Dynamic behavior of the cyanobacterial circadian clock with regulation of CikA∗
Ying Li(李莹), Guang-Kun Zhang(张广鹍), and Yan-Ming Ge(葛焰明)
College of Information Technology,Shanghai Ocean University,Shanghai 201306,China
Keywords: cyanobacterial circadian clock,mathematical model,adaptability,sensitivity analysis
1. Introduction
Circadian clocks are the fundamental process of many organisms to keep the physical and behavior order and adapt to daily environmental variations, which are intrinsic selfsustained oscillators with a period of approximately 24 h. The great progress in molecular biology experiments has enabled researchers to explore the components and molecular mechanisms involved in circadian clocks.[1]Among a wide variety of organisms,cyanobacteria are the simplest organisms to have circadian clocks.
In the cyanobacterial circadian clock, the gene clusterkaiABCand its product proteins KaiA, KaiB, and KaiC are essential for generating the rhythm.[2]KaiC plays a central role and undergoes rhythmic activities in transcription,translation, phosphorylation, and autodephosphorylation in the presence of both KaiA and KaiB.[3,4]The cyanobacterial circadian clock is composed of two coupled components: a post-translational oscillator (PTO) and a transcriptional/translational feedback loop(TTFL).These two components have a hierarchical relationship. The PTO is the core pacemaker to which the TTFL is attached. The TTFL feeds back to the PTO by the synthesis of clock proteins.[5]
In the TTFL,the clock genes,kaiA,kaiB,andkaiC,form a gene cluster, wherekaiBandkaiCare co-transcribed askaiABCmRNA.[2]Biological experiment shows that KaiC over expression consistently reduces kaiBC promoter activity, which implies KaiC regulates kaiBC transcription negatively.[2]This self-repression of KaiC indicates that the negative feedback transcriptional regulation generates oscillation ofkaigene expression. Additionally, KaiA over expression shows different effects on kaiBC transcription depending on KaiC; the transcriptional regulation by KaiC also showskaiAdependency.[6]Based on the fact that KaiA enhances KaiC phosphorylation, this cooperative regulation by KaiA and KaiC is considered to be realized by KaiC phosphorylation.[7]
The PTO is a self-sustained core pacemaker which consists of three core proteins: KaiA, KaiB, and KaiC.[8]KaiC can autophosphorylate and autodephosphorylate. The other clock proteins KaiA and KaiB modulate KaiC autophosphorylation. KaiA enhances the autophosphorylation of KaiC by binding to the A-loop, whereas KaiB inhibits this action of KaiA by sequestering KaiA from the A-loop,[6]which generates the oscillation of KaiC phosphorylation with a circadian period.[9]
Although the Kai proteins alone produce a robust circadian rhythm in vitro,there are several other proteins that have a great influence on the rhythms.[10]CikA,a signal transduction protein, plays an important role in the three processes of cyanobacterial circadian clock: input, oscillator and output.In the input pathway, CikA senses environmental light information indirectly, which entrains the circadian oscillator.[11]CikA also functions as an output pathway component by acting as a phosphatase against RpaA,a transcription factor regulated by the circadian clock.[12]In the central oscillator,CikA affects the phosphorylation and dephosphorylation of KaiC indirectly. Structural analysis reveals that both CikA and KaiA bind competitively to the same site on KaiB that is used to sequester KaiA,[13]which changes the ability of KaiA to stimulate KaiC phosphorylation. This regulation of CikA affects both the amplitude and the period of the circadian oscillation.[14]Considering the important role of CikA on the circadian clock,many biological results have been found.However, the molecular mechanism is not fully understood.To our knowledge, there is no research work on it from the dynamical angle,which may help us more deeply understand the role of CikA in the cyanobacterial circadian clock.
On the investigation of the regulatory mechanism of circadian clocks, mathematical models are useful and powerful,which have been widely used.[11,15–18]To explore the regulation mechanisms of CikA on the phosphorylation and dephosphorylation of KaiC,we bring forward a mathematical model with incorporation of additional regulation of CikA into the cyanobacterial circadian clock model. We will carry out a detailed study of the model to reveal the crucial role of CikA on the cyanobacterial circadian clock. The rest of this article is organized as follows. In Section 2, a detailed mathematical model is developed. Section 3 shows the numerical simulation and analysis. The conclusion and discussion are presented in Section 4.
2. Description of the mathematical model
When CikA regulation is incorporated into the PTO and TTFL circadian network,[8]the model of the cyanobacterial circadian oscillator is schematized in Fig.1. Cooperative regulation of KaiA and KaiC on kaiBC is realized by phosphorylated KaiC.KaiC can autophosphorylate and autodephosphorylate. KaiA promotes the phosphorylation of KaiC,and KaiB inhibits the phosphorylation in a competitive manner. However,CikA weakens the inhibition of KaiB.
It has been observed that the amount of KaiA in a single cell remain constant at a low level in the cytosol, and the amount of KaiB is always kept to be proportional to that of KaiC.[19]The model is described by a three-dimensional system of ordinary differential equations which include the temporal dynamics of kaiBC mRNA and KaiC in two forms: unphosphorylated and phosphorylated. This system is described as follows:
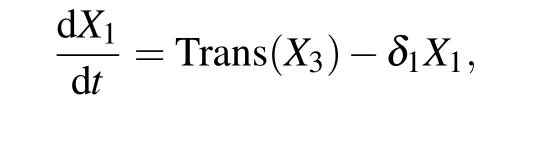
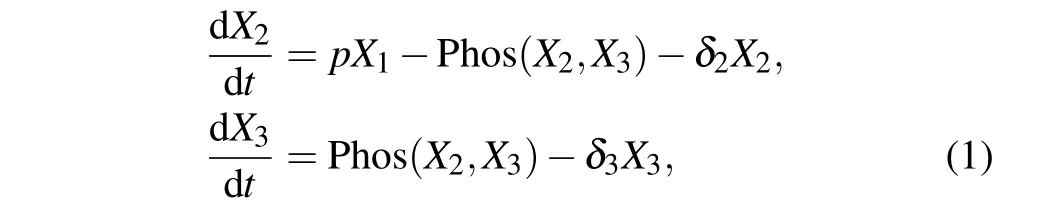
whereX1,X2, andX3are the concentration of kaiBC mRNA,unphosphorylated KaiC(NP-KaiC)and phosphorylated KaiC(P-KaiC),respectively;δ1,δ2,andδ3are the degradation rates of kaiBC,NP-KaiC,and P-KaiC,respectively;pis the translation rate of kaiBC.

Fig.1. Schematic diagram for the cyanobacterial circadian clock with the regulation of CikA, which involves the processes of kaiBC transcription and KaiC phosphorylation.

whereαandKare both nonnegative constants.
Phosphorylation rate of KaiC is described by the Phos function. We suppose that KaiB interacts with KaiA which decreases KaiA amount that can stimulate the phosphorylation of KaiC,and CikA depresses the interaction. KaiA-mediated KaiC phosphorylation is described with the Michaelis–Menten function as follows:
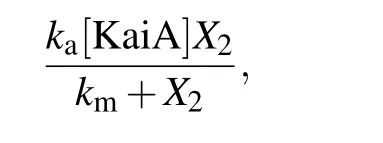
where [KaiA] represents the concentration of KaiA and it is a constant;kaandkmare the maximum phosphorylation rate per KaiA concentration activity and the Michaelis constant,respectively. The dephosphorylation rate is denoted askdX3,wherekdis the dephosphorylation rate per P-KaiC concentration. Without KaiB and CikA,the total change of phosphorylation and dephosphorylation is as follows:

Considering the interaction of KaiB with KaiA decreases KaiA amount and [KaiB] is always proportional to the total amount of KaiC,we use the following equation to describe the total change of phosphorylation and dephosphorylation with KaiB:
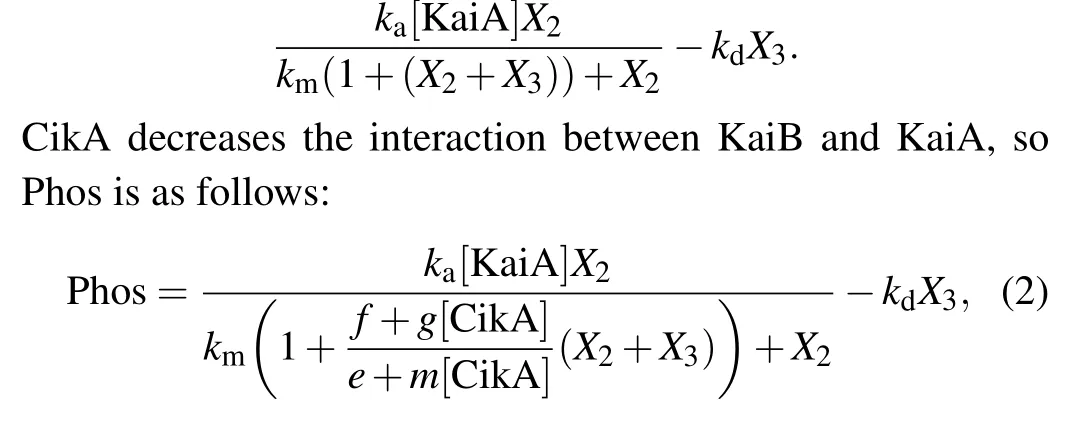
wheref,g,eandmare constants.
In the following numerical simulations, we take the parameter values asδ1= 0.135 h−1,δ2= 0.1425 h−1,δ3=0.1425 h−1,p=36.1 h−1,α=5,K=15µM,ka=110 h−1,km= 1 µM,f= 16.48,g= 3.3,e= 1.6,m= 1,kd=0.105 h−1.
3. Results
3.1. The effect of CikA on the dynamics of the circadian clock
For a mathematical model of the circadian clock, we should first validate whether its dynamic behaviors agree with experimental observations. In order to observe the effect of CikA on KaiC phosphorylation, we take various values of [CikA] in the circadian clock model with fixed [KaiA].Time evolution for the concentrations of P-KaiC is shown in Fig.2(a). The corresponding amplitude and period are shown in Fig. 2(b), from which we can see that CikA affects both the amplitude and the period of the circadian oscillation of KaiC phosphorylation. In particular, increasing amounts of CikA shorten the period of the KaiC phosphorylation oscillation,which is consistent with the experimental results.[14]
Biological experiments showed that the amount of KaiA in the reaction mixture is an important factor for the circadian oscillator of KaiC phosphorylation.[21]Numerical simulation of the system shows that with the increase of [KaiA], the period decreases, and the amplitude increases first and then decreases (see Figs. 2(c)–2(d)). All these results are coincident with experimental findings.[14,21]
Considering the competition of CikA with KaiA for the binding site on KaiB, to explore the effect of CikA, we decrease the concentration of KaiA in the reaction mixture while simultaneously increasing the concentration of CikA.The sum of[KaiA]and[CikA]is maintained. From Figs.2(e)–2(f),we can see that the phosphorylation state of KaiC maintains robust circadian rhythms with a stable circadian amplitude and a relatively stable period with the increase of [KaiA]. That is to say, in the presence of CikA, decreasing [KaiA] lengthens the period slightly. The ability of KaiA to stimulate KaiC phosphorylation is enhanced by CikA,which makes KaiA out of its sequestered state. Therefore,more CikA in the reaction mixture can release more free KaiA by occupying its binding site on KaiB,keeping the kinase activity of KaiC turned on by comparatively small amounts of KaiA.
In order to see the dynamics more clearly, we give the bifurcation diagrams in Fig. 3, which show that only when[KaiA] is large enough the presented model produces selfsustained oscillations, no matter whether CikA presents or not. The bifurcation point in Fig. 3(b) is smaller than that in Fig.3(a),which means that the regulation of CikA expands the range of[KaiA]that makes the system oscillate.
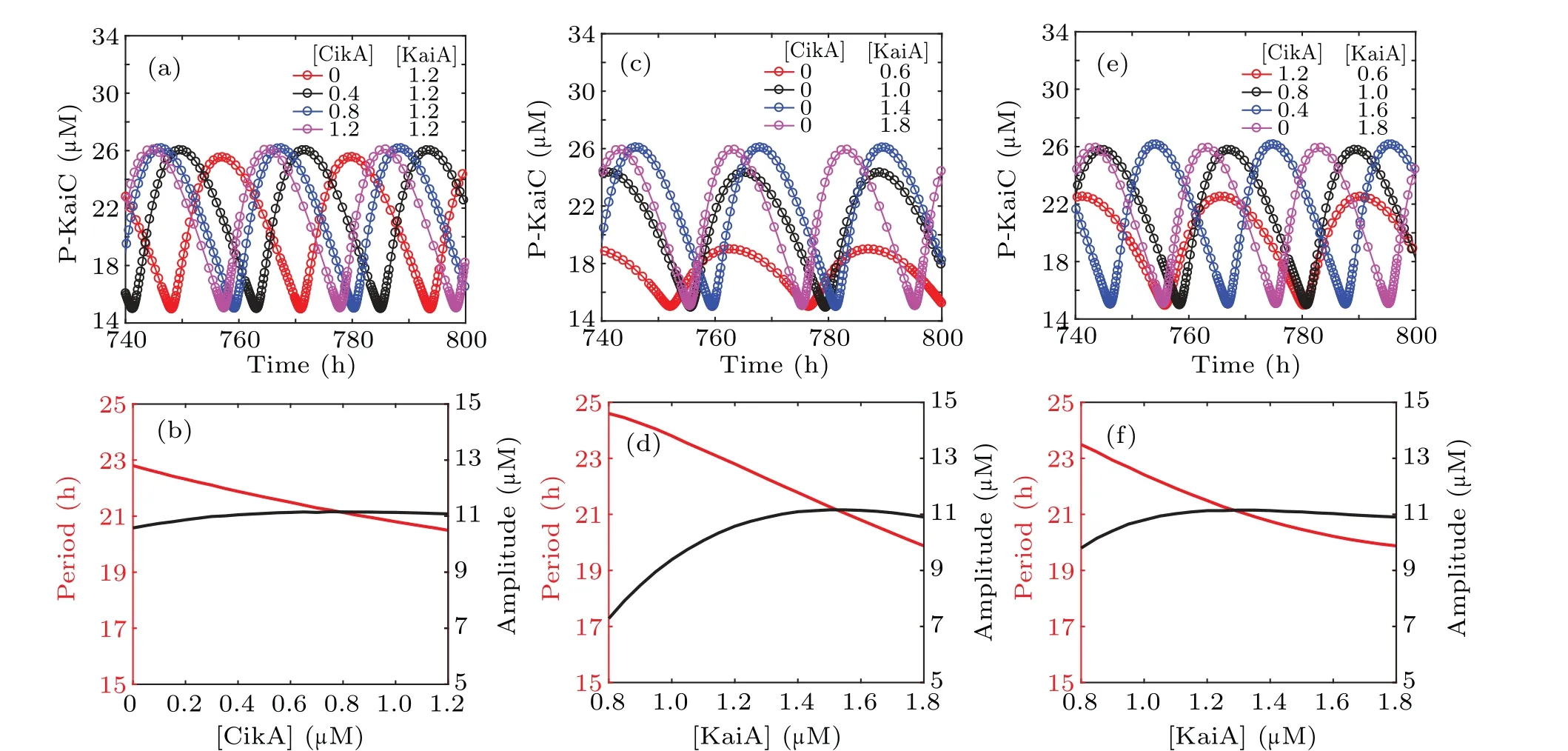
Fig. 2. Effects of varying the amounts of CikA and KaiA on KaiC phosphorylation. (a) Simulation of circadian oscillators of P-KaiC with various[CikA]and fixed[KaiA](1.2µM).(b)Amplitude and period of P-KaiC in(a). (c)Simulation of circadian oscillators of P-KaiC with various[KaiA]and without CikA.(d)Amplitude and period of P-KaiC in(c). (e)Simulation of circadian oscillators of P-KaiC with various[KaiA]and[CikA]. (f)Amplitude and period of P-KaiC in(e),with[CikA]+[KaiA]=1.8µM.
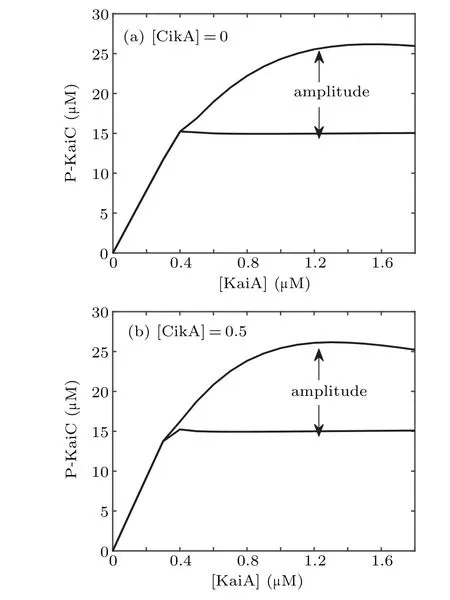
Fig.3.Bifurcation diagrams with[KaiA]as the control parameter in the absence(a)and presence of CikA(b),respectively.
3.2. Sensitivity analysis of the oscillation period to parameter values
Robustness is an intrinsical property of a system, which describes the capability to keep performance facing up to the perturbation of system parameters. In order to study the robustness of the system with the regulation of CikA,we present the dynamic sensitivity analysis. The degree of robustness displays an inverse correlation with that of the corresponding sensitivity. The oscillator period is an important character to quantify the oscillator system,so we investigate the period sensitivity. The period sensitivity shows the variations of period coming from perturbations of the parameters in the model,which is described as follows:[22]

whereTis the period of the circadian clock,andpiis the parameter with indexi. Considering that there are several parameters in the model,it is difficult to fully study the dynamic properties of the system in the whole parameter space.In order to evaluate the period’s sensitivity to changes of the parameters, we vary one parameter value at a time and keep the rest unchanged.
The following system parameters are considered. The first kind is about the production of KaiC and dephosphorylation of KaiC, denoted by parameterspandkdrespectively,which reflect the roles of TTFL on the cyanobacterial circadian clock. The second kind is about the regulation of KaiA,KaiB and KaiC on the phosphorylation of KaiC, denoted by parameterka, which reflects the role of OPT on the circadian clock. The third kind is about the degradation rate of kaiBC,NP-KaiC,and P-KaiC,denoted byδ1,δ2,andδ3,respectively.

Fig.4. Period sensitivity related to the parameters of the system. [(a),(b)] The conditions of +10% perturbation and −10% perturbation to each parameter,respectively. The other parameters are fixed.
Figure 4 shows the period sensitivity to the parameter perturbations with different concentrations of CikA. In Fig. 4(a)and 4(b), +10%perturbation and−10%perturbation to each parameter are introduced,respectively.From Fig.4,we obtain some interesting results. With the regulation of CikA,the system still oscillates when the parameters are set within a certain range. That is to say,the system is robust with the regulation of CikA. With or without CikA, the oscillator period is least sensitive to the perturbation of parameterspandkd, which are associated with TTFL.However,it is most sensitive to the perturbation of parameterka,which is associated to PTO.This finding indicates that the PTO as the core pacemaker plays the key role on deciding the oscillator period,while the TTFL is a slave oscillator. The presence of CikA decreases the sensitivity of the period to most of the parameters related to the TTFL and the sensitivity is inversely proportional to the concentration of CikA.On the contrary,the presence of CikA increases the sensitivity toka, which is proportional to the concentration of CikA.This result indicates that the regulation of CikA further strengthens the crucial role of the PTO. With the regulation of CikA,the system is more sensitive to the core PTO compared with that without CikA.That is to say,with the regulation of CikA,it is easier for the system to modulate its period against the environmental perturbations.
3.3. CikA increases the adaptation to disrupted light-dark cycle
Without the external time cues,the endogenous period of the cyanobacterial circadian clock is not exactly 24 h. The period becomes 24 h when it is exposed to natural environmental cycles. In other words, the oscillator is phase-locked or entrained in the external cycles.[23]The daily light-dark(LD) cycle is the strongest entraining signal. If entrainment is disrupted by a sudden shift in the phase of the LD cycle,the circadian oscillator will adjust its phase until the oscillator is reentrainmented or locked to the new LD cycle.[24]The time for the oscillator to get reentrainment reflects the ability of organisms to adapt to environmental changes,i.e.,adaptation.
Experimental observations show that the light can increase KaiC phosphorylation rate,so we will simulate the effect of light by adding a term to the right-hand side of the differential equation for P-KaiC,i.e.,


Fig.5.Reentrainment of the cyanobacterial circadian clock where the LD cycle is disturbed by 6 h delays.Simulation of circadian oscillators of P-KaiC in the absence of CikA(a)and in the presence of CikA(b),respectively. The solid and dotted lines indicate the oscillator in the normal LD cycle and the disturbed LD cycle respectively. (c)The corresponding circadian cycles in(a)–(b)are projected onto NP-KaiC-P-KaiC plane.(d)Phase shifts between the circadian oscillators in normal LD cycle and that in the disturbed LD cycle.
Figures 5(a)and 5(b)show that the cyanobacterial circadian clock can be entrained by the LD cycle whenL0is set to appropriate values with or without CikA.Given the entrainment,we study the speed at which the system adjusts the phase to get reentrainment when there is a sudden shift in the phase of the LD cycle. The circadian oscillator runs for 20 days in a normal LD cycle. Then 6 h delay for light are added on the 21-th day. The dotted lines in Figs. 5(a) and 5(b) show the process of adjusting the phase of the system to reentrainment with and without CikA, respectively. Six-hour delay of light leads to phase delay of the oscillator. Comparing Figs. 5(a)and 5(b),we find that the phase shift is larger in the situation with the regulation of CikA than that without CikA. In order to see the adjustment speed more clearly, taking the peak of P-KaiC concentration as the phase of the circadian oscillator,we calculate the phase shifts between the circadian oscillator under the new LD cycle and the normal LD cycle, as shown in Fig.5(c). We can see that the presence of CikA speeds up the reentrainment of the circadian system, i.e., the regulation of CikA strengthens the adaptation of the system.
In order to explore the dynamical mechanisms of the effect of CikA,we present the phase plane diagram in Fig.5(c).The presence of CikA weakens the inhibition of KaiB on KaiA which increases the activation of KaiA on promoting the phosphorylation of KaiC; however, the phosphorylated KaiC inhibits the transcription of KaiBC. Consequently, the regulation of CikA brings about a lower concentration of the total KaiC compared with the situation without CikA. Thus, the limit cycle is smaller in the situation with CikA than that without CikA, as shown in Fig. 5(c). On the other hand, the LD cycle directly affects the phosphorylation of KaiC.The phase changing of the LD cycle leads to the phase adjustments until the system resynchronizes with the new LD cycle. However,the amount of total KaiC that can be changed during a period is limited. Therefore, it will take longer time for the larger cycle to adjust the same amount of phase shift. This analysis explains why it is easier for the system to get reentrainment with CikA than that without CikA.
Instead of light delay in the LD cycle, we also studied the reentrainment where the light is advanced by 6 h. We get the same conclusion that CikA increases the speed of adjusting phase shifts,which increase the system’s adaptation to the disrupted LD cycle.
3.4. Amplitude response curve
In addition to period, amplitude also plays an important role in the circadian clock. The degree of circadian rhythm was related to the amplitude of circadian oscillator,which can be response to external stimuli.[25]Researchers experimentally found that higher amplitude of circadian oscillator enhances fitness in cyanobacteriain silico.[26]For mammals, it was found that people with schizophrenia,depression or cancer have no circadian clock or very weak circadian clock(low amplitude). Thus, it is important to explore at what time the external stimulation added helps to increase amplitude and when stimulation inhibits amplitude which should be avoided.In clinical medicine,external stimulation is an important measure to treat related diseases.[27,28]
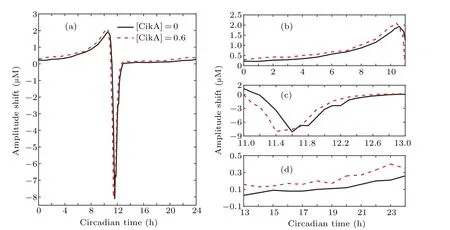
Fig.6. (a)The amplitude response curve(ARC)of the circadian clock. (b)–(d)The partial enlarged details of(a)during different intervals of CT.The horizontal axis represents the circadian time when light pulse is added. Negative(positive)amplitude changes correspond to the increase(decrease)of amplitudes after stimuli.The black line indicates the situation without the regulation of CikA,and the red dotted line indicates that with[CikA]=0.6µM.The pulse duration is 1 h.
Making amplitude response curves(ARCs)is an effective way to study the amplitude changes induced by environmental signals added at different circadian times(CTs).In order to see the difference between the amplitude changes clearly,we draw the ARCs corresponding the situations with and without CikA shown in Fig. 6, where 1 h light pulse is added at different CTs. A complete circadian rhythm cycle includes two parts,the phosphorylation process and the dephosphorylation process,which correspond to the increase and decrease processes of the P-KaiC concentration respectively.The time at which PKaiC reaches its peak level is defined as CT0. The periods of the circadian system are 22.77 h and 21.47 h in the situations with the regulation of CikA and without CikA, respectively.Thus, the interval from CT0 to CT11 corresponds roughly to the dephosphorylation process;and the interval from CT11 to CT22 corresponds roughly to the phosphorylation process.We measured the amplitude of P-KaiC before and after the light pulse. From Fig. 6(a), we can see that the system receiving light stimulation exhibits increase of amplitude at most CTs.However,the system receiving light stimulation at CTs(11 h–13 h) around the trough level of P-KaiC shows significant amplitude reduction (see Fig. 6(c)). This behavior should be avoided because reduction of amplitude decreases the fitness of cyanobacteria. Comparing the black line (without CikA)and the red dotted line(with CikA)in Fig.6(a),the peak and trough of the red dotted curve are slightly larger than those of the black curve. That is to say, with the regulation of CikA,the amplitude increases slightly more and decreases slightly less, which means that CikA slightly increases the fitness of cyanobacteria. Combining Figs. 6(b) and 6(d), we can see that the amplitude increases more when the light stimulation is added during the dephosphorylation process than that when the light stimulation is added during the phosphorylation process.
4. Conclusion and discussion
As an input pathway component, CikA plays essential roles in the cyanobacterial circadian clock by regulating the phosphorylation state of KaiC. However, to our knowledge,the current mathematical models of the cyanobacterial circadian clock have been made only considering interactions between the Kai proteins. In the present paper,to investigate the dynamical potential of CikA in regulating the cyanobacterial circadian clock, a more accurate model of introducing CikA into the cyanobacterial circadian clock is built based in part on the model proposed by Takigawaimamuraet al.[7]A detailed study of the model is carried out and the uncovered dynamic mechanism fits the biological observations on the roles of CikA in regulating circadian clock. Therefore, the model captures the main features of CikA regulation, which can be used to study other biological problems associated with CikA as an input component.
With the model, we get some interesting results about CikA, which affects both the period and amplitude of the cyanobacterial circadian clock. The presence of CikA expands the range of parameters that make the system produce oscillators, which indicates stronger robustness of circadian oscillator. Sensitivity analysis shows that CikA also strengthens the central role of the PTO as a core loop. At the same time, CikA improves the adaptability of the system. In other words,with the regulation of CikA,it is easier for the system to adapt the change of environment. The ARCs tell us that CikA enhances the fitness of cyanobacterial. These findings may help us understand the mechanism of CikA-regulated circadian clock more deeply, which also provides a theoretical clue for biological researchers.
Besides input functionality of CikA as an input pathway component, it also acts to deipnosophist the output pathway transcription factor RpaA.[12]The technique used is also helpful to analyze the output functionality of CikA. In the near future, with the help of mathematical model and dynamical analysis, we will further explore the dynamical mechanisms of CikA as an output pathway component in the cyanobacterial circadian clock.
- Chinese Physics B的其它文章
- Physical properties of relativistic electron beam during long-range propagation in space plasma environment∗
- High winding number of topological phase in non-unitary periodic quantum walk∗
- Widely tunable single-photon source with high spectral-purity from telecom wavelength to mid-infrared wavelength based on MgO:PPLN∗
- Control of firing activities in thermosensitive neuron by activating excitatory autapse∗
- Adaptive synchronization of chaotic systems with less measurement and actuation∗
- Dynamics analysis of a 5-dimensional hyperchaotic system with conservative flows under perturbation∗

