lmpact of intraocular pressure fluctuations on progression of normal tension glaucoma
Susanne Hopf, Doris Schwantuschke, Irene Schmidtmann, Norbert Pfeiffer, Esther Maria Hoffmann
Abstract
● KEYWORDS: intraocular pressure; intraocular pressure fluctuation; glaucoma progression; visual field; optical;optical coherence tomography
INTRODUCTION
Intraocular pressure (IOP) is described as a dynamic value with diurnal amplitude up to 5 mm Hg in healthy subjects[1-2]. The data on circadian fluctuation (amplitude between the maximum and minimum IOP readings) of normal tension glaucoma (NTG) are controversial, and range from being lower (2 to 4 mm Hg) or comparable to healthy subjects[1,3], or even higher[4]. Little is known on IOP fluctuation values calculated as “standard deviation” (SD) which is less sensitive to outliers and therefore more useful[5]. Eyes with NTG may show a larger circadian IOP fluctuation (defined as SD)compared to normal eyes (3.9vs3.0 mm Hg,P=0.040,n=46)[6].We have learned from multicenter randomized clinical trials [Advanced Glaucoma Intervention Study (AGIS),Collaborative Initial Glaucoma Treatment Study (CITGS),Collaborative Normal Tension Glaucoma Study (CNTGS)] that mean IOP is a significant risk factor for progression in NTG[7],while IOP fluctuation is not consistently a proven risk factor for development of visual field defects in preperimetric NTG[8].But several studies indicate that long-term IOP fluctuation is associated with progression of glaucomatous visual field loss in general[9-14]. Furthermore, that large IOP fluctuation is a risk factor for glaucoma progression even or especially at a low IOP level[11,15], specifically in normal-tension glaucoma[16-17].Finally, the role of short- and long-term IOP fluctuation in NTG progression is controversial.In the Early Manifest Glaucoma Trial (EMGT), and partly in the above-mentioned multicenter studies[7]further parameters beyond IOP, such as age, mean deviation, and optic disc hemorrhage are risk factors for progression.
The aim of this retrospective longitudinal cohort study is to investigate the role of short and long-term IOP fluctuation and further parameters in a large NTG cohort undergoing 48-hour IOP profile, independently from therapy regimen,regarding progression. We hypothesize that NTG progression is determined by the SD within the first pressure profile(defined as the short-term IOP fluctuation), and SD over each of the available pressure profiles (defined as the long-term IOP fluctuation).
SUBJECTS AND METHODS
Ethical Approval The study fol lows the Tenets of the Declaration of Helsinki. No formal approval from the Medical Ethical Committee of the State Chamber of Medicine of Rhineland Palatinate in Mainz, Germany was required according to their statute, because of the retrospective study design. All patients gave informed consent about data collection and analysis.
Seventy-five NTG patients of glaucoma patients treated in our clinic were enrolled in this retrospective longitudinal cohort study. Inclusion criteria were: 1) diagnosis of NTG(glaucomatous optic disc, or glaucomatous visual field defect, with normal IOP<21mm Hg) acquired from the first IOP profile (diagnosis taken from the records and from at least 3 IOP measurements), irrespective of the condition of the fellow eye; 2) having more than one 48-hour phasing of IOP during the day and an IOP measurement at night in supine position (with a time-difference between both IOP profiles of a minimum of 6mo); 3) more than three visual field examinations, Heidelberg retina tomograph (HRT) analyses and optic disc photographs per eye available. Both eyes were evaluated individually. The adjustment of anti-glaucomatous treatment was admissible. Basically, although not presented in this study, in case of unmet target IOP, medical treatment was intensified. We excluded patients with ocular hypertension,pseudoexfoliative syndrome/glaucoma, pigment dispersions syndrome/glaucoma, juvenile glaucoma, and myopia of more than -6 D. Lens status was not considered in this study. We also excluded investigations with bad quality findings in visual field analyses (fixation loss, false positive/negative rate of ≥30%) or HRT SD≥50 µm]. We considered 137 eyes of 75 patients with NTG, independently from therapy regimen.
The following methods, especially the device settings, had been used in a similar way in a previously published study[18].
The 48-hour IOP Profiles Goldmann applanation tonometry was used during diurnal and the handheld Perkins applanation tonometer (both Haag-Streit Diagnostics Holding AG, Switzerland) for the night time measurements in supine position. During the 48-hour profiles IOP was measured twice at 8:00a.m., 2:00p.m., 6:00p.m., 9:00p.m., and 12:00a.m.The overnight IOPs were measured in supine position. Ten IOP readings in each IOP profile (5 timepoints per day; each profile lasted two days) were analyzed. We computed IOP fluctuation,maximum, and mean. By defining the IOP fluctuation as SD, it represents a robust value, by taking into account the number of readings, and it is less affected by outliers compared to range[5].The amount of short-term IOP fluctuation was calculated by the SD within the first pressure profile, and long-term IOP fluctuation was calculated by the SD of the mean over each of the available pressure profiles.
Diagnostic Devices Either an Octopus 101 unit (Haag-Streit Int. AG, Switzerland), or a Humphrey Field Analyzer (HFA)II 750i (Carl Zeiss Meditec AG, Germany) was used for the visual field. The programs used were 30-degree Octopus G1, HFA 24-2 SITA-Standard, or HFA 30-2 SITA-Standard.Always the same unit and program were used for one patient.To inspect the optic nerve head three-dimensionally, HRT III(Heidelberg Engineering GmbH, Germany) was performed.
All patients received mydriatic fundus photographs of the optic disc by a Carl Zeiss fundus camera (type FF450 plus iR).
Optical coherence tomography (OCT) imaging (Spectralis OCT, Heidelberg Engineering GmbH, Germany) to measure the peripapillary retinal nerve fibre layer (RNFL) was performed in some but not all patients. The evaluated OCT data was utilized for a sub-group analysis (n=38 eyes), if at least two OCTs per eye were available. Segmentation of RNFL and its thickness determination were facilitated by Heidelberg Eye Explorer (HEYEX, version 1.9.14.0). Quality control of all segmented peripapillary OCT scans were carried out manually by a board-certified ophthalmologist (Hopf S), and the results were revised by a glaucoma specialist (Hoffmann EM). There was no disagreement between the graders in the subgroup-analysis, and no decentered scan, but two eyes were excluded for segmentation errors.
Definition of Normal Tension Glaucoma Progression The evaluation of progression as reproducible deterioration (at least one confirmation) in visual field (based on 2 baseline and 2 further visual field examinations), HRT, or optic disc photographs was performed by two graders: one inexperienced examiner (Schwantuschke D), trained on a data set of 20 visual fields, photographs, and HRT each, and an experienced examiner(glaucoma specialist, Hoffmann EM). Both graders classified progression into “suspect” progression (progression confirmed once), “possible” progression (progression confirmed twice),and “confirmed” progression (at least three confirmations,on the basis of 6 visual fields). In case of discrepancies, the experienced evaluator decided on the rating.Visual field progression was defined as: reduction in sensitivity on the pattern deviation plot, either in at least one test point location withP<0.5%, or in more than one test point location withP<1%, or in at least three test point locations withP<5%.The upper and lower rows of the physiologic blind spot were factored out of the assessment[18]. Analyzing of the visual fields with regard to the formation of clustered points and longitudinal comparing of the mean defect/deviation were applied[19].
To evaluate structural progression of rim thinning in the HRT III, we analyzed the stereometric parameters and the topographic change analysis (TCA). Changes between single scans from follow ups to baseline were detected in the superpixel analysis in a test-retest approach. Repeated deviation confirmation was flagged as “depression” in the report.
On the stereophotographs of the optic disc, progression was to be interpreted as either diffuse or localized neuroretinal rim loss, or change of the position of the vessels at the optic disc, or development of a notch/pit, optic disc hemorrhage, or incident pallor (diffuse or localized).
For the subgroup-analysis of the OCT data, the eyes were classified as having OCT progression, if peripapillary RNFL thickness exhibited a significant trend for thinning (negative slope) over time.
Statistical Analysis We used Statistical Package for Social Sciences (SPSS) for the analysis. Data analysis included descriptive statistics, and analysis of risk factors of patients’general characteristics at baseline using Chi-square test and exact Fischer test. For each general variable and for each investigated IOP parameter [mean, fluctuation, and maximum of the first IOP profile (short-term) and of all available IOP profiles (long-term)], we used univariate Cox-regression to evaluate their contribution to NTG progression. Multivariate cox-regression models tested five risk factors (age, sex,myopia of more than -3 D but no more than -6 D, mean defect/deviation of more than 3.98 dB in the first visual field, and cup-to-disc-ratio ≥0.7 by HRT at baseline) and one shortterm and long-term IOP parameter (fluctuation, maximum, or mean). To determine the progression-free time and ratio, we used a Kaplan-Meier analysis. We adjusted for dependency between eyes of patient.The IOP parameters of patients with progression was compared with those without progression using a Mann-WhitneyUtests.
RESULTS
Data of 137 eyes of 75 NTG patients (63.5% female) with a mean age of 63.3y (range 28-82y) were analyzed. The evaluation of NTG progression was based on 528 48-hour IOP profiles, 1129 visual fields, 564 HRT measurements, 621 optic disc photographs, and 148 OCTs with reliable quality. Table 1 displays the characteristics of the study population.Overall progression occurred in 46 included eyes (this corresponds to a progression rate of 33.6%) with at least one single confirmation (“suspect” progression) within a followup period of 37.6mo in average (SD 18). The total rate of confirmed progression (≥3 confirmations) was 20.4% (28/137).
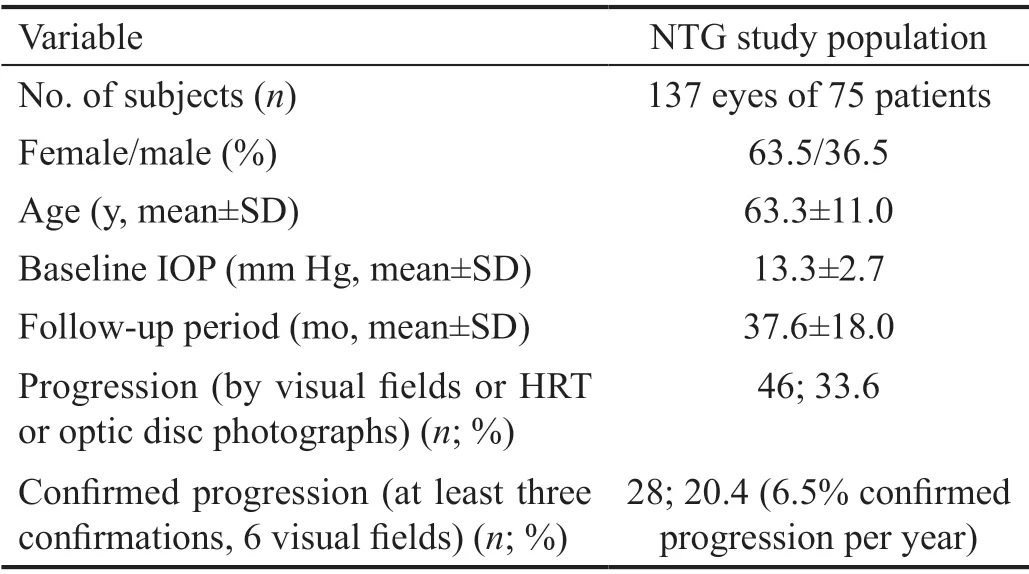
Table 1 Characteristics of the NTG study population
Risk Factors for Normal Tension Glaucoma Progression None of the investigated general characteristics at baseline(sex, myopia, glaucoma in family history, central corneal thickness ≤520 µm, mean defect/deviation ≥3.98 dB, cupto-disc-ratio ≥0.7 in the HRT, migraine, arterial hyper- and hypotension, and autoimmune disease) were significantly more frequent in the progression group than in the group without progression (Chi-square test/exact Fischer test). Circulatory disorders (peripheral vascular diseases) were more frequent in eyes without progression, but the total number was too low to draw conclusions
Myopia (HR 0.484; 95%CI: 0.2-1.0;P=0.063) and age were identified as risk factors for NTG progression in the univariate Cox-regression of the time independent variables [sex, age,central corneal thickness, myopia (SE more than -3 D), arterial hypertension, vascular disorder, migraine, mean defect/deviation (≥3.98 dB), cup-to-disc-ratio ≥0.7 per 0.1]. The risk for progression rose about 4% per year of life (HR 1.036;95%CI: 1.01-1.07;P=0.016; Table 2). The hazard ratios (HR)of the time dependent IOP parameters showed values above 1 for all parameters (Table 3), but without significantP-values.Univariate Cox-regression analyses showing the HR for time independent variables as relative risks for progression with the corresponding 95%CI andP-values.
Multivariate Cox-regression analysis revealed that none of the investigated parameters were predictive of NTG progression(Table 4). Age and myopia were not risk factors for progression in these models.
Diagnostic Devices and Progression-Free Interval Visual field progression was identified in 73.9% (36 of 46 cases).Less frequently, HRT (30.4%, 15 cases) and optic disc photographs (19.6%, 8 cases) detected progression (Figure 1).The optic disc OCT data based sub-group analysis with aresponse rate of 26.3% (n=36/137 eyes) revealed progressive reduction (negative slope) in peripapillary RNFL thickness over time in 8 out of 36 eyes (22.2%), using one confirmation for progression definition; 28/36 (77.7%) remained stable. Out of the 8 progressed eyes, 5 eyes exhibited progression by OCT alone, and not by HRT.

Table 2 Univariate Cox-regression for NTG progression (general characteristics) n=137

Table 3 Univariate Cox-regression for NTG progression (IOP parameters) n=137

Table 4 Multivariate Cox-regression analysis n=137
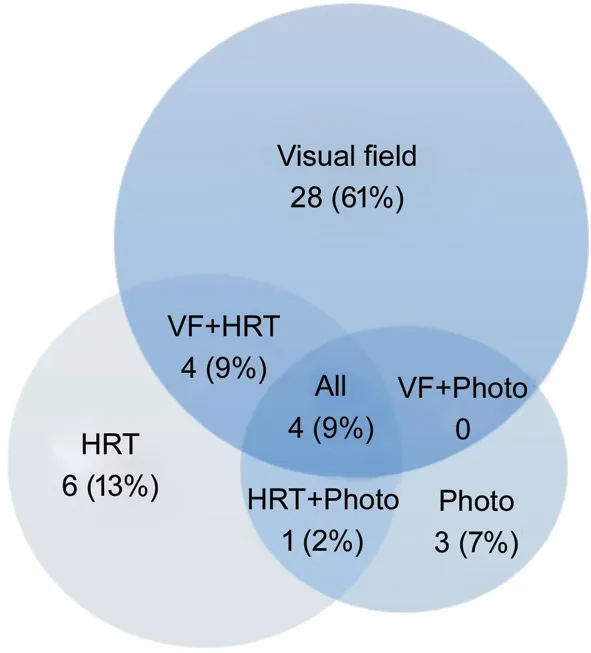
Figure 1 Venn diagram of the diagnostic device for progression detection VF: Visual field; HRT: Heidelberg retinal tomography.
The interval without progression was 60mo (95%CI: 60, 65) in average. The 5-year persistence ratio without progression was 58.2% (SD 6.5), and 75% were progression-free during 43mo.The 5-year persistence ratio without visual field progression was 67.7% (SD 5.8).
IOP Measurements in the Study Population The progression eyes showed a higher average IOP, maximum IOP(P=0.054) and IOP fluctuation at the initial visit and these results could not be proved in the long-term analysis (Table 5).
DISCUSSION
The role of IOP parameters, especially fluctuation, and further demographic and ocular factors on NTG progression was investigated in this retrospective longitudinal study. The main results were that neither the scope of IOP fluctuation over short and long periods, nor other potential ocular, demographic,and health factors we investigated, were predictors for NTG progression.
The literature is controversial on the role of short- and longterm IOP fluctuation. While Kimet al[17]found no effect of short-term fluctuation on progression in NTG, the use of a time-adjusted model revealed long-term fluctuation being a risk factor. Data from the Advanced Glaucoma Intervention Study showed that long-term IOP fluctuation in patients with low mean IOP was associated with visual field progression[11].Another study showed that long-term IOP fluctuation is associated with NTG progression[20]. Fast-progressors had larger IOP fluctuations (defined as SD) than slow-progressors.In contrast, our data showed, that neither short-term nor long-term IOP fluctuation had impact on progression. The progressionvsno progression groups differed slightly withinthe short-term interval (2.4vs2.2 mm Hg), but not in the longterm IOP parameters.
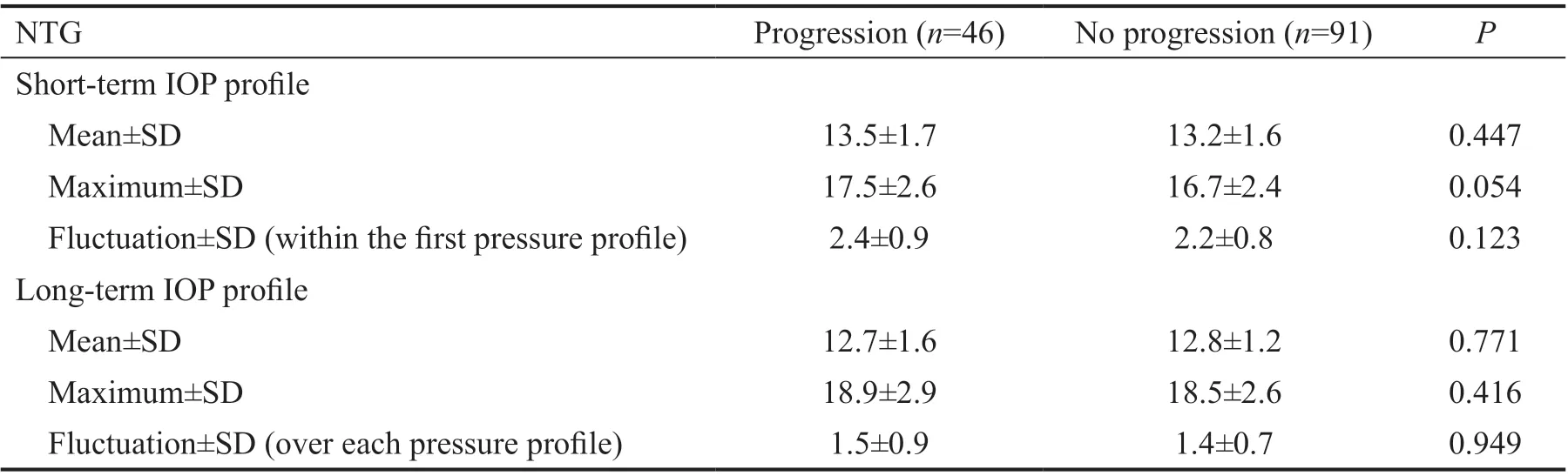
Table 5 IOP profile parameters in NTG n=137, mm Hg
Sawadaet al[8]reported short-term IOP mean but not IOP fluctuation to be strongly associated with visual field defect development in 130 eyes over 5y with preperimetric NTG.Interestingly, in our NTG cohort, IOP mean was not a risk factor for progression.
IOP fluctuation may change after glaucoma surgery such as trabeculectomy[21-22]. Despite lowering IOP surgically to 10 mm Hg, long-term IOP fluctuations (above 2 mm Hg) may be associated with visual field progression. This was shown by Honget al[21]reporting this phenomenon after triple procedures(including cataract surgery and trabeculectomy). Our results on short-term IOP fluctuations were above 2 mm Hg, and below it in the long-term perspective. Moonet al[3]investigated 24-hour IOP measurements of NTG with different optic disc phenotypes. They found differences in their range (peak minus trough), which was 4vs2 mm Hg in the focal ischemic groupvsmyopic glaucomatous group (P=0.013). Progression data were not investigated.
In our study, baseline maximum IOP was higher in patients with progression than without progression (P=0.054), but not significant in the regression models. Similarly, a greater diurnal IOP at baseline was associated with greater probability of disease progression in low-teens NTG (<12 mm Hg) in a recent study by Baeket al[16].
Although we could not find an impact of myopia on progression in NTG respecting the fluctuation we measured,Leeet al[23]reported that in myopic NTG eyes, IOP fluctuation was associated with progression, while it was not in their nonmyopic cohort with NTG.
We found “confirmed” progression in 20.4% (28/137)within 38mo of follow-up (6.5% per year). This is slightly higher than in a recent study from Baeket al[16]reporting that pretreated NTG eyes (with IOP below 12 mm Hg) showed progression in 36 patients (35%) during an average of 8.7y(4.1% per year). Sunget al[24]found a progression rate of 28%in NTG during 6y of follow-up (4.7% per year). Data from the preceding CNTGS showed comparable results with 12%(8/66) progression in treated NTG eyes and 27% (21/79) in untreated eyes within 7y[25]. Visual field testing is basically the device for determine progression by which other instruments are measured. Perimetry detects progression at a greater rate,than HRT or OCT, likewise in our cohort, especially in eyes with advanced NTG[26].
This study was based on a large dataset with a high number of examinations, similar to those in large multicenter studies.By targeting one type of glaucoma makes our study more valuable than studies including all types of glaucoma. Yet,several limitations need to be discussed in this retrospective study. The study underlies a selection bias, as not all patients with NTG receive 48-hour IOP phasing several times. Since IOP fluctuation was measured every 4h in the present study,the time between the measurements is not represented in the data. Contact lens sensors measuring continuous variables of IOP might provide more accurate data on fluctuation. The device Triggerfish™ uses the change in corneal curvature as a measure of change in IOP. However, up to now, the Triggerfish contact lens does not provide IOP data but particular units that might reflect IOP values and its usefulness has to be considered in further studies[27-29].
In addition, our clinical cohort was heterogeneous regarding the spectrum and adjustment of anti-glaucomatous therapy.Since this study has been designed as a real-life study, no restrictions regarding medical therapy modification to prevent further progression was allowed. Blood pressure measurements over 24h were not available, and OCT was not obtainable for each patient at the recruitment phase, which affects the total rate of progression. The grading of progression was without masking, and performed by two graders, without individual test-retest procedure or inter-observer reliability testing.
The scope of IOP fluctuation, mean, and maximum we studied regardless therapy regimen did not have a significant impact on NTG progression in our study. Appropriate glaucoma treatment pursuant to the outcomes of frequently performed pressure profiles and close controls (every three months) might have contributed to low fluctuations. Our analysis of risk factors revealed that none of the investigated demographic,general health and ocular parameters were associated with NTG progression. Low fluctuations in this study have pointed to pressure control, eventually facilitated by the close followups. As functional changes were most likely, NTG should be monitored with visual field testing more often than with other devices (HRT, OCT, optic disc photograph). We would endorse a long-term study to consider therapy status and vascular glaucoma aspects besides IOP parameters in NTG.
ACKNOWLEDGEMENTS
Authors’ contributions:Hopf S drafted the manuscript. Hopf S and Schwantuschke D analyzed and interpreted the data.Schmidtmann I supported the statistical analyses. Hoffmann EM designed the study and made substantial contributions to conception and interpretation of the data and revising critically the manuscript for important intellectual content. Pfeiffer N supported the study design, enabled data collection, revised,and supervised critically the work. All authors read and approved the final manuscript.
Foundation:Supported by a DFG (German Research Foundation) Grant (HO 3277/2-1).
Conflicts of Interest:Hopf S, None; Schwantuschke D, None;Schmidtmann I, None; Pfeiffer N, None; Hoffmann EM, None.
 International Journal of Ophthalmology2021年10期
International Journal of Ophthalmology2021年10期
- International Journal of Ophthalmology的其它文章
- Exosomal miR-29b found in aqueous humour mediates calcium signaling in diabetic patients with cataract
- Intraluminal stenting versus external ligation of Ahmed glaucoma valve in prevention of postoperative hypotony
- Visual acuity after intravitreal ranibizumab with and without laser therapy in the treatment of macular edema due to branch retinal vein occlusion: a 12-month retrospective analysis
- Dexamethasone intravitreal implant (Ozurdex) in diabetic macular edema: real-world data versus clinical trials outcomes
- Comparative analysis of the clinical outcomes between wavefront-guided and conventional femtosecond LASlK in myopia and myopia astigmatism
- Reliability of the ocular trauma score for the predictability of traumatic and post-traumatic retinal detachment after open globe injury
