Transcranial direct current stimulation for auditory verbal hallucinations: a systematic review of clinical trials
Samaneh Rashidi, Myles Jones, Eric Murillo-Rodriguez, Sergio Machado,Youguo Hao , Ali Yadollahpour,
Abstract Transcranial direct current stimulation (tDCS) has been reportedly beneficial for different neurodegenerative disorders. tDCS has been reported as a potential adjunctive or alternative treatment for auditory verbal hallucination (AVH). This study aims to review the effects of tDCS on AVH in patients with schizophrenia through combining the evidence from randomized clinical trials (RCTs). The databases of PsycINFO (2000–2019), PubMed(2000–2019), EMBASE (2000–2019), CINAHL (2000–2019), Web of Science (2000–2019),and Scopus (2000–2019) were systematically searched. The clinical trials with RCT design were selected for final analysis. A total of nine RCTs were eligible and included in the review. Nine RCTs were included in the final analysis. Among them, six RCTs reported a significant reduction of AVH after repeated sessions of tDCS, whereas three RCTs did not show any advantage of active tDCS over sham tDCS. The current studies showed an overall decrease of approximately 28% of AVH after active tDCS and 10% after sham tDCS.The tDCS protocols targeting the sensorimotor frontal-parietal network showed greater treatment effects compared with the protocols targeting other regions. In this regard,cathodal tDCS over the left temporoparietal area showed inhibitory effects on AVHs. The most effective tDCS protocol on AVHs was twice-daily sessions (2 mA, 20-minute duration)over 5 consecutive days (10 sessions) with the anode over the left dorsolateral prefrontal cortex and the cathode over the left temporal area. Some patient-specific and diseasespecific factors such as young age, nonsmoking status, and higher frequencies of AVHs seemed to be the predictors of treatment response. Taken together, the results of tDCS as an alternative treatment option for AVH show controversy among current literatures, since not all studies were positive. However, the studies targeting the same site of the brain showed that the tDCS could be a promising treatment option to reduce AVH. Further RCTs,with larger sample sizes, should be conducted to reach a conclusion on the efficacy of tDCS for AVH and to develop an effective therapeutic protocol for clinical setting.
Key Words: auditory verbal hallucinations; dorsolateral prefrontal cortex; effective protocol; randomized clinical trial, schizophrenia; temporoparietal area; transcranial direct current stimulation; treatment efficacy
Introduction
Auditory verbal hallucinations (AVHs) are auditory perceptions involving a verbal aspect in the absence of a provoking external stimulus (Liester, 1998). AVHs are a core symptom of schizophrenia spectrum disorders, but they also frequently occur in other psychiatric disorders as well as in non-psychiatric general population (Maijer et al., 2018). In schizophrenia, AVHs are referred to a multi-dimensional and heterogeneous group of symptoms that could be categorized into different subtypes according to certain phenomenological features such as subjective loudness, acoustic clarity, and the perceived location of the voices.
Schizophrenia is a mental disorder characterized by disruptions in thought processes, emotional responsiveness,perceptions, and social interactions (Andreasen and Flaum,1991; Wong and Van Tol, 2003). Schizophrenia affects more than 26 million people worldwide with about 1%prevalence in adult population worldwide. As the etiologies of schizophrenia are still unknown, the current treatments focus on eliminating the symptoms of the disease. Antipsychotic medications are effective in treating acute psychotic episodes and improve symptoms of early schizophrenia in 85% of patients (Andreasen and Flaum, 1991; Hugdahl and Sommer,2018). About 60–80% of patients affected by schizophrenia experience AVHs during the course of the disease (Andreasen and Flaum, 1991; Hugdahl and Sommer, 2018), but AVHs also frequently occur in other psychiatric disorders and in the non-psychiatric general population. In schizophrenia, AVHs comprise a multi-dimensional group of symptoms that can be differentiated by certain phenomenological aspects such as subjective loudness, acoustic clarity, location and subjective reality (Fleischhacker et al., 2014).
Treatment of AVHs is still a major clinical challenge (El-Mallakh and Walker, 2010). The standard treatment option currently recommended by the American Psychiatric Association is antipsychotic medications, which could effectively inhibit AVHs in most of the patients. However, 25–30% of the patients do not respond to the medications (Sommer et al., 2012; Hugdahl and Sommer, 2018). Considering the severe and debilitating effects of the AVH symptoms in the treatment-resistant patients, developing new treatments is necessary.
Transcranial direct current stimulation (tDCS) is a non-invasive cost-effective and safe neuromodulation technique that acts through delivering a weak direct current (typically 1–2 mA) via two surface electrodes placed on scalp (Fregni et al., 2008).The current passes through the brain regions and changes the neural and cortical excitability of the stimulated neurons in a polarity dependent manner. Anodal tDCS (positively charged electrode) increases the neural excitability, whereas the cathodal electrode (negatively charged) decreases it(Koop and Spangenberg, 1989; Wachter et al., 2011; Woods et al., 2016). The effects of tDCS on cortical excitability have been reportedly attributed to the depolarization or hyperpolarization shifts in the resting membrane potentials(Stagg and Nitsche, 2011; Pellicciari et al., 2013; Romero Lauro et al., 2014; Woods et al., 2016).
Since the development of tDCS in the 1960s, it has been used for different disorders and for different purposes in healthy individuals. The current evidence has suggested the potential therapeutic outcomes of tDCS in depression, addiction,tinnitus, Alzheimer’s disease, attention deficit hyperactivity disorder, and stroke (Gandiga et al., 2006; Suemoto et al.,2014; Cosmo et al., 2015; Wu et al., 2015; Lefaucheur et al.,2017; Yadollahpour et al., 2017; Yuan and Yadollahpour, 2018).This modality has been used for improving different cognitive functions in healthy individuals and also improving athletic and artistic performance (Borducchi et al., 2016; Edwards et al., 2017). Several studies have reported the beneficial effects of tDCS on chronic tinnitus (Yuan et al., 2018; Lee, 2019).These positive impacts of tDCS on tinnitus have encouraged researchers to investigate the possible therapeutic effects of tDCS for treatment of AVHs.
The main rationale for this motivation was the similarity
between the underlying mechanism of subjective tinnitus and AVHs in patients with schizophrenia (Mori et al., 2016). In this regard, several studies have investigated the effects of tDCS on AVHs in patients with schizophrenia (Agarwal et al., 2013;Fitzgerald et al., 2014; Slotema et al., 2014; Mondino et al., 2015a; Smith et al., 2015; Kantrowitz et al., 2019). The initial studies have shown controversial findings on the efficacy of tDCS in AVHs. However, some studies have reported that tDCS could reduce AVHs and other positive symptoms in patients with schizophrenia (Brunelin et al., 2012; Mondino et al.,2014; Kantrowitz et al., 2019). The studies are ongoing to find whether tDCS could be used as an alternative or add-on treatment for refractory AVHs. Conducting systematic reviews on the current literature on tDCS applications for AVHs could help researchers design further trials to reach definitive answer to this question. Therefore, this systematic review aims to investigate the therapeutic effects of tDCS on AVHs in patients with schizophrenia. We will review randomized clinical trials (RCTs) that investigated the effects of tDCS on AVHs symptoms.

Figure 1 |Flow diagram for study selection of studies addressing the effects of tDCS on AVH.
Data and Methods
Search strategy
This study was performed in accordance with Preferred Reporting Items for Systematic review and Meta-Analysis(PRISMA) protocol. The databases of PsycINFO (2000–2019),PubMed (2000–2019), EMBASE (2000–2019), CINAHL (2000–2019), Web of Science (2000–2019), and Scopus (2000–2019)were systematically searched using the search terms “auditory verbal hallucination” OR “AVH” OR “auditory hallucination”“hallucinate” OR “hallucinated” OR “hallucinating” OR“hallucination” OR “hallucinations” OR “hallucinatory” OR“hallucinatory” OR “voice hearing” AND “schizophrenia” OR“schizophrenic” AND “transcranial direct current stimulation”OR “transcranial direct current stimulus” OR “transcranial electrical stimulation” OR “tDCS”. The articles written in English were eligible for evaluation and the last search was performed on March 30, 2019. The clinical trials with RCT design were selected for final analysis. The reference lists of the tDCS review articles and the retrieved papers were also searched for additional records.
Eligibility criteria
Studies investigating the effects of tDCS on AVH in patients with schizophrenia were included. Only RCTs that assessed the effects of tDCS on AVHs taking a sham treatment as control were considered for inclusion. Studies that contained populations of patients with schizophrenia accompanied by neuropsychiatric comorbidities were excluded. Other types of study rather than RCT were excluded (open label, case report, case series, cohort, and reviews), as well as studies with populations younger than 18 years, or studies that investigated tDCS in combination with a medication or other non-medication modality. Furthermore, letters to editors,editorials, commentaries, and conference abstracts were excluded. The titles and abstracts of all retrieved records were reviewed and the eligible records were entered in the final review based on the inclusion and exclusion criteria.Figure 1 shows the details of the literature search. A total of 354 articles were identified through the search; 39 additional articles were identified through a search of the references of selected articles resulting in 359 records. After removing duplications, 256 records were entered in the next step.Following review of titles and abstracts, 38 articles were selected for full articles review. Finally, nine articles met the criteria for full analyses and entered into the final analysis.The basic information and characteristics of the reviewed studies are presented in Table 1.
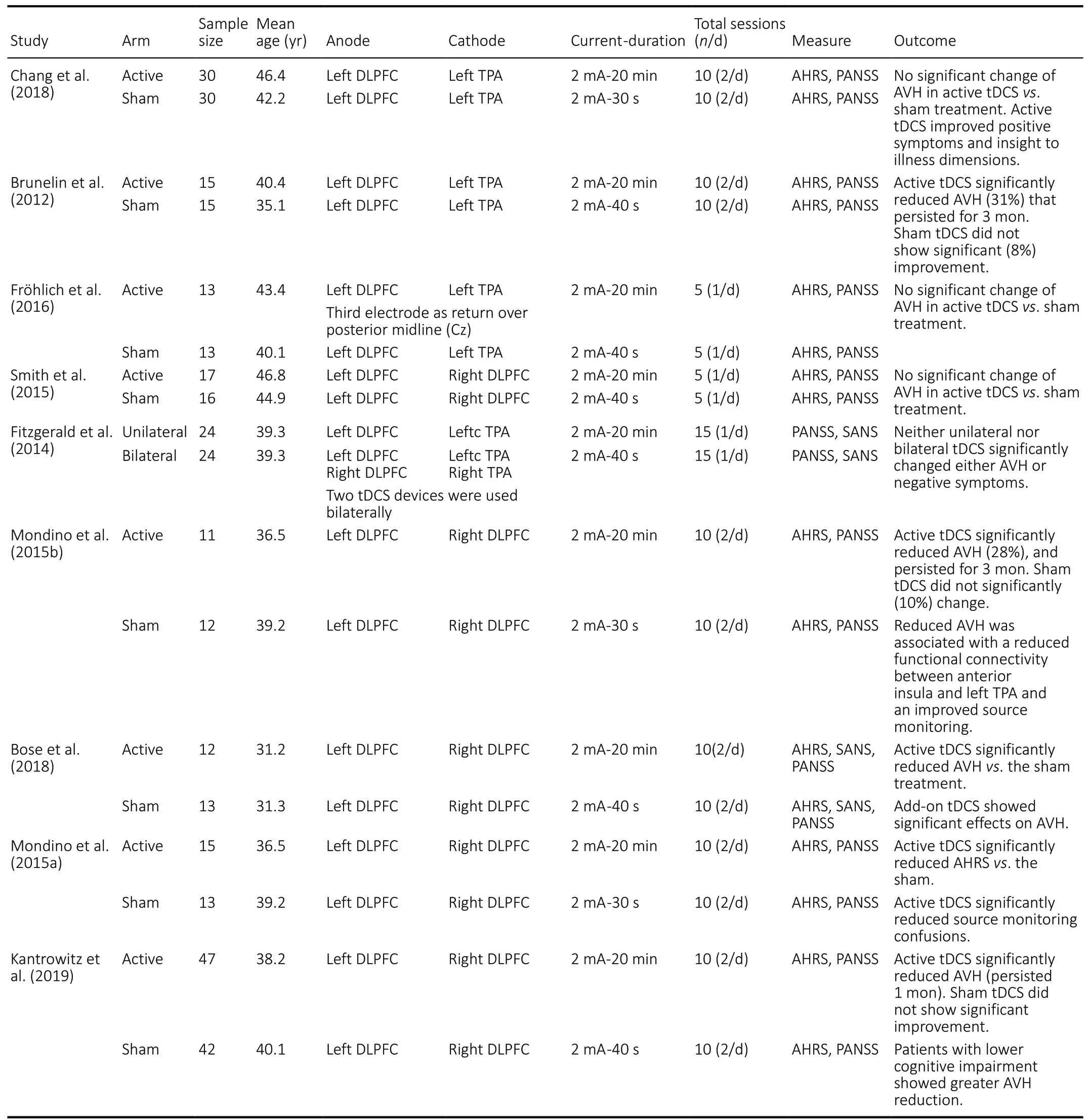
Table 1 |Clinical parameters of studies obtained for review
Results
Totally, nine RCTs (Brunelin et al., 2012; Fitzgerald et al., 2014;Mondino et al., 2015a, b; Smith et al., 2015; Fröhlich et al.,2016b; Bose et al., 2018; Chang et al., 2018; Kantrowitz et al., 2019) were reviewed in this study (Table 1). Among these studies, six reported a significant reduction of AVH following repeated sessions of tDCS (Brunelin et al., 2012; Mondino et al., 2015a, b; Bose et al., 2018; Chang et al., 2018; Kantrowitz et al., 2019), whereas three RCTs did not show any advantage of active tDCS over sham tDCS (Fitzgerald et al., 2014; Smith et al., 2015; Fröhlich et al., 2016b). However, most of the studies with negative findings reported the beneficial effects of tDCS on other symptoms of schizophrenia.
The most effective tDCS protocol of AVH was twice-daily sessions (2 mA, 20-minute duration) over 5 consecutive days (10 sessions) with the anode over the left dorsolateral prefrontal cortex (DLPFC) and the cathode over the left temporoparietal area (TPA). The current studies showed an overall decrease of approximately 28% of AVH after active tDCS and 10% after sham tDCS. The therapeutic effects are more significant when the stimulation sites and protocols are targeting at the sensorimotor frontal-parietal network compared with the situations not targeting at the sensorimotor frontal-parietal network. In this regard, cathodal tDCS over left TPA showed inhibitory effects on AVH.
The first RCT on the effects of tDCS on AVH in patients with schizophrenia was performed by Brunelin group. In a double blind sham RCT, Brunelin et al. (2012) investigated the effects of anodal tDCS over frontotemporal region in patients with treatment-resistant AVH (n= 15). The tDCS consisted of a 20 minute tDCS treatment session twice daily at an intensity of 2 mA, for 5 consecutive days. They applied the sham tDCS in the counterpart subjects (n= 15). They reported a significant reduction in the severity of AVH in 31% patients receiving active frontotemporal tDCS stimulation, compared to the sham tDCS with 10% mean reduction (Brunelin et al.,2012). They reported that the beneficial impacts of tDCS on AVH symptoms remained significant 3 months after the tDCS regimen. Bose et al. (2018) in a double blind placebo RCT examined the effect of add-on tDCS (cathode over the left TPA and anode over the left DLPFC; 2 mA, twice-daily sessions for 5 days; a total of 10 sessions) on refractory AVH in patients with schizophrenia (n= 25). Following the RCT phase, the patients that had < 30% reduction in AVH severity underwent an open-label extension active stimulation to evaluate the effect of cross-over to active tDCS. In the RCT phase, the active(n= 12) group showed significantly greater reduction of AVH score compared to the sham (n= 13) group. In the open-label extension phase, sham patients who crossed over to active treatment (n= 13) showed significantly greater reduction in AVH severity than the corresponding change during RCT phase. The add-on tDCS has beneficial effects on refractory AVH in schizophrenia.
Kantrowitz et al. (2019) in a large sample sized (n= 89)double blinded RCT investigated the effects of frontotemporal tDCS on antipsychotic-resistant AVH in schizophrenia. They randomly divided the patients into active (n= 47) and sham (n= 42) groups. The tDCS protocol was the same as that in the previous studies consisting of 5 days of twicedaily 20-minute sessions with an intensity of 2 mA. The AVH severity was assessed using the Auditory Hallucination Rating Scale (AHRS) total score. They reported a statistically significant, moderate effect-size change in AHRS total score across 1-week and 1-month in the active tDCS compared with the baseline values. The greatest change was on the AHRS loudness domain and the greatest reductions of AHRS were reported in patients with lower cognitive symptoms. This was the largest-sample-size study of tDCS for persistent AVH conducted to date. Their findings supported the previous reports of significant therapeutic benefits. However, they suggested that the medication dosage in the patients should be considered in the study design as their findings showed that the patients receiving lower medication dosage showed greater therapeutic effects. Moreover, they reported that the response was greatest in patients with lowest levels of cognitive symptoms. They concluded that overall, these findings support continued development of tDCS for persistent AVH, but also suggest that response may be influenced by specific patient and treatment characteristics.
In contrary to the positive effect of tDCS in the above studies,some studies have reported no improvements from tDCS in AVHs in patients with schizophrenia (Fitzgerald et al., 2014;Smith et al., 2015; Fröhlich et al., 2016b). Among the nine RCTs reviewed in this review, three studies (Fitzgerald et al.,2014; Smith et al., 2015; Fröhlich et al., 2016b) did not show significant reduction in AVHs in patients compared with the sham treatment.
Chang et al. (2018) in a double blind sham RCT investigated the therapeutic effects of frontotemporal tDCS on the severity of AVHs, other schizophrenia symptoms, and insight into illness of patients with schizophrenia. They randomly assigned 60 patients to receive active (n= 30) or sham (n= 30) treatments.The tDCS protocol and electrode sites were the same those in a previous study (Chang et al., 2018). They reported that the frontotemporal tDCS did not cause significant changes in the severity of AVHs and other schizophrenia symptoms. However,the levels of insight into illness and positive symptoms were significantly improved after tDCS as compared to the sham treatment. The beneficial effects on the 2 insight dimensions remained 1 month after DCS.
Fitzgerald et al. (2014) reported no significant therapeutic effects of bimodal tDCS (anodal stimulation to the DLPFC and cathodal stimulation to the TPA in schizophrenic patients with refractory AVH using Positive and Negative Syndrome Scale(PANSS) and Scale for Assessment of Negative Symptoms(SANS) scales. They suggested neither unilateral nor bilateral tDCS resulted in a significant change in either hallucinations or negative symptoms. (Fröhlich et al., 2016b) in a double blind sham RCT investigated the effects of the same bimodal tDCS(anodal over the DLPFC and cathodal over the TPA) with daily single session (2 mA, 20-minute duration, 5 days per week,3 consecutive weeks, a total of 15 sessions) in schizophrenic patients with AVH. They reported no significant improvement compared with the placebo group, in the severity of overall schizophrenia symptoms, assessed by PANSS. It seems that the more intensive application of tDCS may be therapeutically significant, especially as studies targeting the sensorimotor cortex have shown that repeated tDCS sessions at a short interval may be more effective than a single session paradigm.
Discussion
AVH is the core and main symptom of schizophrenia with high prevalence during the disease course (Hugdahl and Sommer,2018). The standard treatment option currently recommended by the APA is antipsychotic drugs, but 25–30% of the patients do not respond to them. The neurobiological basis of AVH is complex and remains unclear. However, studies assessing structural and functional connectivity in patients with schizophrenia suggested that during the occurrence of AVH,affected subjects may demonstrate significant hyperactivity in wide brain networks consisting of different areas such as leftinferior frontal (precentral gyrus, Broca’s area, and anterior insula, precentral gyrus), and left TPA (Wernicke’s area and temporal gyri) regions. These findings suggested that cortical areas involved in speech perception and production may be affected during the occurrence of AVH in schizophrenia(Fitzgerald et al., 2014; Mondino et al., 2015a; Mori et al.,2016).
Although tDCS has been extensively used in different psychiatric disorders, its application as a therapeutic modality for schizophrenic patients with AVH has just been started in recent years and most of the studies in this regard have been conducted in patients with AVH in the context of psychotic disorders. The current review describes seven randomized clinical trials, all at the experimental phase, which sought to evaluate the therapeutic effects of tDCS on AVH in schizophrenia. Our analysis showed that in eight RCT studies investigating the effects of tDCS on AVH, the cathode was placed over the left TPA and the anode over the left DLPFC for multiple sessions of 20-minute tDCS at an intensity of 2 mA. TPA is a location that has been linked to the experience of AVH, and then its stimulation may have a positive effect on AVH severity. Furthermore, the excitatory effect of the anode on the DLPFC is crucial to induce a beneficial effect on AVH. This effect can be explained by a positive effect from anodal stimulation of the left DLPFC on working memory(Fregni et al., 2008; Stagg and Nitsche, 2011; Impey et al.,2017). In conclusion, among nine RCTs reviewed in this study, six reported a significant reduction of AVH following repeated sessions of tDCS (Brunelin et al., 2012; Mondino et al., 2015a,b; Bose et al., 2018; Chang et al., 2018; Kantrowitz et al., 2019), whereas three RCTs (Fitzgerald et al., 2014;Smith et al., 2015; Fröhlich et al., 2016b) did not show any advantage of active tDCS over sham tDCS. Altogether, current studies showed an overall decrease of approximately 28% of AVH after active tDCS and 10% after sham tDCS. This study has some limitations. First, we were not able to do meta-analysis because of the heterogeneities in the design of the studies,different protocols and the different sites of stimulation.Second, we only included the studies with RCT design, which decreased the number of studies included in this review. We did not include the studies that assessed the tDCS effects using biological and laboratory surrogates
The treatment effects are more significant when the stimulation sites and protocols are targeting at the sensorimotor frontal-parietal network (Mondino et al., 2015a,b; Bose et al., 2018; Kantrowitz et al., 2019) compared with the situations not targeting at the sensorimotor frontalparietal network. In this regard, cathodal tDCS over the left TPA showed inhibitory effects on AVH. The most effective tDCS protocol on AVH was twice-daily sessions (2 mA, 20-minute duration) over 5 consecutive days (10 sessions) with the anode over the left DLPFC and the cathode over the left TPA(Mondino et al., 2015b, 2015a; Bose et al., 2018; Kantrowitz et al., 2019). Moreover, reviewing the findings of the conducted RCTs showed that some of the patients’ specific and disease specific factors such as young age, nonsmoking status, and higher frequencies of AVH seemed be the predictors of treatment response. Conducting further studies with larger sample sizes and robust design could help determine the factors that are of predictive value on treatment response.
Mechanisms of action
The exact mechanisms of therapeutic effects of tDCS on AVH are not fully understood. There are different hypotheses proposed to explain the therapeutic effects of tDCS on AVH.The first hypothesis is based on the inhibitory effects of cathodal tDCS over specific brain regions, particularly the leftTPA. Four first line studies of tDCS on AVH have targeted the left TPA (Brunelin et al., 2012; Fitzgerald et al., 2014; Fröhlich et al., 2016b; Chang et al., 2018). Two studies that used cathodal stimulation to the left TPA have reported beneficial effects on AVH (Brunelin et al., 2012; Chang et al., 2018), and the other two studies showed no beneficial effects (Fitzgerald et al., 2014; Fröhlich et al., 2016b). Therefore, it can be concluded that application of cathodal tDCS over both left TPA and left DLPFC sites contributes to the beneficial effects.
The second hypothesis on the mechanisms of therapeutic action of tDCS on AVH is that improvement of executive control by tDCS over specific regions, particularly DLPFC, acts as a mediator for the beneficial effects of tDCS on AVH (Heeren et al., 2014). This hypothesis also explains the therapeutic effects of anodal tDCS over DLPFC on tinnitus. Tinnitus,like schizophrenia, is accompanied by reduced executive control (Heeren et al., 2014). Moreover, studies have shown therapeutic effects of tDCS over the DLPFC on tinnitus symptoms (De Ridder and Vanneste, 2012; Frank et al., 2012;Lefaucheur et al., 2017). These therapeutic effects could be explained by the hypothesis of improved executive control as a mediator of tDCS effects.
Several studies have demonstrated that the excitatory effects of anodal tDCS on DLPFC could induce a beneficial effect on AVH (Brunelin et al., 2012; Mondino et al., 2016; Bose et al.,2018; Kantrowitz et al., 2019). This effect could be explained by a positive effect from anodal stimulation to the left DLPFC on working memory. Different studies have reported that excitation of DLPFC achieved by anodal tDCS reduces the negative symptoms of schizophrenia (Brunelin et al., 2012;Bose et al., 2018). Interestingly, applications of anodal tDCS over the more neutral brain regions that are irrelevant to the AVH symptoms, such as left mastoid did not have beneficial effects on AVH (Andrade, 2013). Anodal tDCS over the left DLPFC improved working memory that plays an important role in the executive control of information (Brunoni and Vanderhasselt, 2014). Therefore, it can be concluded that anodal tDCS over the left DLPFC inhibited the AVH symptoms through enhancing the inhibition of irrelevant verbal information (Homan et al., 2011). In addition to the direct effects of tDCS, exerted on the local exposed regions under the two electrodes, it also exerts indirect effects that could alter the functions of other brain regions and distinct neural networks.
Adverse effects and tolerability
The studies conducted on the safety and possible adverse effects of tDCS have reported that this technique is generally safe with few mild side effects. Some of the current studies have assessed the side effects and tolerability of the technique. Brunelin et al. (2012) did not report side effects.They described the tDCS a low-cost and user friendly modality with few side effects. Smith et al. (2015) assessed the adverse effects with customized open-ended questionnaire at each session. They reported that the patients tolerated the treatment well and there was no significant difference in side effects between the active and sham tDCS groups. Fröhlich et al. (2016a) reported no differences between the tDCS and sham groups on the experienced side effects.
The other studies that assessed the tolerability to and adverse effects of the tDCS reported some mild side effects of a transient nature in association with tDCS including a slight headache, itching or tingling sensation and redness of skin at the location of the electrodes, and slight sense of burning(Matsumoto and Ugawa, 2017; Bayat et al., 2018; Rashidi et al., 2019). However, most of these studies have reported that such side effects are also reported after the sham treatment.The tDCS interventions were generally well tolerated by the patients with no serious adverse effects. This makes the tDCS modality a promising alternative or adjunctive to the conventional medications for AVHs particularly in those patients who do not respond to the conventional medications or could not tolerate such drugs.
The present study reviewed the current literature of RCTs on the effectiveness of tDCS on treatment-resistant AVH in patients with schizophrenia. Taken together, the current literature shows controversy regarding the results of tDCS as an alternative treatment option for AVH, since not all studies were positive. Some of them show significant results when targeted the left TPA and others the left DLPFC. These findings showed that the tDCS could be a promising treatment option to reduce AVH. In addition, it should be noted that the results of the tDCS studies are scarce and the technique is a young treatment method. The tDCS interventions were generally well tolerated by the patients with no serious adverse effects. Therefore, caution is necessary when interpreting the results presented, and does not disrupt tDCS as a promising alternative or adjunctive to the conventional medications for AVH particularly in those patients who do not respond to the conventional medications or cannot tolerate such drugs.Further RCTs, with larger sample sizes, should be conducted to reach a conclusion on the effectiveness of tDCS on AVH and to develop an effective therapeutic protocol for clinical setting.
Author contributions:SR, MJ, AY searched the databases and retrieved the records. SR, MJ, SM, YH, EMR, and AY reviewed the titles and abstracts to choose the papers for analysis. SR, SM, YH, and EMR wrote the manuscript draft. MJ, YH, and AY performed the critical reviews on the manuscript. All authors read and approved the final manuscript.
Conflicts of interest:The authors declare no conflict of interest.
Financial support:None.
Reporting statement:This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic reviews andMeta-Analyses (PRISMA) guidelines.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Giovanni Assenza, Universita Campus Bio-Medico di Roma, Italy.
- 中国神经再生研究(英文版)的其它文章
- The use of hydrogel-delivered extracellular vesicles in recovery of motor function in stroke: a testable experimental hypothesis for clinical translation including behavioral and neuroimaging assessment approaches
- Advances in human stem cell therapies: pre-clinical studies and the outlook for central nervous system regeneration
- MicroRNAs in laser-induced choroidal neovascularization in mice and rats: their expression and potential therapeutic targets
- The emerging role of probiotics in neurodegenerative diseases: new hope for Parkinson’s disease?
- The phenotypic convergence between microglia and peripheral macrophages during development and neuroinflammation paves the way for new therapeutic perspectives
- Modeling subcortical ischemic white matter injury in rodents: unmet need for a breakthrough in translational research

