Effects of Dust on Male and Female Floral Organs and the Pollination of the Walnut
CHEN Hong, LV Wei, YANG Li, PAN Cun-de
College of Forestry and Horticulture, Xinjiang Agricultural University, Key Laboratory of Forestry Ecology and Industry Technology in Arid Region, Education Department of Xinjiang, Urumqi 830052, PRC
Abstract The Tarim Basin, Xinjiang, China, a region important to the walnut industry, is affected by frequent heavy dust storms. Dust, including micro-particles (diameter <10 μm), covers the surface of the walnut tree, thereby changing the surface-atmosphere interface microenvironment, which, in turn, influences the exterior and interior structure of the tree. Dust storms occur in spring and summer, during the flowering period of walnut, which is the key developmental stage leading to fruit formation. This study investigated the effects of dust on female flowers, male flowers, and the pollination of walnut. The morphological changes in the stigma during pollination were recorded. Stigma receptivity was studied via the benzidine–H2O2 method. Morphological features of the female floral organs and pollen were investigated using scanning electron microscopy. Pollen germination and pollen tube growth were examined by fluorescence microscopy. The results showed that dust had a significant inhibitory effect on male and female flowers, resulting in decreased catkin growth, reduced pollen viability (pollen viability was 20.13%), blocked pollen apertures, a reduced pollen germination rate on the stigma, and increased time needed for pollen tube appearance. Dust also had an inhibitory effect on stigma length and receptivity of female walnut flowers, with the length of the walnut stigma being reduced by 0.25~0.80 mm during the flowering process. In addition, there was decreased stigma mucus, resulting in stigma atrophy, decreased amount of pollen on the stigma, weakened stigma receptivity, and accelerated drying of female flowers. In the Tarim Basin, walnut flowering occurd at the same time as dust storms do, which had a negative impact on the floral organ, flowering and pollination of walnut.
Key words Walnut; Dust fall; In situ pollen germination; Pollen viability; Stigma morphology; Stigma receptivity
1. Introduction
The Tarim Basin in Xinjiang China which is frequently affected by major sandstorms, has an annual average of more than 60 sandstormcaused dusty days. This dusty weather occurs mostly in the spring and summer, especially in the late spring and early summer, which is the walnut growing season. Furthermore, the flowering period of the walnut and the formation of young fruit coincide with the reproductive growth and embryogenesis of the walnut trees.
The Tarim Basin has limited precipitation and the dust there has a high pH and a high salt content, which, together with the limited precipitation, could directly affect the growth and development of crops. Studies have shown that dust can cause varying degrees of damage to plants. Dust can affect light absorption by forming a barrier on leave, blocking the stomata, reducing stomatal conductance, and increasing the transpiration rate. Atmospheric particulate matter with a diameter of less than 2.5 µm (PM 2.5) can enter the interior of the tissue of the leaves through stomata and induce impairment of the cell membrane and the blade structure, resulting in the reduction of chlorophyll content, protein denaturation, and damage to photosynthetic structures. As a consequence, multiple physiological and ecological processes, such as the absorption, transmission, transformation, and allocation of assimilation of light energy by leavesare compromised, which will directly or indirectly affect photosynthesis, respiration, and the transport of nutrients generated, ultimately influencing the yield and quality of fruit trees.
As it is well-known that dust is harmful to plants, few studies have focused on the effects of dust on the floral organs, pollination, and fertilization of fruit trees. For this reason, in this study, we investigated the effects and mechanism by which dust affected the male and female organs of the walnut, as well as the pollination and fertilization process. The results of this study have practical significance for dust control and prevention, as well as the cultivation of walnut trees in this region.
2. Materials and Methods
2.1. Experimental materials
This study was carried out in a walnut orchard in Aksu, Xinjiang, China (N 41°12′53.69′′~41°13′12.26′′, E 79°15′47.24′′~79°16′02.13′′) with uniform 12-yearold walnut trees (Juglans regia
L. “Xinxin 2”). 6 walnut trees were selected, and the effects of dust on the male and female flowers and the process of pollination and fertilization were analyzed by comparative experiments. The experimental trees were treated by being exposed to dust during dusty weather. The control trees were provided with manual dust prevention via bagging of the male and female floral organs. For the controls, to ensure that both ends of the bags were ventilated and to prevent dust from entering the bags, the bottom end of the bag was fixed with a pin and the top was slightly folded.2.2. Morphological observation of the male and female floral organs of walnut trees
From April 13to 20(initial flowering stage to last flowering stage), the morphological features of the treatment and control walnut trees were investigated according to the instructions described in "Chinese Walnut". Specifically, we observed the shape and color change of the female floral organs and measured the size of both the male and the female floral organs. Furthermore, the morphological features of the female floral organs and the pollen were examined under a scanning electron microscope.
2.3. Determination of stigma receptivity of female walnut flowers
During the flowering process, the benzidine–hydrogen peroxide method was used to measure the stigma receptivity of treated and control trees.
2.4. Pollen viability test
Pollen viability for the treated and control trees was tested using the MTT (methylthiazoletrazolium) staining method. Subsequently, pollen was observed under a microscope and three random fields were selected for evaluating pollen viability. There were at least 30 pollen grains in each field.
Pollen viability (%) = (the number of stained pollen grains / total pollen number) × 100%
2.5. In situ pollen germination on stigma
For both treatment and control trees, when stigma lobes had expanded to an angle of 45°, the pollen of ‘Wen 185,’ which was collected from catkins that were bagged before the anther opened, was employed to perform artificial pollination. 10 female flowers were picked and fixed in a FAA fixative solution from 8 h to 240 h after pollination. Then, 0.1% aniline blue staining was used to observe pollen tube germination. Germination percentages and pollen tube length were determined under fluorescence microscopy following a previously described method.
2.6. Data analysis
Student’st
test was used to compare the treated and control walnut trees in terms of the length of their female stigma during the flowering process. Multivariate analysis of variance (MANOVA) was used to determine the morphological differences among walnut pollen.3. Results
3.1. Effects of dust on the morphological features of female floral organs
The flowering of female walnut floral organs can be divided into 6 distinct periods (Table 1), namely, the period before which the stigmas have expanded (Period I), the period when stigma lobes begin to separate (Period II), the period when stigma lobes have expanded to an angle of 15° (Period III), the period when stigma lobes have expanded to an angle of 30° (PeriodⅣ), the period when stigma lobes have expanded to an angle of 45° (PeriodⅤ), and the period when stigma lobes begin to wither (Period Ⅵ). Hereafter, we termed these Period I to PeriodⅥ, respectively. During Period I and Period II, the appearance of the stigma was not affected by dust because the female floral organs had just emerged from the leaf buds, and the stigma lobes were not separated. The surface of the protruding stigma glandular cells was not exposed to dust (Fig. 1: a1~a2 and b1~b2). Further measurement showed that during these two periods, there was no significant difference in the length of the female stigma between the treated and control trees (Table 1) (P
>0.05). From Period III to Period V, the feathery stigmas cracked and the opening angle gradually increased. Compared with the control trees, the stigma length of treated trees was significantly influenced by the dust (P
<0.05), with the protruding stigma glandular cells withered and died prematurely (Fig. 1: a3~a4 and b3~b4). From Period III on, the results of thet
-test showed that there was a significant difference in the length of the female stigmas between the treatment and control trees (Table 1) (P
<0.05), indicating that dust which occurred during the flowering period may have a negative impact on the length of the stigma from Period III to Period V. The dust also decreased stigma receptivity and stigma mucus. When entering Period VI, pollination was completed; therefore, the length of the stigma of treatment and control trees showed no significant difference during this period (Table1) (P
> 0.05) (Fig. 1: a5 and b5).
Table 1 Differences in stigma activity between the control and treated walnut trees
3.2. Effects of dust on stigma receptivity
The benzidine-hydrogen peroxide method wasemployed to measure stigma receptivity from Period I to Period V. The results stated that for both the control trees and the treated trees during Period I and Period II, the stigmas were in a cohesive state, without mucus production and air bubbles, and the stigma color presented no significant change (Fig. 1: a1~a2 and b1~b2). These observations indicated that the dust had no effect on stigma receptivity. From Period III to Period V, the receptivity and mucus secretion of the stigma gradually increased, but the surface of the protruding glandular cells on the stigma of the treated female flowers was streaked with more dust, leading to a slow discoloration of the stigma. There were fewer bubbles generated around the stigma and the bubbles were generated at a slow rate (Fig. 1: a3~a4 and b3~b4). During Period VI, for both the control and treated trees, the stigmas began to wither and secreted almost no mucus due to water loss (Fig. 1: a5 and b5). When the benzidine-hydrogen peroxide method was used for the analysis, with the treated trees, the stigmas produced fewer bubbles at a slow pace and the staining time became longer with poor quality. During Period VI, the stigma receptivity of the control and treated trees were similar, and only some of the stigmas were receptive to pollen.

Fig. 1 Differences in stigma activity between treated trees (a1~a5) and control trees (b1~b5)
A further examination of the stigmas with a scanning electron microscope showed that for the control trees, pollen germination was supported by the large surface area exposed to the air and the strong pollen adhesion capacity of the stigmas. The outline of the surface cell of the female flower stigma of the control plant was clear, the protruding glandular cells on the stigma were clearly visible, and the cell structure was relatively complete and flat. In addition, the stigma cell volume was large and full, the surface area in contact with the air was large, and the stigma had strong adhesion properties, which provided suitable conditions for pollen germination (Fig. 2: A~B). In contrast, in treated trees, the dust covered the surface of the gland-like stigma cells, resulting in cell shrinkage and reduced stigma surface area. Some stigma cells cracked, thereby narrowing the contact area between the pollen and the stigmas, reducing the ability of the pollen to adhere to the stigma. The surface of the stigma shrank significantly, resulting in a reduction in the volume of the stigma. In more serious cases, the surface of the stigma cracked and died due to water loss. The overall regionalization was negatively impacted, resulting in the reduction of the surface area of the stigma that was exposed to air, which reduced the area to which the pollen could adhere. As the amount of dust on the surface of the stigma increased, the negative effects of the dust on the stigma became more severe (Fig. 2: A'~B').

Fig. 2 Differences in micromorphological characters of stigma between control trees (A~B) and treated trees (A'~B')
3.3. Effects of dust on the growth and pollen dissemination of the male floral organs
The length of the catkins was measured from the time the calyxes of male flowers cracked to pollen dissemination (Fig. 3). The results showed that the average catkin length of the test trees was 95.43±2.35 mm, and the average length of the catkins of the control trees was 123.45±5.32 mm (Table 2). The difference between the catkin length of the treatment and the control trees was significant (P
<0.05). The length of the daily catkin growth was also different. On the 2, 8, and 10day, an extremely significant difference on the 2day average growth was found between the treatment and the control trees (P
<0.01), while on the 6day, the difference was only significant (P
<0.05). On the 12day, the inflorescences of the treated trees had already fallen off.
Fig. 3 Difference in average periodic growth of male flowers between control trees and treated trees
Dust was not conducive to the dissemination of pollen by male flowers. Further analysis found that there was an extremely significant difference between the pollen number (per individual flower bud) of the treated and the control trees (P
<0.05) (Table 2). The number of pollen grains (per individual flower bud) of the treated trees was 58.7% that of the control trees and the number of pollen grains was (2.96±0.54)×10.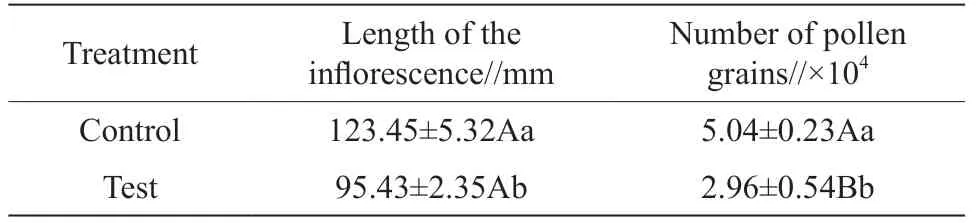
Table 2 Differences of morphological inflorescence between the control and treated walnut trees
3.4. Effects of dust on pollen grains
Walnut pollen viability was estimated by judging the depth of the color using the MTT method. The pollen stained dark pink indicated that it was vigorous, while the pollen stained light pink indicated the opposite. The results of the MTT staining showed that for the control trees, the pollen was stained dark red, and the morphological examination also revealed that the stained pollen grains were plump (Fig. 4: A). In contrast, the pollen grains of the dust treated trees were primarily light red and the walls of some pollen grains were shrunken (Fig. 4: B). There was an extremely significant difference between the control and treated trees in terms of average pollen viability (P
<0.01) (Table 3). In micromorphology, the pollen grains of the control trees showed no obvious difference compared to the pollen grains of the test trees (Table 3). The pollen grains of both were round, with a granulated surface, and had uniform apertures (Fig. 5). Multivariate analysis of the morphological data showed that for the healthy pollen grains of trees in both groups, there were no difference in the polar axis length, equatorial axis, P/E value, long and short axes of the pollen apertures, aperture distance, and ridge width. There was no significant difference between spacing and mesh ridge width (P
>0.05). These results showed that dust had no impact on healthy pollen. However, the examination of the surface of the pollen revealed that compared with the control trees (Fig. 5: A~C), the surface of the pollen of the dust treated trees was covered with a large number of particles, which covered the apertures (Fig. 5: A'~C'), lessening the viability of the pollen.
Table 3 Differences in pollen morphology between the control and treated walnut trees
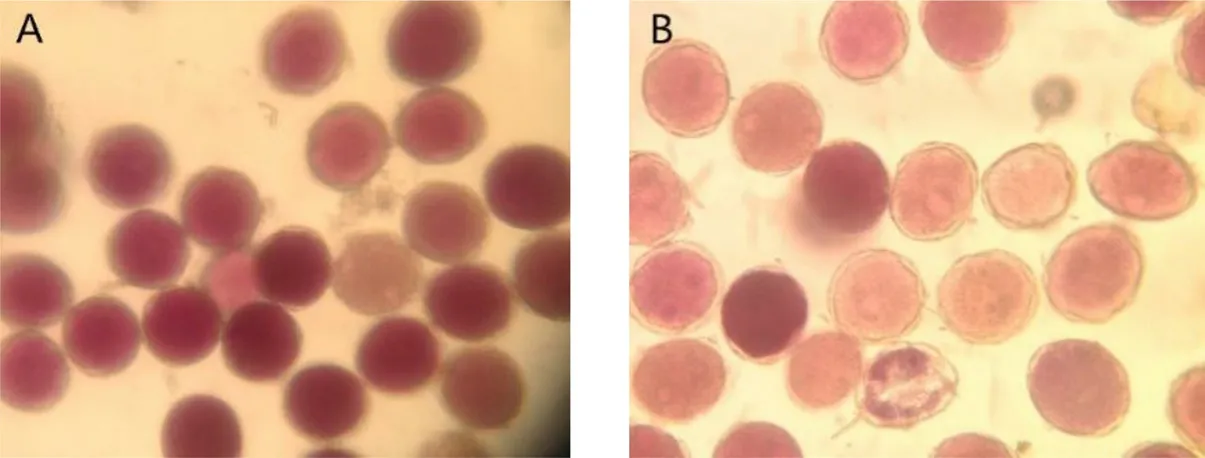
Fig. 4 The difference in pollen viability between the control (A) and treated walnut trees (B)
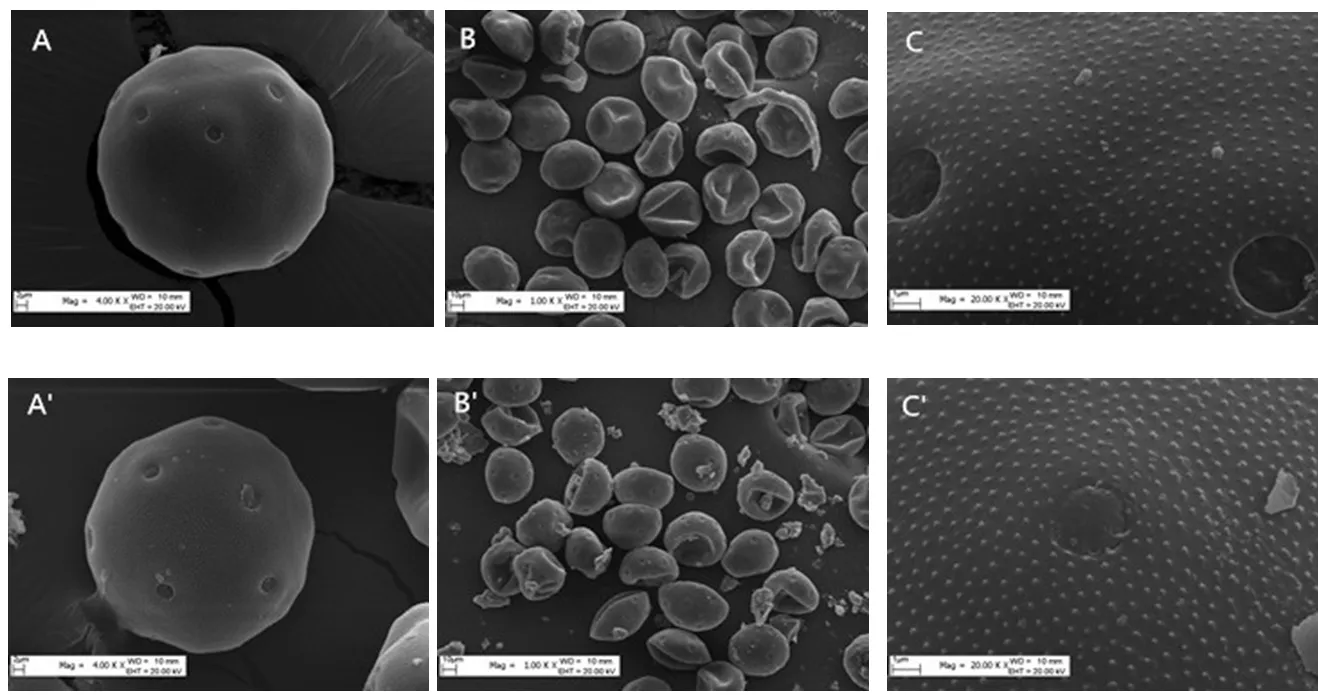
Fig. 5 Differences in pollen viability between the control (A~C) and treated walnut trees (A'~C')
3.5. Effects of dust on pollination and fertilization
To understand the effects of dust on pollination and fertilization, the pollen grain germination percentage and pollen tube lengths of the control and treated trees were determined using fluorescence microscopy. 8 h after pollination, the pollen grains on the stigma began to germinate. However, there was a lower number of pollen grains on the stigmas of the treated trees and the germination rate was low (Fig. 6: A). In contrast, 95% of the pollen grains on the stigmas of the control trees germinated (Fig. 6: A'). 36 h after pollination, the pollen tubes of the control trees began to elongate towards the style (Fig. 6: B), whereas there was no fluorescence in the same location on the treated trees (Fig. 6: B'). In addition, 60~100 h after pollination, the elongation (towards the style) of the pollen tubes of the treated trees was completed (Fig. 6: C~F), while the pollen tubes of the control trees had started to grow downwards to enter the ovary cavity (Fig. 6: C'~F'). The pollen tubes of the treated trees did not reach the ovary cavity until 144 h after pollination (Fig. 6: G), whereas the pollen tubes of the control trees had passed through the ovary cavity, reaching the top of the receptacle and the outer integumental layer (Fig. 6: G'). Then, 240 h after pollination, the embryo sac had merged with the sperm to form a fertilized nucleus (Fig. 6: H'), while the pollen tube of the treated tree began to turn upwards to reach the embryo sac (Fig. 6: H). Through the above analysis, it was found that dust could reduce the viability of the pollen grains on the stigma and delay the pollen tube, thus affecting the fertilization of female walnut flowers.

Fig. 6 Differences in pollen germination after allogamy between the control trees (A~H) and treated trees (A'~H')
4. Discussion
For most plants, normal pollination and fertilization are prerequisites for fruit formation. With regard to the conditions for successful fertilization, except for some internal conditions such as fertile pollen grains and stigmas with good receptivity, external environment factors such as temperature, humidity, wind, and insects impact successful fertilization. Therefore, the sexual reproductive phase in plants might be particularly vulnerable to the effects of environmental change, which has been documented in previous studies. For example, under high temperature conditions, the pistils of many plants significantly withered during their flowering period. Due to water loss, the secretions on the surface of corn silk were reduced and the corn silk withered, resulting in a shortened life span of the flower. Under high temperature conditions, the rate of blasted pistils and curled corollas of female sweet cherry flowers increased. It was found that when dust fell on the stigmas of the walnut, the stigma receptivity was significantly affected, especially during Period III (Table 1; Fig. 1). Although stigma mucus secretions reached the maximum level and both the opening angle and contact area were the largest, the dust caused a reduced stigma length, a lower amount of secretions, brown streaks on the surface, early withering, and the death of the protruding glandular cells. Thus, the ability of pollen to adhere to stigmas was weakened. This was similar to the results of studies on the effects of other adverse weather conditions, especially high temperature, on the mucus secretion and receptivity of female flowers.
The quantity and morphology of pollen, anther dehiscence, and pollen wall architecture, as well as the chemical composition and metabolism of pollen, have been shown to be affected by environmental change, especially high temperatureor heavy metal conditions. In this study, it was found that dust can cause a much shorter flowering phase of the male flowers of the walnut. The amount of pollen grains disseminated from the flower buds and the viability of the pollen were both significantly lower in the dust treated trees than in the control trees (Table 3), and the pollen malformation rate was significantly higher in the treated trees than in the control trees. Thein situ
pollen gemination experiment confirmed that natural dust would reduce the germination rate of the pollen grains that land on the stigma and delay the downwards elongation of the pollen tubes, thus affecting the fertilization of female walnut flowers. A large number of particles matter covered the pollen apertures, hampering the germination of the pollen. The tapetum was easily degraded in the early period, resulting in the inability of the pollen to absorb nutrients, leading to pollen dysplasia, low vitality, and a low germination rate. After being subjected to a high temperature treatment (35°C, 4 h) for 5 d, the pollen germination rate of rice decreased by 33.22% compared to the control. In some extreme cases, due to the degradation that occurred during pollen development, the anthers were deflated, resulting in complete male sterility. In addition, under high temperature stress, male organs of rice showed a blockage of the cracking of anthers and the number of pollen grains that landed on the stigmas was insufficient. Also, stress due to adverse weather conditions can cause the pollen mother cells to produce polyads rather than the normal tetrads during meiosis. For example, in the sweet cherry, meiosis produced 14 times more polyads (84.3% of total) than the control did. Our results indicated that during the flowering period of walnut trees, long-term dust exposure had an adverse impact on the development of floral organs and affected pollination and fertilization. Regarding the effects of dust on the development of female flowers and male pollen, the development of post-anthesis embryo sacs, and the fruit setting rate, further observation was needed. In addition, future studies should be conducted on how to overcome the negative effects of dust on the pollination and fertilization of walnuts.5. Conclusions
In the Tarim Basin, the flowering period of the walnut occurred at the same time as dust storms do, which adversely affected the flower organs of the walnut by reducing the stigma secretion, decreasing the pollen germination rate, hindering pollen tube germination, and prolonging the pollen tube elongation time. Overall, the pollination and fertilization of walnuts were negatively affected by the presence of dust.
 Agricultural Science & Technology2021年2期
Agricultural Science & Technology2021年2期
- Agricultural Science & Technology的其它文章
- Effects of Different Soil Preparation and Fertilizer Application Methods on the Growth and Yield of the Rice-Crayfish Rotation Direct Seeding Rice Nongxiang 32
- Influence of Seedling Age and Seeding Rate on Grain Yield in Double Cropping Rice with Machine Transplantation
- Genotype Analysis of Yuzhenxiang, a Long Grain, High Quality Aromatic Rice Cultivar
- Comparision of the Leaf Morphology of Two Phoebe bournei Varieties
- Effects of Biochar on Physiological Characteristics of Lonicera Japonica and Accumulation of Cd and Pb in Cd and Pb Contaminated Soil
- Effects of Different Extract of Pseudostellaria Heterophylla on Immunological Function in Mice based on Meta-analysis and Network Meta-analysis
