Deletion of phosphatidylserine flippase β-subunit Tmem30a in satellite cells leads to delayed skeletal muscle regeneration
Kuan-Xiang Sun, Xiao-Yan Jiang, Xiao Li, Yu-Jing Su, Ju-Lin Wang, Lin Zhang, Ye-Ming Yang,Xian-Jun Zhu,3,4,5,*
1 Health Management Center, Sichuan Provincial People's Hospital, School of Medicine, University of Electronic Science and Technology of China, Chengdu, Sichuan 610072, China
2 Sichuan Provincial Key Laboratory for Human Disease Gene Study, Center for Medical Genetics, Sichuan Provincial People’s Hospital,University of Electronic Science and Technology of China, Chengdu, Sichuan 610072, China
3 Research Unit for Blindness Prevention of Chinese Academy of Medical Sciences (2019RU026), Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital, Chengdu, Sichuan 610072 China
4 Department of Ophthalmology, First People’s Hospital of Shangqiu, Shangqiu, Henan 476000, China
5 Key Laboratory of Tibetan Medicine Research, Chinese Academy of Sciences and Qinghai Provincial Key Laboratory of Tibetan Medicine Research, Northwest Institute of Plateau Biology, Xining, Qinghai 810008, China
ABSTRACT Phosphatidylserine (PS) is distributed asymmetrically in the plasma membrane of eukaryotic cells.Phosphatidylserine flippase (P4-ATPase) transports PS from the outer leaflet of the lipid bilayer to the inner leaflet of the membrane to maintain PS asymmetry. The β subunit TMEM30A is indispensable for transport and proper function of P4-ATPase. Previous studies have shown that the ATP11A and TMEM30A complex is the molecular switch for myotube formation. However, the role of Tmem30a in skeletal muscle regeneration remains elusive. In the current study, Tmem30a was highly expressed in the tibialis anterior (TA) muscles of dystrophin-null (mdx) mice and BaCl2-induced muscle injury model mice. We generated a satellite cell (SC)-specific Tmem30a conditional knockout(cKO) mouse model to investigate the role of Tmem30a in skeletal muscle regeneration. The regenerative ability of cKO mice was evaluated by analyzing the number and diameter of regenerated SCs after the TA muscles were injured by BaCl2-injection. Compared to the control mice, the cKO mice showed decreased Pax7+ and MYH3+ SCs,indicating diminished SC proliferation, and decreased expression of muscular regulatory factors(MYOD and MYOG), suggesting impaired myoblast proliferation in skeletal muscle regeneration. Taken together, these results demonstrate the essential role of Tmem30a in skeletal muscle regeneration.
Keywords: Tmem30a; Skeletal muscle regeneration; Knockout mouse model; Atp11a;Satellite cell
INTRODUCTION
Skeletal muscle is widely distributed in human tissue and plays a significant role in regulating basic life activities, such as respiration, metabolism, body temperature maintenance,and exercise. The strong regenerative capacity of skeletal muscle is important for maintaining skeletal muscle tissue homeostasis and ensuring normal physiological function under disease and trauma conditions. Regeneration of skeletal muscle occurs mainly through satellite cells (SCs), which are localized between the muscle and basement membranes during the resting state (Feige & Rudnicki, 2018; Sambasivan& Tajbakhsh, 2007). Resting SCs have a large nucleoplasmic ratio but very few mitochondria, and express marker genes such as Pax7, M-cadherin, syndecan 4, CD34, integrin-A7,CXCR4, and Myf5 (Alfaro et al., 2011; Kuang et al., 2006;Seale et al., 2000), but not MYOD (Cornelison & Wold, 1997;Zammit et al., 2004). In response to muscle injury or other pathological conditions, SCs are activated to proliferate and differentiate, and then repair damaged muscle tissue by fusing to existing myofibers or forming new ones. After activation, the SC nucleoplasmic ratio decreases, but pre-activation marker genes are still expressed. In particular, Myf5 expression is greatly increased in activated skeletal muscle stem cells,resulting in MYOD expression (Crist et al., 2012; Gayraud-Morel et al., 2012). SCs are regulated by a series of myogenic regulatory factors, such as MYOD, myogenin (MYOG), and Myf5, during differentiation. MYOD is a decisive master regulator of muscle lineage, and the expression of exogenous MYOD in non-muscle cells can lead to their transdifferentiation into muscle cells (Bichsel et al., 2013). MYOD and Myf5 can activate the expression of key downstream transcription factors, such as MYOG, and promote SC differentiation(Füchtbauer & Westphal, 1992; Londhe & Davie, 2011;Tapscott et al., 1988). These downstream transcription factors further activate the expression of muscle-specific genes, such as myosin, and promote the terminal differentiation of muscle cells. Among the terminally differentiated muscle cells,myoblasts fuse with each other to form myotube cells. These cells then form myofibers via orderly arrangement and finally muscle tissues, which play critical roles in many functionsin vivo(Odelberg et al., 2000).
In mammalian plasma membranes, lipids are distributed asymmetrically, mainly in the form of phosphatidylethanolamine (PE) and phosphatidylserine (PS)in the cytoplasmic leaflet, and sphingomyelin (SM) and phosphatidylcholine (PC) in the exoplasmic leaflet (Bretscher,1972). The asymmetric distribution of PS is largely dependent on P4-ATPase, which can transport PS from the outer leaflet of the lipid bilayer to the inner leaflet in the membrane(Holthuis & Levine, 2005). Mounting evidence suggests that P4-ATPase plays important roles in various disease conditions. For instance, mutations inATP8B1can cause progressive familial intrahepatic cholestasis type I and benign recurrent intrahepatic cholestasis (Bull et al., 1998). Mutations inATP8A2can lead to cerebellar ataxia, mental retardation,and disequilibrium (Tadini-Buoninsegni et al., 2019).Furthermore,ATP11Adeficiency may affect myotube formation and cell surface PS maintenance (Tsuchiya et al.,2018), whileATP11Cdeficiency can cause congenital hemolytic anemia (Arashiki et al., 2016).
Similar to the sodium-potassium pump, P4-ATPase is also heterodimeric and requires the β-subunit for physiological function. The highly conserved transmembrane CDC50 protein family interacts with P4-ATPase and plays a crucial role in maintaining normal physiological function as well as proper cellular localization of P4-ATPase (Bryde et al., 2010;Saito et al., 2004; van der Velden et al., 2010). For proper cellular localization, P4-ATPase exportation from the endoplasmic reticulum requires the CDC50 subunit, which also plays an important role in the establishment of cell polarity. The CDC50 protein family contains three homologous proteins CDC50A, CDC50B, and CDC50C (also named TMEM30A, TMEM30B, and TMEM30C, respectively).TMEM30A is the main β-subunit that interacts with P4-ATPase(Sebastian et al., 2012). Indeed, increasing data support the key roles ofTMEM30Ain multiple tissues. For example,TMEM30A-deficient cells can cause severe damage in the formation of membrane ruffles, leading to cell migration inhibition (Kato et al., 2013; Paulusma et al., 2008). In addition,TMEM30AKO cells contain PS on their surface and are engulfed by macrophages (Segawa et al., 2014).Tmem30adeficiency can also lead to intrahepatic cholestasis due to the mislocalization of the PS transporter protein (Liu et al., 2017). Moreover,Tmem30ais essential for the function of retinal bipolar cells and photoreceptor cells in the retina (Yang et al., 2019; Zhang et al., 2017).Tmem30adeficiency inhibits vascular endothelial growth factor (VEGF)-induced signaling,leading to reduced endothelial cell proliferation and impaired retinal vascular development (Zhang et al., 2019). Recent research also reports thatTmem30aplays a role in insulin processing and secretion (Yang et al., 2021), and loss ofTmem30ain podocytes can lead to albuminuria and glomerulosclerosis in murine KO models (Liu et al., 2021).Neuronal-specific KO ofTmem30aresults in exposure of PS on the neuronal outer membranes and loss of inhibitory postsynapses and seizures (Park et al., 2021).
Regeneration of skeletal muscle involves massive proliferation and migration of SCs and fusion of myoblasts,which requires the synthesis and fusion of many cell membranes. Abnormalities in cell membrane function are likely to affect skeletal muscle regeneration. Recent study found that the phospholipid flippase complex of ATP11A and TMEM30A regulates myoblast fusion and syncytium elongation by controlling the Ca2+channel PIEZO1, a key regulator of myotube formation in C2C12 cells (Tsuchiya et al.,2018). As the ATP11A and TMEM30A complex is closely associated with myotube formation, we wondered whether this complex also plays a crucial role in the process of skeletal muscle regeneration. Previous study found that ATP11Amediated PIEZO1 activation plays an important role in proper morphogenesis during myofiber regeneration (Tsuchiya et al.,2018). However, theinvivofunctions ofTmem30ain skeletal muscle regeneration remain elusive.
In this study, we generated a novel SC-specificTmem30aKO mouse model using Pax7CreER(Murphy et al., 2011) and investigated the roles ofTemem30ain skeletal muscle regeneration. Our data showed that specific deletion ofTemem30ain mouse SCs reduced the expression of key regenerative marker proteins, such as Pax7, MYH3, MYOD,and MYOG, which impaired the proliferation and differentiation of SCs. Taken together, our study showed thatTmem30ais indispensable for skeletal muscle regeneration.
MATERIALS AND METHODS
Animal model and genotyping
All animal experiment protocols (approval No.: LS2019-044)were approved by the Animal Care and Use Committee of Sichuan Provincial People’s Hospital, China. All experimental operations were performed in accordance with the approved study protocols and relevant regulations. All mice were housed in a specific pathogen-free (SPF) barrier facility with a standard diet and environment.
FloxedTmem30amice with a loxP site inserted to flank exon 3 of theTmem30agene have been described previously(Zhang et al., 2017). To generate a SC-specific KO mouse model, we mated Pax7CreERmice (Jackson Laboratory,https://www.jax.org/strain/017763) toTmem30aloxP/loxPmice to yield heterozygous progeny (Tmem30aloxP/+; Pax7CreER). TheTmem30aloxP/+; Pax7CreERmice were mated toTmem30aloxP/loxPmice to generateTmem30aloxP/loxP; Pax7CreERmice (designated cKO mice). TheTmem30aloxP/loxPandTmem30aloxP/+;Pax7CreERmice were designated as wild-type (WT) mice. To monitor the efficiency of Cre enzyme expression in SCs, the tdTomato reporter (strain name: B6.Cg-Gt (ROSA) 26Sortm14(CAG-tdTomato) Hze/J, http://jaxmice.jax.Org/strain/007914.html) was used.
Mice were genotyped by polymerase chain reaction (PCR).Genomic DNA templates were obtained from mouse tails.Mice were screened using the primers:Tmem30a-loxP-F, 5'-ATTCCCCTTCAAGATAGCTAC-3',Tmem30a-loxP-R, 5'-AAT GATCAACTGTAATTCCCC-3'; Cre-F, 5'- GAACGCACTG ATTTCGACCA-3', Cre-R, 5'- GCTAACCAGCGTTTTCGTTC-3'; Rosa-tdt-wt-F, 5'-CACTTGCTCTCCCAAAGTCG-3', Rosatdt-wt-R, 5'-TAGTCTAACTCGCGACACTG-3'; and Rosa-tdt-R-KI, 5'-GTTATGTAACGCGGAACTCC-3'.
Tamoxifen injection and skeletal muscle injury
Tamoxifen salt (Sigma Aldrich, USA) was dissolved in anhydrous ethanol at a concentration of 10 mg/mL as a stock solution. On the day of injection, the stock solution was diluted in corn oil (Sigma Aldrich, USA) at a ratio of 1:9 as an injection solution. Mice received an intraperitoneal injection with a daily injection solution dosage of 15 μL/g body weight for five consecutive days.
We dissolved BaCl2powder (Sigma Aldrich, USA, 10 361-37-2) in normal saline to create a BaCl2solution with a concentration of 1.5%. Skeletal muscle was injured by injection of 50 μl of 1.5% BaCl2solution in the right tibialis anterior (TA) muscles.
Quantitative real-time PCR (qRT-PCR)
Extraction of RNA from the TA muscles was performed using TRIzol reagent (Life Technologies, USA, catalog # 15 596-026). cDNA was synthesized using a reverse transcription kit(Ambion, USA, catalog # 18 090 010). In addition, qRT-PCR was performed with a SYBR Green kit (Bio-Rad Laboratories,USA; catalog # KK4607), and the values were normalized to the GAPDH transcript as an internal control. The following primers were used for qRT-PCR:Tmem30a-F, 5'-CAAA CAGCAACGGCTACCC-3',Tmem30a-R, 5'-GTTGTTGGAG GTGACGAAGAT-3V; GAPDH-F, 5'-TGTGTCCGTCGTGGAT CTGA-3', and GAPDH-R, 5'-TTGCTCTTGAAGTCGCAGG AG-3'.
Immunoblotting
The TA muscles were lysed in RIPA lysis buffer with protease inhibitor cocktail and ethylenediaminetetraacetic acid (EDTA)(TransGen, China). Protein concentration was determined using a BCA Protein Assay (TransGen, China). Equal amounts of protein were loaded onto a 10% polyacrylamide gel, then separated and transferred to nitrocellulose membranes (Merck Millipore, Germany). Blots were blocked with 8% non-fat dry milk in Tris-buffered saline and Tween 20(TBST) for 2 h at room temperature and then incubated with primary antibodies in blocking solution overnight at 4 °C. The primary antibodies included TMEM30A (rabbit polyclonal,ab217330, Abcam, USA) and GAPDH (mouse monoclonal,60004-1-Ig, Proteintech, USA). Primary antibodies were detected with either an anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (Bio-Rad Laboratories, USA), and signals were developed using a SuperSignal West Pico Chemiluminescent Substrate (Pierce Protein Biology, USA). ImageJ (v1.8.0) was used to calculate the relative density of the protein.
Immunohistochemistry
For immunohistochemical analysis, mice were first anesthetized with ketamine (16 mg/kg body weight) and perfused transcardially with phosphate-buffered saline (PBS),followed by 4% paraformaldehyde (PFA) in PBS. The right hind limbs of the mice were removed from the skin and gastrocnemius muscle and immersed in 4% PFA overnight.The tibia and excess muscles were then removed, and the TA muscles were fixed for 3 h in 4% PFA, then dehydrated with 30% sucrose for 24 h. The TA muscles were then embedded in optimal cutting temperature solution (OCT), frozen, and sectioned at a thickness of 10 μm. The sections were blocked and permeabilized with 10% normal bovine serum and 0.2%30%Triton X-100 in PBS for 2 h, then incubated overnight at 4 °C with primary antibodies. Subsequently, the sections were washed with PBS and stained with the Alexa-Fluor-488 or Alexa-Fluor-594-labeled goat anti-mouse or rabbit Ig secondary antibodies (diluted 1:500). For section immunofluorescence, the following antibodies were used:TMEM30A (rabbit polyclonal, ab217330, Abcam, USA), Pax7(rabbit polyclonal, AF7584, Affinity, USA), Laminin (rabbit polyclonal, L9393, Sigma, USA), MYH3 (rabbit polyclonal,22287-1-AP, Proteintech, USA), MYOD (rabbit polyclonal,18943-1-AP, Proteintech, USA), and MYOG (rabbit polyclonal,bs-3550R, Bioss, USA).
Histological staining and cell quantification
For hematoxylin-eosin (H&E) and Sirius Red staining, mice were anesthetized with ketamine (16 mg/kg body weight) and perfused transcardially with PBS followed by 4% PFA. The isolated TA muscles were fixed in 4% PFA overnight at 4 °C,then embedded in paraffin, cut into 10 μm sections, and stained using H&E and Sirius Red staining protocols. The H&E and Sirius Red stained sections were viewed and imaged using a microscope and used to count and measure the number and diameter of regenerating SCs in injured skeletal muscle.
Statistical analysis
Statistical analysis was performed using Student’st-test.Quantitative data are expressed as mean±standard error of the mean (SEM), as shown in the figure legends. Differences were considered statistically significant atP<0.05. Statistical analyses were performed using GraphPad Prism 6 software.
RESULTS
Tmem30a was highly expressed in regenerating skeletal muscle
Previous studies have suggested that both Duchenne muscular dystrophy (DMD) patients and dystrophin-null (mdx)mice exhibit sustained activation of SCs and muscle regeneration (Bulfield et al., 1984; Sacco et al., 2010).Therefore, we examined the expression ofTmem30ain the skeletal muscles ofmdxmice. Results showed thatTmem30awas highly expressed (Figure 1A, C, D), thus suggesting the potential role ofTmem30ain skeletal muscle regeneration. To verify whetherTmem30ais highly expressed during skeletal muscle regeneration, the TA muscles of WT mice were injured with an injection of BaCl2. At 5 days post-injury (dpi), the TA muscles were harvested and subjected to qRT-PCR,immunoblotting, and immunohistochemical analysis. The mRNA expression ofTmem30ashowed a 6-fold increase in the injured muscle (Figure 1E). The TMEM30A protein levels were also higher in 5 dpi mice (Figure 1B, F). The immunohistochemical results verified that the TMEM30A protein was up-regulated in 5 dpi mice (Figure 1G). Therefore,Tmem30amay play a role in skeletal muscle regeneration.
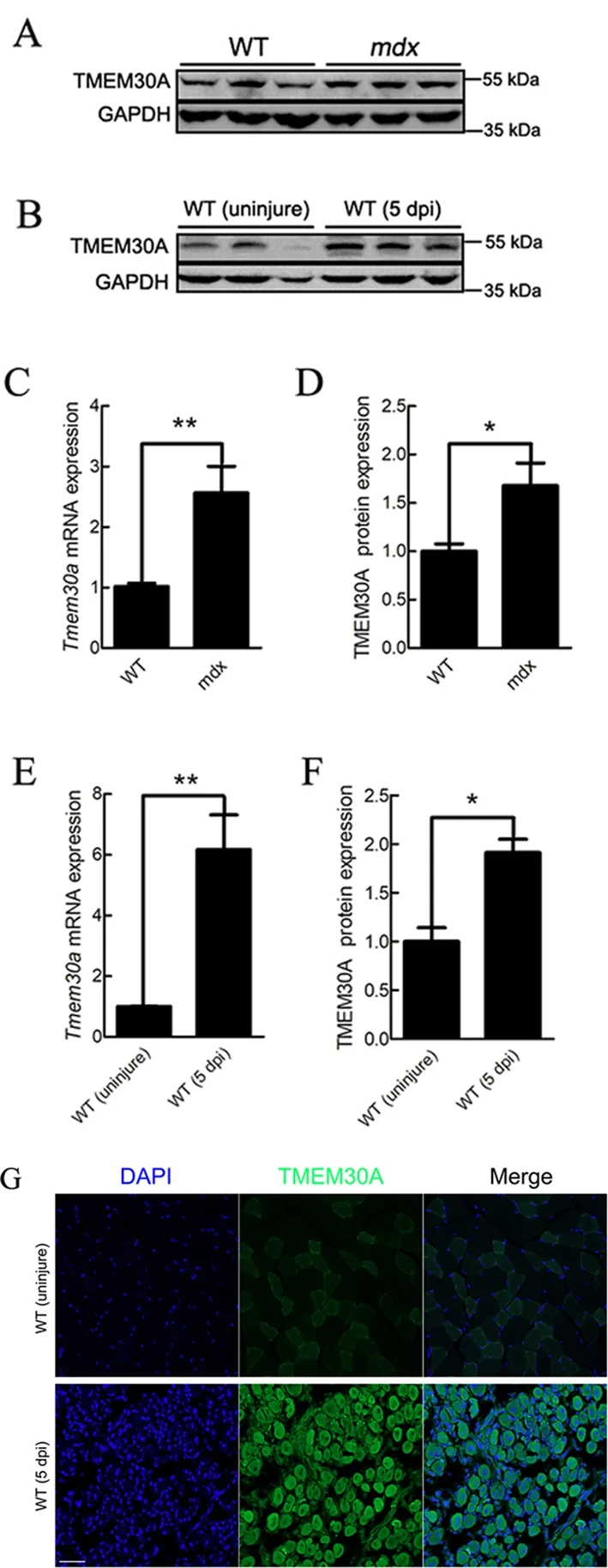
Figure 1 Tmem30a was up-regulated during skeletal muscle regeneration
Generation of inducible SC-specific Tmem30a-deleted mice
Previous studies have demonstrated thatTmem30aglobal KO results in embryonic lethality (Zhang et al., 2017). To investigate the role ofTmem30ain skeletal muscle regeneration, we crossed Pax7CreERmice expressing Cre recombinase from thePax7locus (Murphy et al., 2011) with mice bearing floxedTmem30aalleles to generateTmem30aloxP/loxP; Pax7CreER(cKO) mice (Figure 2A). PCR was used to identify the genotypes of the offspring (Figure 2B).
Cre reporter line ROSA26-tdTomato was used to verify the specific expression of Pax7CreERin SCs. We crossed ROSA26-tdTomato reporter mice with cKO mice to generateTmem30aloxP/loxP; Pax7CreER; Rosa-tdTomato mice(Supplementary Figure S1). Cre enzyme expression was a prerequisite for the expression of tdTomato. A loxP-flankedSTOP sequence before the tdTomato expression cassette was deleted in the Cre-expressing cells, and tdTomato was subsequently expressed. Next, 7 dpi TA muscle sections expressing tdTomato were stained with a specific antibody against Pax7. Results showed that tdTomato was expressed distinctly in Pax7-positive SCs, suggesting that Pax7CreERdrives deletion ofTmem30ain SCs specifically (Figure 2D).Immunofluorescence staining of TMEM30A in 7 dpi mice revealed that TMEM30A was barely expressed in the SCs of the cKO mice (Figure 2E). Immunoblot analysis of proteins extracted from the TA muscles at the injury site further confirmed that TMEM30A expression in the cKO mice was reduced to 76% that of the controls (Figure 2F, G). This KO efficiency is meaningful, asTmem30ain the cKO mouse model was only deleted in the SCs andTmem30ais expressed in other non-SCs, which account for a large proportion of the TA muscles.
Loss of Tmem30a led to impaired skeletal muscle regeneration
We first investigated whether deletion ofTmem30ain SCs affects muscle development. Deletion ofTmem30awas induced with an intraperitoneal injection of tamoxifen on postnatal days 1 to 3 (P1-P3). No visible gross abnormalities were observed and no visible differences in body weight were detected between the WT and cKO mice from P30 to P60(Supplementary Figure S2).
To investigate the role ofTmem30ain skeletal muscle regeneration, cKO and WT mice were induced with an intraperitoneal injection of tamoxifen at 9 weeks of age,followed by BaCl2-induced damage (Figure 2C). The TA muscles were subjected to histological analysis at 3, 5, 7, 10,and 14 dpi. Based on H&E staining, the histological appearance of skeletal muscle was compared between the WT and cKO mice. Uninjured skeletal muscle cells exhibited a well-organized pattern with dark-blue stained nuclei mainly located at the cell periphery (Figure 3A). At 3 dpi,inflammatory cells and necrotic myofibers were prominently observed, as well as a small number of proliferating SCs in WT mice (Figure 3A). At 5 dpi, myofibers with centralized nuclei proliferated from SCs replaced most of the necrotic myofibers in WT mice. In contrast, few proliferating myofibers were observed in theTmem30acKO mice (Figure 3A, B). At 7-10 dpi, newly formed myofibers with larger diameters were
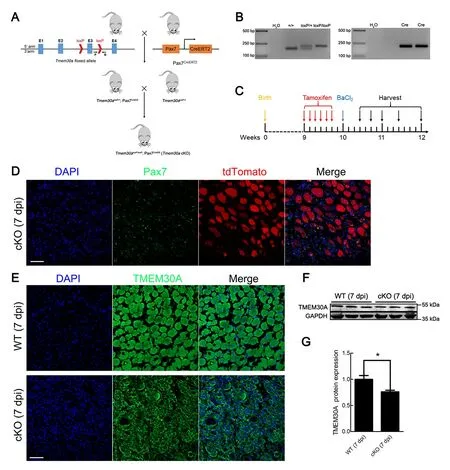
Figure 2 Generation of Tmem30a conditional knockout (cKO) mice
observed in WT mice, whereas these myofibers were barely visible in the cKO mice (Figure 3A, C). At 14 dpi, the WT mice displayed tightly packed, well-formed myofibers, whereas the cKO mice exhibited numerous small myofibers with single nuclei (Figure 3A).
Connective tissue fibrosis can reflect the status of skeletal muscle regeneration (Murphy et al., 2011). We histologically analyzed Sirius Red-stained TA muscle sections. We obtained similar results as H&E staining regarding the number and diameter of regenerating myofibers (Supplementary Figure S3A). The number of regenerating myofibers in the cKO mice was significantly less than that in the WT mice at 5-14 dpi(Supplementary Figure S3A, B). Similarly, in the cKO mice,more small-diameter regenerating myofibers were observed at 7-14 dpi (Supplementary Figure S3A, C). In summary,pathological section-staining revealed that loss ofTmem30ain SCs impaired skeletal muscle regeneration.
Tmem30a deficiency impaired SC proliferation
To further investigate the mechanism by which deletion ofTmem30adelays skeletal muscle regeneration, we examined Pax7, a transcription factor involved in the regulation of SC proliferation (Diao et al., 2012). During skeletal muscle regeneration, SCs first proliferate rapidly and Pax7 expression increases. Most SCs begin to differentiate into myoblasts and myofibers (which down-regulate Pax7), while the remaining Pax7+SCs located under the basement membrane of the myofibers return to quiescence (Seale et al., 2000). Here, we performed immunohistochemical analysis of TA muscles at 3,5, 7, 10, and 14 dpi. No differences in Pax7+SCs were noted between the WT and cKO mice when SCs were at the resting state (Figure 4A-C). In the WT mice, Pax7+SCs were mainly concentrated in the regenerating myofiber region and proliferated rapidly during the early stage of injury, with a peak level at 5 dpi (Figure 4A). Subsequently, Pax7 expression gradually decreased. By 14 dpi, the number of quiescent Pax7+SCs returned to uninjured levels (Figure 4A). In comparison to the WT mice, the cKO mice showed a significant decrease in the number of Pax7+SCs at 3, 5, 7, 10,and 14 dpi (Figure 4B). Quantification of Pax7+SCs revealed a significant reduction in Pax7 expression in cKO mice at 3-5 dpi, a critical period of SC proliferation (Figure 4C).
We next explored the expression of MYH3, an embryonic myosin heavy chain expressed in embryonic and regenerating myofibers only (Schiaffino et al., 2015). In the WT mice,regenerating myofibers began to express MYH3 at 3 dpi,followed by a gradual increase in expression until 14 dpi, after which the mature regenerated myofibers no longer expressed MYH3 (Figure 5A). In contrast, the cKO mice exhibited delayed and lower expression of MYH3 (Figure 5B, D). The number of regenerating myofibers was also investigated. In the cKO mice, few large-diameter myofibers with centralized nuclei were observed and those myofibers were morphologically disorganized (Figure 5C). Furthermore, fewer myofibers with centralized nuclei were observed in the cKO mice than in the WT mice at the different periods after injury(Figure 5E). The diameters of myofibers with centralized nuclei were smaller in the cKO mice than in the WT mice

Figure 3 Myoblast regeneration was impaired in Tmem30a cKO mice after injury
(Figure 5F). These data suggest that deletion ofTmem30ainhibits Pax7 and MYH3 expression in activated SCs, thus affecting the SC proliferation process.

Figure 4 Pax7 expression was decreased in Tmem30a cKO mice
Tmem30a was essential for myoblast differentiation
As previously noted, ATP11A plays an essential role during myofiber fusion (Tsuchiya et al., 2018). Here, we further explored the function ofTmem30ain the differentiation of myoblasts. The expression of MYOD, a master regulator of muscular regulatory factors (MRFs), was evaluated using immunofluorescent staining. Results showed that fewer MYOD+cells were observed in the cKO mice (Figure 6A), with a ~70% reduction in number (Figure 6C). We also examined the expression of MYOG, which can promote differentiation of SCs. Fewer MYOG+cells were detected in the cKO mice than in the WT mice at 7 dpi (Figure 6B, D). These results indicate that deletion ofTmem30ain SCs impairs MRF expression. To investigate the effects of reduced MRF expression on myoblast differentiation, we histologically analyzed the TA muscles at 14 dpi. Both H&E and Sirius Red staining revealed abnormally fused myofibers in the cKO mice (Figure 6E).These results suggest thatTmem30adeficiency decreases MRF expression, which ultimately impairs myoblast differentiation.
DISCUSSION
In this study, we investigated the role ofTmem30ain skeletal muscle regeneration by generating SC-specificTmem30aKO mice. The important function ofTmem30ain skeletal muscle regeneration was suggested by the up-regulation ofTmem30aexpression in themdxand 5 dpi mice (Figure 1). Furthermore,loss ofTmem30aimpacted skeletal muscle regeneration by reducing activated SCs and inhibiting proper myoblast fusion.Studies have indicated that SCs proliferate mainly by asymmetric division after skeletal muscle injury (Gurevich et al., 2016; Kuang et al., 2007; Shinin et al., 2006; Webster et al., 2016). Asymmetric division of SCs is primarily maintained by intracellular polarity (Dumont et al., 2015). Asymmetric distribution of PS on the plasma membrane mediated by flippase is essential for cell polarity (Chen et al., 2006; Das et al., 2012). Thus, inTmem30acKO mice, the absence of a functional flippase complex due toTmem30adeficiency may result in the disruption of the asymmetric distribution of PS on the plasma membrane, which may disturb cell polarity.Ultimately, the asymmetric division of SCs may be affected,leading to inhibition of proliferation. Thus, the relationship between PS distribution and SC regeneration warrants further investigation. As shown in Figures 3B, 5E, the number of regenerating myofibers was reduced in the cKO mice and SC proliferation was impaired.

Figure 5 Deletion of Tmem30a impaired SC proliferation
Cell migration is inhibited by severe defects in the formation of membrane ruffles in TMEM30A-deficient cells (Kato et al.,2013; Paulusma et al., 2008). After muscle injury, most activated SCs migrate to damaged myofibers, where they further differentiate and fuse together (Tidball, 2011). Reduced migration of SCs impairs skeletal muscle regeneration (Li et al., 2021). Here, loss ofTmem30adecreased the number of regenerating myofibers that could fuse with damaged myofibers, possibly by reducing SC migration, which resulted in smaller diameter regenerating myofibers in the cKO mice(Figures 3, 5F).
Calcium signaling is a critical element in the development,maintenance, and regeneration of skeletal muscle (Tu et al.,2016) and perturbing Ca2+release from internal stores impairs the regenerative process (Tu et al., 2016).Tmem30a-mutant cells exhibit abnormally high intracellular calcium levels (Cao et al., 2019), indicating disturbance of calcium efflux.Therefore,Tmem30aregulation of skeletal muscle regeneration may be related to intracellular Ca2+concentrations. Recent research suggests thatAtp11aandTmem30aregulate myotube fusion through control of the calcium channel (Tsuchiya et al., 2018). Moreover, abolishing Ca2+transients in regenerating muscle cells decreases the number of activated SCs in regenerating skeletal muscle (Tu& Borodinsky, 2014). Calcium and calcineurin can also regulate skeletal muscle differentiation by activating the MYOD transcription factor (Friday et al., 2003). Therefore, in the current study, the intracellular Ca2+concentration in the cKO mice may be abnormal, resulting in the observed decrease in MYOD expression (Figure 6A, C). MYOG can be activated by MYOD and serves as a master regulator of MRFs to promote myoblast differentiation. This explains why MYOG expression was markedly lower than that of MYOD in the cKO mice (Figure 6B, D).
In the current study, we discovered a novel role ofTmem30ain SC regeneration. Notably,Tmem30adeficiency in SCs inhibited their activation and proliferation and myoblast differentiation was also impaired by reduced MYOD and MYOG expression. Collectively, our results indicate thatTmem30ais an important factor in skeletal muscle regeneration.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHOR CONTRIBUTIONS
X.J.Z conceived the research. K.X.S., X.L., Y.J.S, X.Y.J.,J.L.W., L.Z., and Y.M.Y. conducted the experiments. X.J.Z.and K.X.S. wrote the manuscript. All authors read and approved the final version of the manuscript.

Figure 6 Specific deletion of Tmem30a in SCs impaired differentiation of myoblasts
ACKNOWLEDGEMENTS
The authors gratefully acknowledge the members of the Animal Facility of the Institute of Laboratory Animal Research of the Sichuan Academy of Medical Sciences and Sichuan Provincial People’s Hospital for excellent animal welfare and husbandry. The authors would like to thank Chengdu LiLai Biotechnology Co., Ltd. for technical assistance with histological analysis.
- Zoological Research的其它文章
- Chromosome-scale genome assembly of brownspotted flathead Platycephalus sp.1 provides insights into demersal adaptation in flathead fish
- Global view on virus infection in non-human primates and implications for public health and wildlifeconservation
- Captopril alleviates lung inflammation in SARS-CoV-2-infected hypertensive mice
- Role of juvenile hormone receptor Methoprene-tolerant 1 in silkworm larval brain development and domestication
- Molecular phylogeny and morphological comparisons of the genus Hebius Thompson, 1913 (Reptilia:Squamata: Colubridae) uncover a new taxon from Yunnan Province, China, and support revalidation of Hebius septemlineatus (Schmidt, 1925)
- Prosecution records reveal pangolin trading networks in China, 2014-2019

