under visible light irradiation.As an n-type semiconductor with nontoxic and strong oxidation properties,Bi2WO6has attracted a lot of attention(Wu et al.,2008;Zhang et al.,2010;Huang et al.,2019).With a forbidden bandwidth of approximately 2.8 eV,Bi2WO6has a layered structure of perovskite WO2-4located between Bi2O2+2layers(Zhang et al.,2011).Up to now,various methods have been developed to prepare Bi2WO6with different morphologies and crystal sizes to obtain different photocatalytic efficiencies(Ge and Liu,2011;Tang et al.,2004;Zhang and Chu,2020).Considerable progress has been achieved in the preparation of Bi2WO6nanoparticles(Xu et al.,2009)and hierarchical superstructures(Tian et al.,2011),and the specific surface area has been greatly increased,thereby improving the photocatalytic performance.In particular,the superstructures have become an important alternative because they not only exhibit the advantages of nanomaterials but also overcome the difficulty of recycling nanoparticles from degradation solution(Zhang et al.,2011).Compared with other superstructures,the flower-like Bi2WO6is more promising owing to the advantages of its large surface area and ability to be easily recycled.
The hydrothermal route is an effective method of controlling the size and morphology of photocatalysts.The visible light response region of pure Bi2WO6is only 460 nm,and the high recombination rate of photogenerated electron-hole pairs also limits the photocatalytic activity.To improve its photocatalytic performance and meet the requirements of environmental applications,several modifications of Bi2WO6have been performed.It has recently been demonstrated that modifying Bi2WO6with metal oxide is an effective means of improving its optical and photocatalytic properties.The drawbacks of single-component photocatalysts can be eliminated by synthesizing a heterogeneous photocatalyst with integrated functional components.A multi-component photocatalyst combines the advantages of single-component photocatalysts(Chen et al.,2016).The different band gap potentials between heterostructures can induce irreversible charge carrier separation and transfer on the interface,thereby reducing the recombination process and resulting in improved photocatalytic activities(Chang et al.,2020;Lu¨et al.,2019).Some MxOy/Bi2WO6heterostructures have been successfully fabricated,such as Ti O2/Bi2WO6(Murcia L´opez et al.,2011)and Ag2O/Bi2WO6(Chen et al.,2015).As an n-type semiconductor with a narrow band gap of 2.2 eV,α-Fe2O3can respond to visible light with a wave length of up to 600 nm(Matmin et al.,2018).The modification of Bi2WO6byα-Fe2O3is a rational strategy of broadening the range of visible light response and prolonging the lifetime of photogenerated carriers.Some researchers have synthesizedα-Fe2O3/Bi2WO6composite material,which exhibits high photocatalytic activities for the degradation of Rhodamine B(RhB)(Guo et al.,2013;Wu et al.,2014),and Fe2O3/Bi2WO6composites,which have demonstrated high photocatalytic efficiency in degrading doxycycline(Azzouzi et al.,2020).However,studies on the degradation of microcystins have not been reported.
In this study,pure Bi2WO6was prepared with the hydrothermal method,and Fe2O3/Bi2WO6composite samples were prepared with the dipping-roasting method.Afterward,the Bi2WO6and Fe2O3/Bi2WO6samples were characterized through X-ray diffraction(XRD),scanning electron microscopy(SEM),ultraviolet-visible diffuse reflectance spectroscopy(UV-Vis DRS),photoluminescence(PL)spectroscopy,specific surface area,and aperture analysis.The adsorption and degradation performance of Fe2O3/Bi2WO6composite photocatalysts for MC-LR under visible light were investigated.The parameters influencing the degradation capacity of Fe2O3/Bi2WO6for MC-LR,including catalyst dose,initial pH value,and initial MC-LR concentration,were investigated.
2.Materials and methods
2.1.Materials
Analytical-grade Bi(NO3)3·5H2O,Na2WO4·2H2O,and Fe(NO3)3·9H2O were purchased from Sinopharm Chemical Reagent Co.,Ltd.A standard sample of MC-LR(250μg,purity≥95%)was purchased from Express Technology Co.,Ltd.and stored at-20°C.
2.2.Preparation of pure Bi2WO6 and Fe2O3/Bi2WO6 composite
All chemicals used in this work were of analytical grade without further purification.In a typical procedure,9.7-g Bi(NO3)3·5H2O was dissolved in 60 mL of deionized water.Subsequently,3.3-g Na2WO4·2H2O was added into the suspension under magnetic stirring.After continuous stirring for 30 min,the mixture was transferred to a 100 mL stainless steel autoclave at 160°C for hydrothermal treatment for 12 h.After natural cooling to room temperature,the products were centrifuged and separated.Precipitates were washed several times with deionized water and dried at 80°C in a vacuum drying oven for 20-30 min.A flower-like Bi2WO6sample was obtained and is referred to here as S1.Fe2O3/Bi2WO6heterojunction catalysts were synthesized by depositing Fe2O3nanoparticles on the surface of Bi2WO6in situ.0.1 g of flowerlike Bi2WO6powder was put into an evaporating dish,in which 5 mL of Fe(NO3)3·9H2O stock solution(1.8 mmol/L)containing 0.1%of Fe element were added.The mixture was stirred and evaporated to dryness at 70°C,and the collected residue in powder form was impregnated and roasted for 1 h at 200°C.Fe2O3/Bi2WO6catalyst with 0.1%weight ratio of Fe/Bi2WO6was obtained,and the sample is referred to here as S2.With some modifications,these procedures were modified based on the work of Wu et al.(2014).
2.3.Characterization of Fe2O3/Bi2WO6
According to the XRD data,the crystallite size was calculated using the Scherrer equation.The crystalline phase of the prepared samples was characterized using an X-ray diffractometer(XRD-6100,Shimadzu,Japan,with operating parameters of 40 kV and 100 mA)with Cu-Kα1(with a wavelength of 1.540 6Å)radiation.The scanning angle(2θ)was in a range from 10°to 70°with a step size of 0.02°,and the scanning speed was 5°/min.The sample morphology was observed with SEM(JSM-5610LV).UV-Vis DRS was used to investigate the optical properties and band gaps of the samples.The reflectance spectra of the samples were recorded in the range of 200-800 nm on a Shimadzu UV-3600 UV-Vis spectrometer with BaSO4as the reference material.Specific surface area,pore volume,and pore size distribution of the powder were determined with a Brunauer-Emmett-Teller(BET)surface area analyzer(Nova 2000 series,Quantachrome Instruments,UK).The PL measurements were carried out on a fluorescence spectrophotometer(F-7000,Shimadzu,Japan)at room temperature.
2.4.Adsorption and photocatalytic degradation of MC-LR by Fe2O3/Bi2WO6
0.05-g S1 and S2 samples were added to tubes(10 mL),which were filled with 5 mL MC-LR test solution(2 mg/L),and pH was adjusted to approximately 3.7 to make a comparison between pure Bi2WO6and Fe2O3/Bi2WO6.These tubes were placed in a jacketed beaker,which was connected with cooling water and cooled with fans to prevent solution evaporation.The tubes were covered with a UV block filter(UV420).As a visible light source,a 500-W Xe lamp(GXZ500)was placed 20 cm above the beaker.The suspensions were vigorously stirred in darkness for 60 min in order to assess the adsorption performance.
The photocatalytic experiments were conducted under identical conditions.After the suspensions were vigorously stirred in darkness to establish an adsorption-desorption equilibrium,visible light irradiation was turned on.At 10-min intervals from 0 to 60 min,0.5 mL of reaction solution was taken and centrifuged at 10 000 rpm for 15 min.The supernatants were filtered through a Millipore filter(0.45μm),and the filtrates were analyzed using a high-performance liquid chromatograph(Agilent 1260,USA)equipped with a photodiode array detector operated at 238 nm for the quantification of MC-LR.An Agilent Octadecylsilane(ODS)C18 column(250 mm×4.6 mm,5μm)was used as a stationary phase,and a mixture of 0.05%trifluoroacetic acid(TFA)in water and TFA acetonitrile(with a volume ratio of 85:15)was used as the mobile phase.The applied flow rate was 1 mL/min with an injection volume of 20μL,and column temperature was set to 30°C.
2.5.Mechanism of photocatalytic degradation of MC-LR by Fe2O3/Bi2WO6
Radical species trapping experiments were conducted to investigate the photocatalytic reaction mechanism of various species(Chen et al.,2014;Jiang et al.,2015).Isopropanol(IPA)was introduced as the quencher of hydroxyl radicals(·OH),potassium iodide(KI)acted as the scavenger of h+and·OH,and benzoquinone(BQ)was used to ascertain the·O-2.The dosages of all these scavengers were 0.1 mol/L.
The formed·OH was detected with the PL technique(Cao et al.,2012).Terephthalic acid,which readily reacts with·OH to produce a highly fluorescent product,2-hydroxyterephthalic acid,was used as a probe molecule to detect·OH.The fluorescence intensity at wavelength of 425 nm,which could be ascribed to the characteristic of 2-hydroxyterephthalic acid,was proportional to the amount of·OH formed in the solution.The evaluation process was similar to that of the photodegradation experiment,except that the MC-LR suspension was replaced by the alkaline terephthalic acid solution.The concentration of terephthalic acid was set at 0.5 mmol/L in a diluted NaOH solution(2 mmol/L).Samples were collected periodically,centrifuged,and filtered through a 0.22-μm filter(PTFE Hydrophilic,Millipore,USA).The filtrate was then measured on a Hitachi F-4500 fluorescence spectrophotometer with an excitation wavelength of 315 nm.
2.6.Effects of different factors on MC-LR degradation by Fe2O3/Bi2WO6
The experiments also investigated the influence of factors such as the initial pH,initial Fe2O3/Bi2WO6dose,and initial MC-LR concentration on the degradation performance of MCLR.The 50-mg/L MC-LR stock solution was diluted with distilled water to obtain the concentration gradient(1,2,4,and 8 mg/L).The initial pH values of MC-LR solutions were 1.8,3.7,5.6,9.7,and 11.5,and were adjusted by 1-mol/L NaOH and 1-mol/L HNO3solutions.0.05-g Fe2O3/Bi2WO6catalyst samples were added to 5 mL MC-LR test solutions(2 mg/L).The initial Fe2O3/Bi2WO6doses were 0,0.025,0.05,0.075,and 0.1 g,respectively.The pH was adjusted to be 5.8.Four groups of solutions with initial MC-LR concentrations of 1,2,4,and 8 mg/L were added to 0.05 g of Fe2O3/Bi2WO6to adjust the pH values to 3.7.The influence of each factor on the MC-LR degradation was calculated using the pseudo-firstorder kinetic model:

where C0and Ctare the concentrations of reactant at initial time and time t,respectively;and k1is the pseudo-first-order rate constant,which was obtained from experimental data through linear regression.
3.Results and discussion
3.1.Characterization of produced samples
As shown in Fig.1,the XRD patterns of the prepared S1 and S2 samples were characterized.The results show that the S1 samples agreed with the orthorhombic phase of Bi2WO6(Joint Committee on Powder Diffraction Standards(JCPDS)No.73-1126),and all the diffraction peaks at 2θvalues of 28.3°,32.9°,47.1°,55.9°,and 58.6°corresponded to the index values of(113),(200),(220),(313),and(226),respectively.The peaks were sharp with no impurity peaks,indicating that the samples showed good crystallization and high purity.The characteristic peaks corresponding to orthorhombic Bi2WO6were observed in the diffraction patterns of sample S2.This indicated that the modification of Fe2O3did not affect the crystal structure of Bi2WO6.In addition,the intensities of the diffraction peaks at 2θof 23.2°and 35.6°were relatively weak,and could be used as index values of the orthogonal phase of Fe2O3(JCPDS No.33-0064),corresponding to the index values of(012)and(110)(Zeng et al.,2010).The possible reason for the almost undetectable Fe2O3diffraction characteristics in S2 samples was that Fe2O3was weakly crystallized at low annealing temperatures,and the small amount of Fe2O3was beyond the detection limit of XRD measurement(Wu et al.,2014).
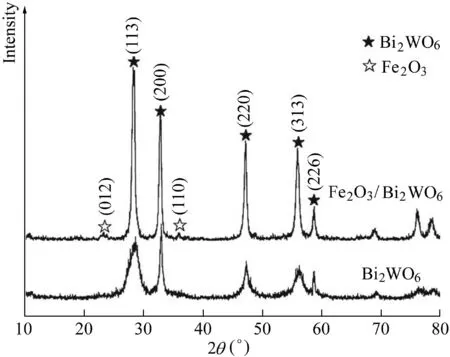
Fig.1.XRD patterns of pristine Bi2WO6 and Fe2O3/Bi2WO6 composite.
SEM was used to observe the as-prepared samples to identify the morphology of S1 and S2 samples.Fig.2(a)shows the scanned images of Bi2WO6samples,and Fig.2(b)shows the scanned images of Fe2O3/Bi2WO6samples.Fig.2(a)shows that Bi2WO6has flower-like microspheres with an average diameter of 4.0μm.The microsphere was assembled from smooth nanoplates through a self-assembly and Ostwald ripening process.Therefore,Bi2WO6can be used as a transmission path for small molecules due to its large number of pores in the overlapping nanosheets.It benefited the reactant and product molecules by moving into or out of the material and causing the chemical reactions to occur easily(Wang et al.,2013).Fig.2(b)demonstrates that the composite retained the hierarchical structure,and the low-temperature calcination treatment had no significant effect on the overall morphology of the flower-like superstructure.Several protruding islands were sparsely deposited onto the surface of nanosheets,and these islands may be Fe2O3nanoparticles resulting from the impregnation of Bi2WO6in Fe(NO3)3·9H2O solution followed by thermal treatment.
Fig.3(a)and(b)shows the nitrogen adsorption-desorption isotherms at standard temperature and pressure(STP)and their corresponding pore size distribution curves for S1 and S2 samples,respectively.All samples had type IV isotherms(Brunauer-Deming-Deming-Teller(BDDT)classification)with a hysteresis loop at high relative pressures,which is the characteristic isotherm of mesoporous materials.The specific surface areas of S1 and S2 samples were 9.052 and 7.363 m2/g,respectively.The pores of samples ensued from the aggregation of neighboring Bi2WO6nanoplates and the stack of adjacent Bi2WO6layers.The pore size distribution of S1 and S2 samples was obtained through the Barrett-Joyner-Halenda (BJH)method for desorption branch.As shown in Fig.3(b),the average pore diameter D of S1 samples was 17.807 nm,and the pore volume V was 0.040 cm3/g.The average pore diameter D of S2 samples was 29.959 nm,and the pore volume was 0.055 cm3/g.This result indicates that the surface and porous parameters of pristine Bi2WO6changed after heat treatment and Fe2O3deposition.Compared with pure Bi2WO6,the specific surface area of Fe2O3/Bi2WO6decreased,and average pore size and pore volume increased.This may be attributed to the fusion of neighboring Bi2WO6plates,resulting in smaller pores becoming lager pores.The mesopores of S1 and S2 samples were both centralized at 3.29 nm,and there was a weak distribution of macropores up to 100 nm.To accelerate diffusion of active species in the photocatalytic process,it is favorable to have many pores with different diameters,which can serve as transport paths for small molecules(Shang et al.,2009).
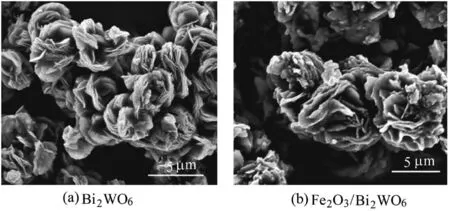
Fig.2.SEM images of Bi2WO6 and Fe2O3/Bi2WO6 samples.
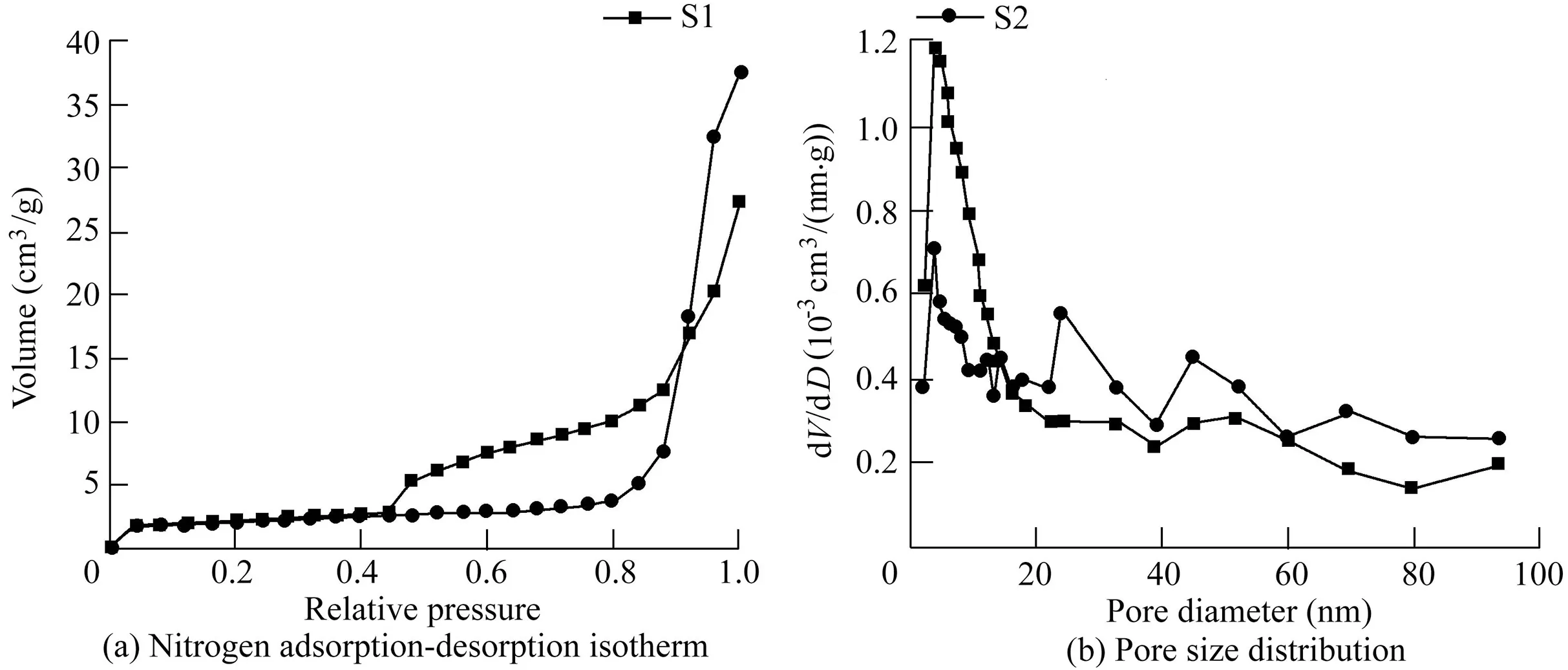
Fig.3.Nitrogen adsorption-desorption isotherms and pore size distribution curves of pristine Bi2WO6 and Fe2O3/Bi2WO6 composite.
Fig.4(a)shows the UV-Vis DRS of the prepared S1 and S2 samples.According to the spectra,the pristine Bi2WO6presented a photo-absorption region in the range of ultraviolet to visible light shorter than 460 nm,whereas the optical absorption of Fe2O3/Bi2WO6composite displayed a clear redshift in the visible light region.Loading Fe2O3on Bi2WO6(with a weight ratio of Fe2O3to Bi2WO6of 0.1%)enlarged the applied range of visible light to 520 nm and improved the performance of light absorption.This can be ascribed to the synergistic photo-sensitization of Bi2WO6and Fe2O3.Light absorption of the composite sample was enhanced,and more electron-hole pairs were generated under the same visible light irradiation,resulting in higher photocatalytic activity.The optical band gap could be calculated from the absorption spectra as follows:whereα,h,v,B,and Egare the absorbance,Planck constant,light frequency,physical constants of related materials,and band gap energy value,respectively;and n is a coefficient related to semiconductor type(n=1/2 in this study).(αhv)1/2and hv were adopted as the ordinate and abscissa,respectively,and the linear section was fitted to obtain the linear equation.As shown in Fig.4(b),Egvalues for S1 and S2 samples were estimated to be approximately 2.72 eVand 2.57 eV,respectively.

To further prove that S2 samples improved the separation efficiency of electron-hole pairs,the PL spectra of the hierarchical structure of Fe2O3/Bi2WO6composite and pristine Bi2WO6were measured at the excitation wavelength of 320 nm.Fig.5 shows that S1 and S2 samples had similar spectra in the emission wavelength range of 375-550 nm,indicating that PL spectra of Fe2O3/Bi2WO6samples mainly depended on the Bi2WO6body.Compared with pure Bi2WO6,the photoluminescence intensity of Fe2O3/Bi2WO6composite was significantly reduced.A weaker intensity in the peak represented a lower recombination probability of free charges.The Fe2O3crystal could effectively inhibit the recombination of photogenerated charge carriers between the hybrid orbitals of Bi 6s and O 2p to the empty W 5d orbital,thereby improving the separation efficiency of electron-hole pairs(Guo et al.,2013).
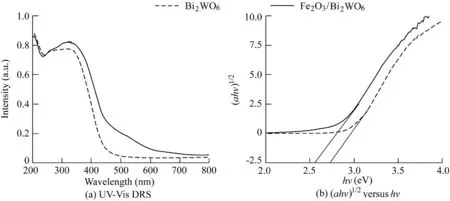
Fig.4.UV-Vis DRS of Bi2WO6 and Fe2O3/Bi2WO6 composite and relationship between(αhν)1/2 and photon energy(a.u.means arbitrary units).
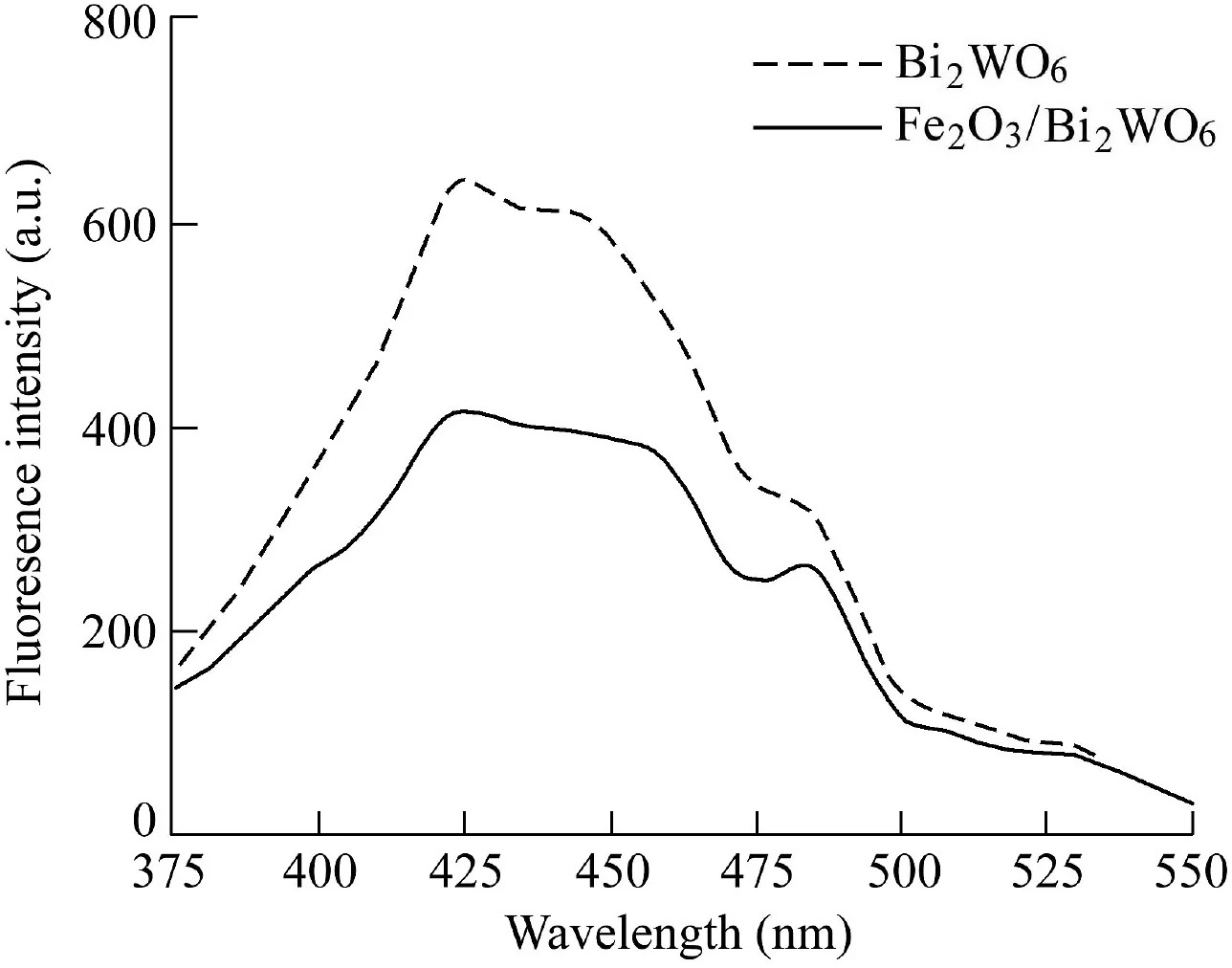
Fig.5.PL spectra of Bi2WO6 and Fe2O3/Bi2WO6 excited at 320 nm.
3.2.Investigation of adsorption and photocatalytic activity of MC-LR with Fe2O3/Bi2WO6
S1 and S2 samples were evaluated for their adsorption ability.The MC-LR concentration variation and adsorption efficiency for S1 and S2 samples in darkness for 60 min are presented in Fig.6(a)and(b).Both of them reached adsorption-desorption equilibrium in 30 min,and they had similar removal rates of MC-LR(approximately 20%and 19.8%,respectively)after 60 min.This indicates that Bi2WO6had a strong adsorption capacity.According to the aforementioned characterization(XRD patterns and SEManalysis),the modification of Fe2O3on Bi2WO6did not clearly affect the overall morphology of the flower-like superstructure.Therefore,the adsorption performance of Fe2O3/Bi2WO6was similar to that of Bi2WO6.Fig.6(c)displays the fitted pseudosecond-order adsorption kinetic curves of S1 and S2 samples based on kinetic data.The pseudo-second-order adsorption kinetic equation is as follows:

where qtis the adsorption amount at time t(mg/g),qeis the adsorption amount when adsorption-desorption is at equilibrium(mg/g),and k2is the pseudo-second-order kinetic rate constant(g/(mg·min)).According to the fitted kinetic equations shown in Fig.6(c),the rate constants of Bi2WO6and Fe2O3/Bi2WO6were estimated to be 8.43 g/(mg·min)and 15.06 g/(mg·min),respectively,and the equilibrium adsorption amounts for Bi2WO6and Fe2O3/Bi2WO6were 0.042 mg/g and 0.041 mg/g,respectively.
The results mentioned above indicated that although the adsorption rate of Fe2O3/Bi2WO6composite was higher than that of Bi2WO6,the overall adsorption capacity was similar to that of Bi2WO6.In general,a strong degree of absorptivity of catalyst could contaminate molecules on the catalyst surface.However,excessive adsorption also caused the active sites to be occupied,diminishing the photocatalytic performance.The addition of Fe2O3ensured that Fe2O3/Bi2WO6composite utilized the adsorption ability of the flower-like Bi2WO6microsphere and did not inhibit the separation and transfer of photogenerated carriers.Thus,the catalytic performance of the composite was enhanced.
As shown in Fig.7(a),S2 samples displayed a higher removal rate of MC-LR(nearly 80%)in 60 min than S1 samples(56%)under visible light.The Langmuir-Hinshelwood model was used to fit the experimental data,and the quasifirst-order linear relationship of the plots of ln(C0/Ct)versus time is shown in Fig.7(c).According to the fitted equations in Fig.7(c),the experimental data were well fitted,with correlation coefficients(R2)of 0.988 and 0.992,respectively.The calculated rate constants for MC-LR degradation by S1 and S2 samples under visible light for 60 min were 0.013 7 and 0.026 min-1,respectively.The photocatalytic reaction rate of Fe2O3/Bi2WO6was 1.9 times that of pure Bi2WO6.This indicated that the addition of Fe2O3improved the photocatalytic performance of Bi2WO6.
3.3.Mechanism of photocatalytic degradation of MC-LR by Fe2O3/Bi2WO6
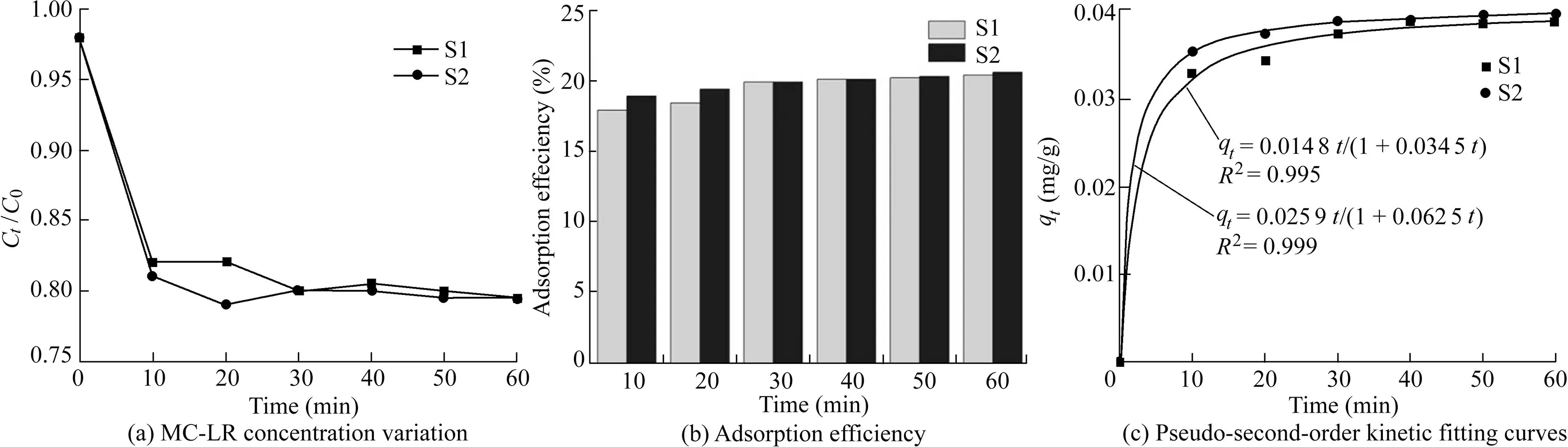
Fig.6.Characteristics of S1 and S2 adsorbing MC-LR in darkness.
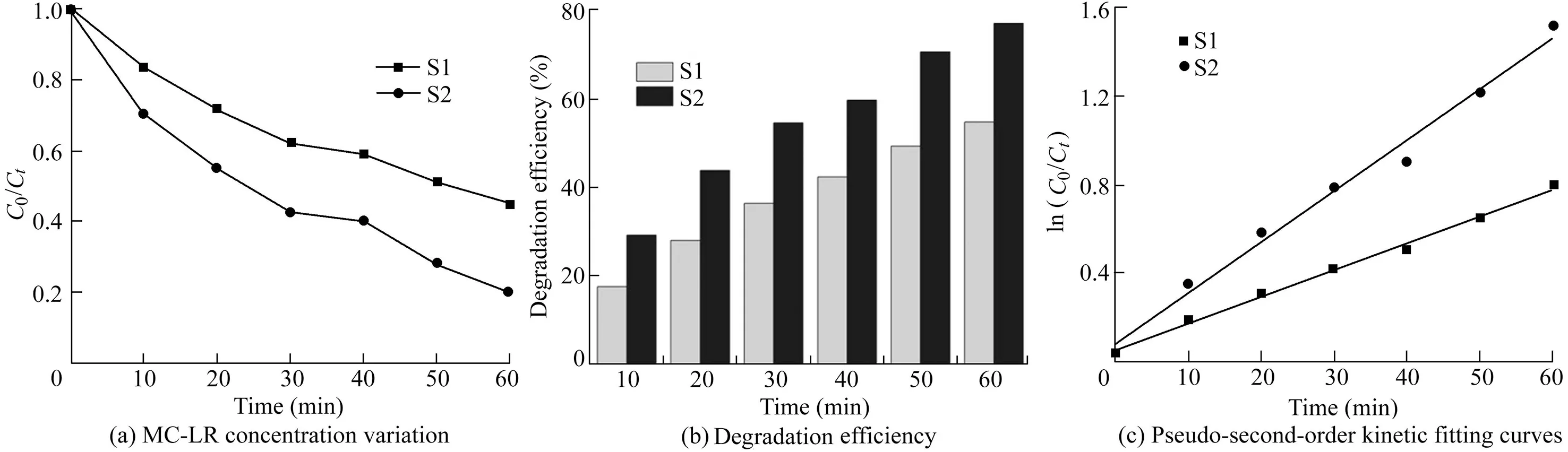
Fig.7.Characteristics of S1 and S2 degrading MC-LR under visible light.
To find the main active species for photocatalytic degradation of MC-LR,IPA was used as a scavenger of·OH radicals,KI was used as a scavenger of h+and·OH radicals,and BQ was used as a scavenger of·O-2radicals in the photoreaction system.The degradation rates of MC-LR in the blank group,·O2-radicals group,·OH radicals group,and h+and·OH radicals group were 80.1%,78.3%,37.5%,and 9.8%,respectively.It was found that adding BQ to the system did not cause any major changes.This indicated that the free·O-2radicals were not the main active species in the Fe2O3/Bi2WO6photocatalytic reaction.When KI and IPA were added,the reaction was strongly inhibited,indicating that the main active species were·OH and h+.
Although the results implied that the·OH may be involved in the degradation of MC-LR by Fe2O3/Bi2WO6,the real ability of S2 samples to generate·OH was still unclear.Therefore,the PL technique was used to detect·OH formed on the surface of S2 samples.The fluorescence intensity of S2 samples under visible light irradiation was observed in a basic terephthalic acid(TA)solution.Fig.8 shows that the PL intensity around 425 nm was proportional to the time of exposure to light irradiation by S2,indicating the continual production of·OH.·OH may be derived from the transformation of H2O2and the direct reaction between water and photogenerated holes.
It is well known that photocatalytic activity is governed by various factors,such as surface area,phase structure,interfacial charge transfer,and separation efficiency of photoinduced electrons and holes(Zhu et al.,2007).Composite semiconductor photocatalysts can be considered one of the most promising methods of improving charge separations,to increase the lifetime of charge carriers,and to enhance the efficiency of interfacial charge transfers to adsorbed substrate in a photocatalytic reaction(Bessekhouad et al.,2004).

Fig.8.PL spectra of·OH trapping in TA solution under visible light irradiation with Fe2O3/Bi2WO6.
The adsorption activities of Fe2O3/Bi2WO6and Bi2WO6were similar.Therefore,the enhanced photocatalytic activity of Fe2O3/Bi2WO6may be primarily attributed to the broadened adsorption in the visible light region and the effective inhibition of charge recombination.To further understand the mechanism of the enhanced photocatalytic activity of the Fe2O3/Bi2WO6heterojunction,the relative band positions of the two semiconductors were investigated.The conduction band edge of a semiconductor(ECB)at pH with a zero-point charge(pHzpc)was calculated as follows:

where E0is the energy of free electrons on the hydrogen scale(-4.5 eV),and X is the absolute electronegativity of a semiconductor(eV).The X value of Bi2WO6was calculated to be 6.197 eV,and the band gap energy of Bi2WO6was 2.7 eV(Wu et al.,2014).According to Eq.(4),the ECBof Bi2WO6was calculated to be 0.347 eV,which was lower than that of Fe2O3.Fig.9 shows the calculated energy band diagram for Fe2O3and Bi2WO6,demonstrating that the positions of the conduction band(CB)and valence band(VB)for Bi2WO6were both more anodic than those of Fe2O3.Therefore,with the band gap potential difference,the interface of Fe2O3/Bi2WO6underwent an irreversible carrier transfer(Col´on et al.,2010).Both Fe2O3and Bi2WO6were easily excited in order to generate photoinduced electrons and holes under visible light irradiation.Because of the existence of the internal electric field,some electrons in the CB of Fe2O3was quickly injected into that of Bi2WO6.There was greater potential energy when electrons jumped from VBs to CBs and left holes in their respective VBs.Simultaneously,holes on the VB of Bi2WO6could migrate to that of Fe2O3with lower potential energy.Hence,the charge transfer process strengthened the separation of photogenerated electrons and holes,and electrons and holes were captured by O2,H2O,and other substances adsorbed on the surface of Fe2O3/Bi2WO6composite to generate H2O2and·OH.Holes and·OH are strong oxidizing agents,which can degrade MC-LR into small molecules.The possible photocatalysis mechanism of Fe2O3/Bi2WO6catalyst is as follows:
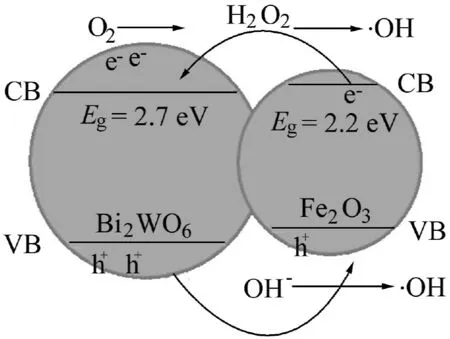
Fig.9.Schematic diagram of band structure and charge migration process of Fe2O3/Bi2WO6.
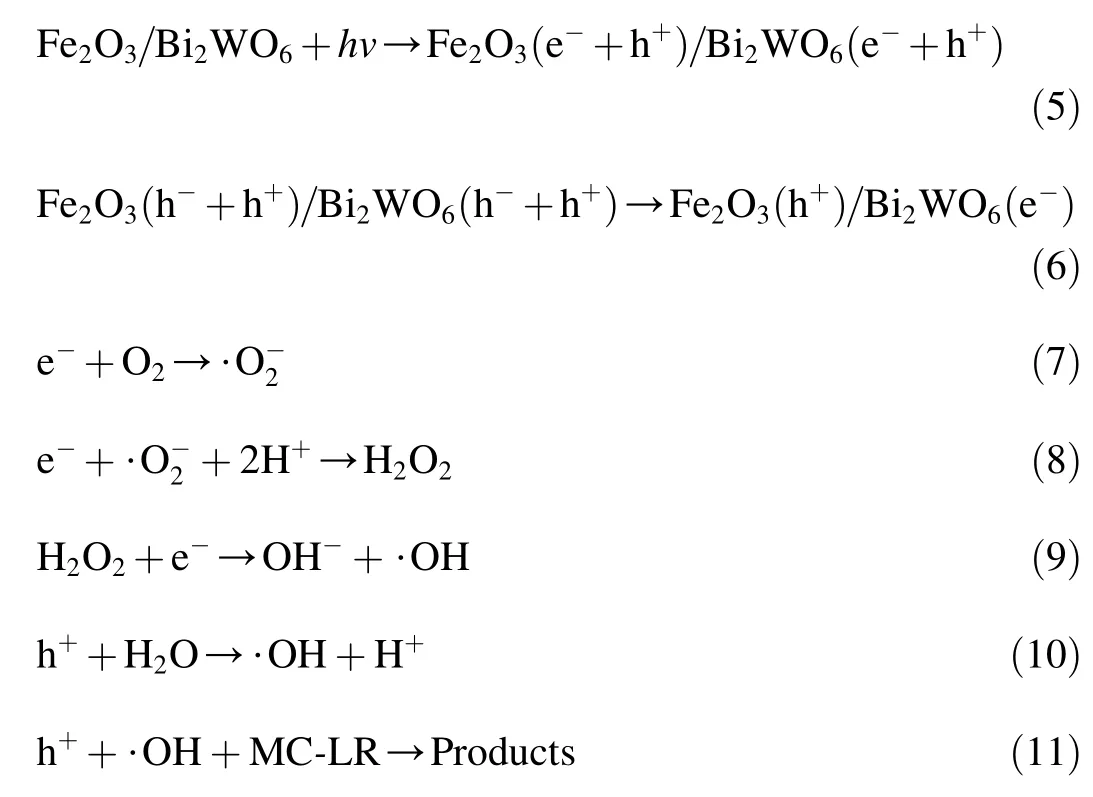
3.4.Effect of different factors on MC-LR degradation by Fe2O3/Bi2WO6
3.4.1.Initial pH
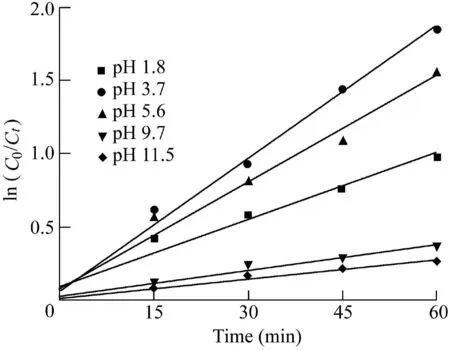
Fig.10.Pseudo-first-order kinetic curves of MC-LR degradation under initial pH values of 1.8,3.7,5.6,9.7,and 11.5.
Fig.10 shows the pseudo-first-order kinetic curves for the degradation of MC-LR by Fe2O3/Bi2WO6under various initial pH values.The rate constant values for the photocatalytic degradation of MC-LR were 0.015,0.030,0.024,0.006,and 0.004 min-1with pH values of 1.8,3.7,5.6,9.7,and 11.5,respectively.As pH decreased from 11.5 to 3.7,k increased from 0.004 min-1to 0.030 min-1.However,when pH further decreased to 1.8,k did not rise but decreased to 0.015 min-1.This result reveals that photocatalytic degradation rates were higher under acidic conditions than under neutral and alkaline conditions.pH had effects on the hydrophobicity of MC-LR.In an acidic environment,MC-LR was more likely to be adsorbed on the catalyst surface from the bulk of aqueous solvents because of its higher hydrophobicity.With the increase in pH,the surface hydrophobicity of MC-LR decreased,and most of the MC-LR was dispersed in the solution,leading to a lower degradation rate.On the other hand,the pH of solution affected the surface charge,band edge positions,and the particle size of the catalyst.
The uncharged pH on the oxide surface is defined as pHzpc(Malato et al.,2009).When the pH is lower than pHzpc,the catalyst surface is positively charged and presents electrostatic attraction towards negatively charged species.The pHzpcof as-prepared Fe2O3/Bi2WO6was prepared with the mass titration method(Xu et al.,2009).The corresponding pH value became constant at approximately 4.5.For MC-LR,it was positively charged below a pH value of 2.1 and negatively charged above this point(Lawton et al.,2003).Accordingly,the degradation rate of MC-LR was higher at a pH value of 3.7.This may be due to the electrostatic attraction between the negatively charged molecules of MC-LR and the positively charged catalyst surface.At a pH value of 1.8,the attractive force between the catalyst and MC-LR was expected to be weak because both MC-LR and the catalyst were positively charged.At higher pH values(9.7 and 11.5),the hydrophobicity of MC-LR was lower,and fewer MC-LR molecules participated in the photocatalytic reaction on the surface of Fe2O3/Bi2WO6.
3.4.2.Catalyst dosage
Fig.11 shows the kinetic curves for the degradation of MCLR under various dosages of Fe2O3/Bi2WO6when the pH was adjusted to 3.7.The control experiment showed that the MCLR removal in the reaction solution without Fe2O3/Bi2WO6was almost negligible under visible light irradiation.The photocatalytic degradation rate constants of MC-LR were 0.016,0.023,0.008,and 0.007 min-1with catalyst dosages of 0.025,0.05,0.075,and 0.10 g,respectively.These results indicated that when the catalyst dosage reached the optimal value,the degradation rate of MC-LR was no longer enhanced with increasing dosage.Under the experimental conditions in this study,the optimal amount of Fe2O3/Bi2WO6was 0.05 g.The active sites provided by the catalyst were raised as the amount of catalyst increased.However,turbidity also increased with catalyst loading,which reduced the penetration intensity of light due to the scattering effect(Wang et al.,2013).Meanwhile,excessive catalyst application also induced the aggregation of catalyst,decreasing the total active surface area available for absorbing organics and light radiation(Zhou et al.,2010).In this study,the reaction rate decreased from 0.023 min-1to 0.007 min-1when the Fe2O3/Bi2WO6dosage increased from 0.05 g to 0.10 g.This may be explained by the absorption of photon energy when it reached saturation at a certain value of catalyst dosage.The excessive catalyst caused the light scattering effect,affecting the light transmittance of the solution(Zanjanchi et al.,2010).In addition,the aggregation of catalyst resulted in fewer active sites available for MC-LR.
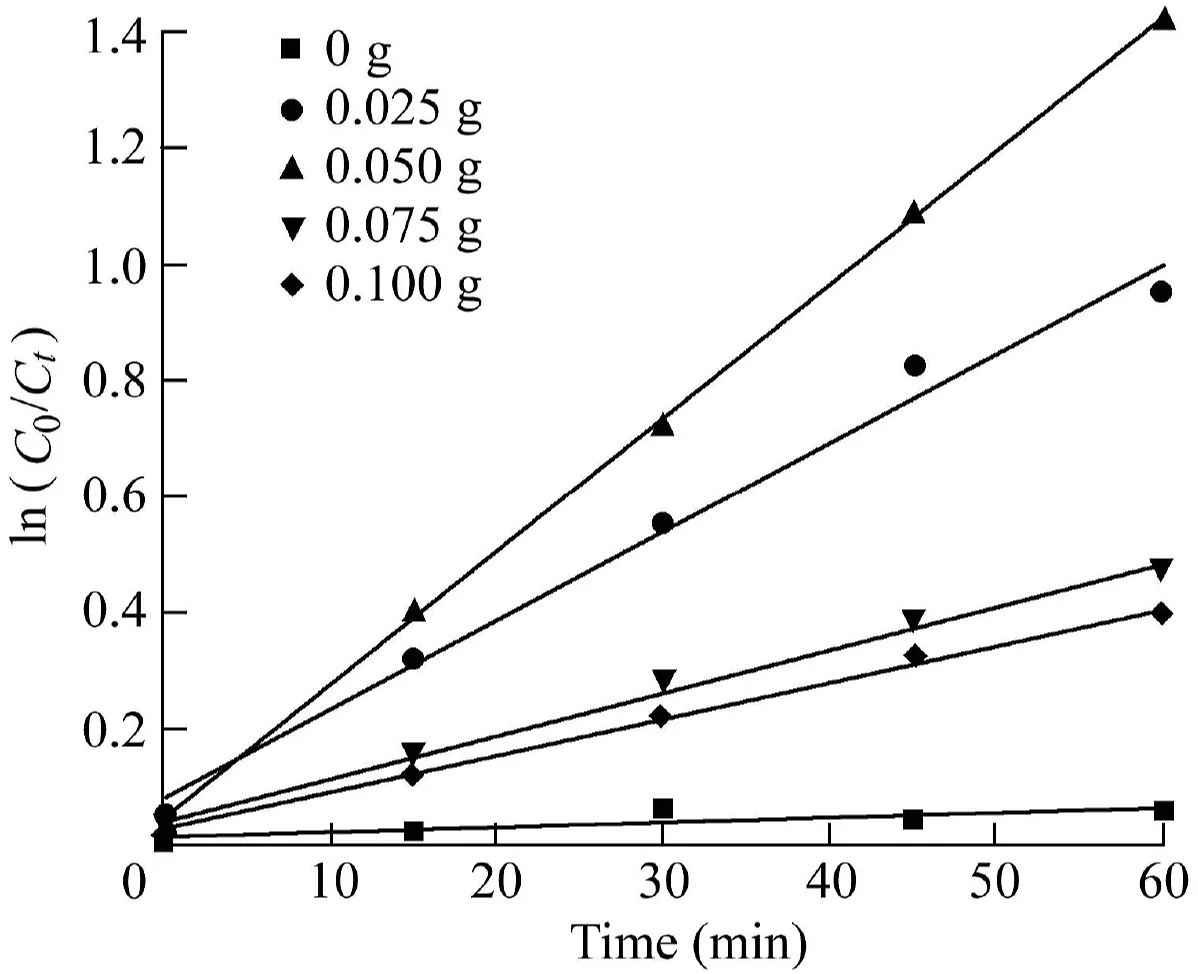
Fig.11.Pseudo-first-order kinetic curves of MC-LR degradation under different Fe2O3/Bi2WO6 dosages of 0,0.025,0.05,0.075,and 0.1 g.
3.4.3.MC-LR concentration
Fig.12 shows the degradation rate at different initial MCLR concentrations in the case of a Fe2O3/Bi2WO6dosage of 0.05 g and a pH value of 3.7.The figure indicates that the initial MC-LR concentration in the reaction solution had a significant effect on the degradation rate.The MC-LR photocatalytic degradation presented a pseudo-first-order reaction with rate constants of 0.031,0.023,0.013,and 0.007 min-1for the initial MC-LR concentrations of 1,2,4,and 8 mg/L,respectively.Moreover,the removal efficiency after a 60-min reaction was found to decrease from 85.56%to 36.46%when the initial MC-LR concentration increased from 1 mg/L to 8 mg/L.These results implied that the degradation rate decreased with increasing MC-LR concentration.The reason may be that the number of h+pairs generated by the Fe2O3/Bi2WO6catalyst in the solution was constant under a certain catalyst dosage and pH value,and the amount of oxidized MCLR generated by the reaction of active material with MC-LR was also constant per unit time.With the increase of MCLR concentration,the proportion of oxidized MC-LR in the total MC-LR decreased.
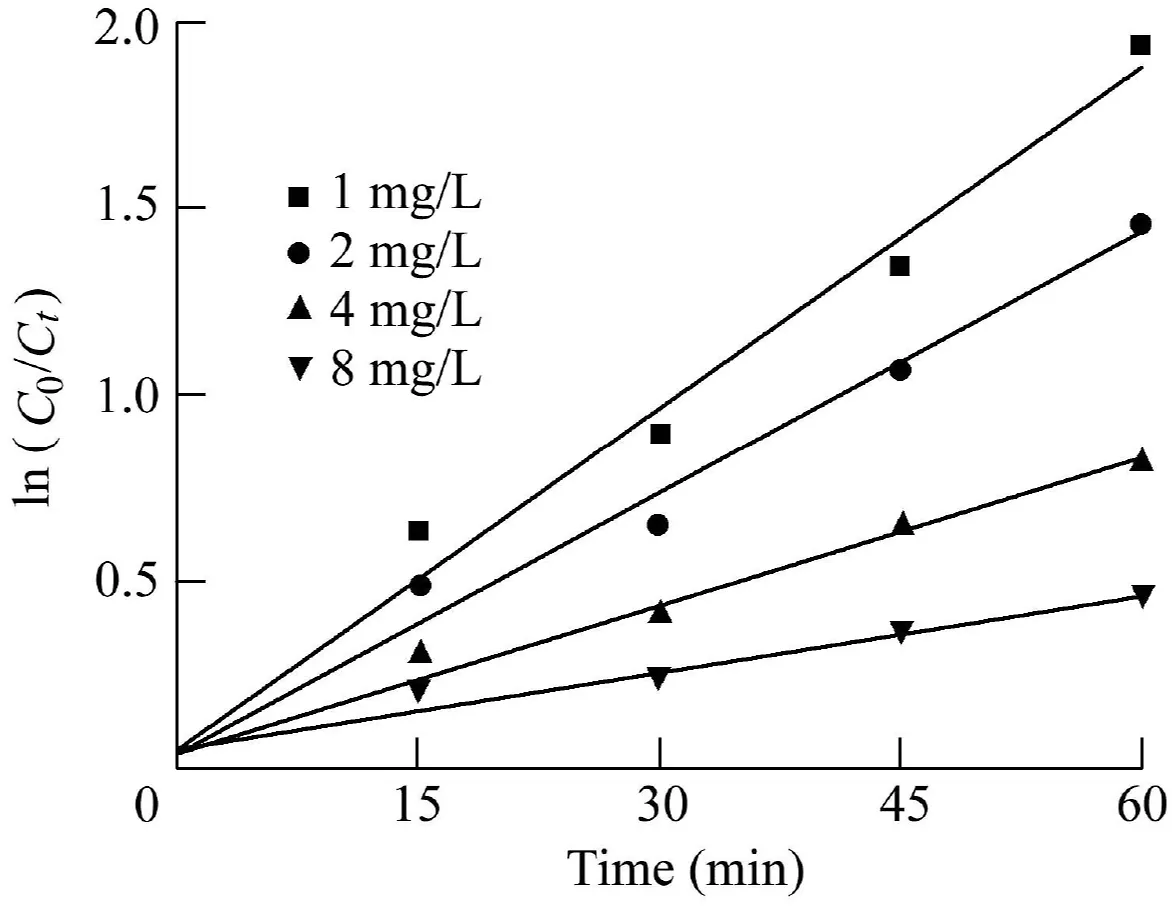
Fig.12.Pseudo-first-order kinetic curves of MC-LR degradation under initial MC-LR concentrations of 1,2,4,and 8 mg/L.
4.Conclusions
Bi2WO6and Fe2O3/Bi2WO6samples were characterized,and their adsorption and degradation of MC-LR under light as well as the factors affecting their ability to degrade MC-LR were investigated.Compared with Bi2WO6,the flower-like Fe2O3/Bi2WO6samples have a smaller specific surface area and larger pore size.The adsorption ability of Fe2O3/Bi2WO6was not significantly different from that of Bi2WO6.However,the reaction rate constant of Fe2O3/Bi2WO6composite samples was approximately 1.9 times that of pure Bi2WO6.This indicates that the addition of Fe2O3is conducive to improving the photocatalytic performance of Bi2WO6.Based on spectral analysis and theoretical calculation,the enhanced photocatalytic performance of Fe2O3/Bi2WO6composite was primarily attributed to the broadening of absorption in the visible light region and the effective inhibition of photogenerated electrons and hole recombination.In addition,h+and·OH were the main active species in the photocatalytic process.The degradation rate of MC-LR was mainly influenced by the catalyst dosage,initial pH,and initial MC-LR concentration.the degradation rate of MC-LR was the highest when the pH was 3.7,the Fe2O3/Bi2WO6dosage was 10 g/L,and the MCLR concentration was 1 mg/L.
Declaration of competing interest
The authors declare no conflicts of interest.
 Water Science and Engineering2021年2期
Water Science and Engineering2021年2期
- Water Science and Engineering的其它文章
- Flood disaster monitoring based on Sentinel-1 data:A case study of Sihu Basin and Huaibei Plain,China
- Influence of cascade reservoirs on spatiotemporal variations of hydrogeochemistry in Jinsha River
- Photodegradation of reactive blue 19 dye using magnetic nanophotocatalyst α-Fe2O3/WO3:A comparison study ofα-Fe2O3/WO3 and WO3/NaOH
- Enhancement of removal efficiency of heavy metal ions by polyaniline deposition on electrospun polyacrylonitrile membranes
- Assessment of physicochemical properties of water and their seasonal variation in an urban river in Bangladesh
- Estimation of unloading relaxation depth of Baihetan Arch Dam foundation using long-short term memory network
