Experimental study on the parameter optimization and application of a packed-bed dielectric barrier discharge reactor in diesel particulate filter regeneration
Yunxi SHI(施蕴曦),Yirui LU(卢奕睿),Yixi CAI(蔡忆昔),Yong HE(何勇),Yin ZHOU (周银), Yi CHEN (陈祎) and Huarong QIU (邱华荣)
1 School of Automotive and Traffci Engineering,Jiangsu University,Zhenjiang 212013,People’s Republic of China
2 Vehicle Measurement, Control and Safety Key Laboratory of Sichuan Province, School of Automobile and Transportation, Xihua University, Chengdu 610039, People’s Republic of China
Abstract To compensate for the shortcomings of the thermal and catalytic regeneration of the diesel particulate filter (DPF), a self-designed packed-bed dielectric barrier discharge (DBD) reactor for DPF regeneration was developed.The DBD reactor with the main active substance of nonthermal plasma (NTP) as the target parameter was optimized by adjusting the feed gas,packing particles (material or size), and cooling water temperature.Moreover, a set of optimal working parameters (gas source, O2; packing particles, 1.2–1.4 mm ZrO2; and cooling water temperature, 20 °C) was selected to evaluate the effect of different O3 concentrations on DPF regeneration.The research results showed that selecting packing particles with high dielectric constant and large particles, as well as reducing the cooling water temperature, with oxygen as the feed gas, contributed to an increase in O3 concentration.During DPF regeneration, the following changes were observed:the power of the NTP reactor decreased to lower than 100 W,the O3 concentration increased from 15 g m−3 to 45 g m−3,the CO and CO2 volume fractions of the particulate matter decomposition products increased, and the peak regeneration temperature increased to 173.4 °C.The peak temperature arrival time was 60 min earlier, indicating that the regeneration rate of DPF increased with the increase in O3 concentration.However, the O3 utilization rate (the amount of carbon deposit removed per unit volume O3) initially increased and then decreased; when the O3 concentration was set to 25 g m−3, the highest O3 utilization rate was reached.The packed-bed DBD technology contributed to the increase in the concentration of NTP active substances and the regeneration efficiency of DPF.It provides a theoretical and experimental basis for high-efficiency regeneration of DPF at low temperatures(<200 °C).
Keywords: dielectric barrier discharge packing particles, ozone, diesel particulate filter,nonthermal plasma
1.Introduction
Diesel engine exhaust particulate matter (PM) pollutes the environment and seriously threatens human health [1].Scholars have opined on the composition of PM.Babaie et al and Kittelson [2, 3] suggested that diesel exhaust particles mainly consist of highly agglomerated solid carbonaceous material and ash, in addition to volatile organic and sulfur compounds.Salvat et al [4] determined that PM is composed mainly of solid carbon microspheres (about 0.01–0.08 μm in diameter),gaseous hydrocarbons from diesel fuel and lube oil, sulfur dioxide(SO2),and water vapor.Maricq et al[5]demonstrated that PM consists of two types of particles: (a) fractal-like agglomerates of primary particles measuring 15–30 nm in diameter, composed of carbon and traces of metallic ash, and coated with condensed heavier end organic compounds and sulfate and (b) nucleation particles composed of condensed hydrocarbons and sulfate.Therefore, PM is a complex aggregate.The diesel particulate filter (DPF) is currently the most effective method to reduce PM emissions from diesel engines,with a collection efficiency exceeding 90%[6,7].However,as PM is deposited in the DPF pores, the DPF blocks and increases the exhaust back pressure of the diesel engine,affecting the normal operation of the engine.Thus,the DPF has to be regenerated in time [8].
The use of a dielectric barrier discharge (DBD) is an important technique to prepare nonthermal plasma(NTP).NTP contains numerous active substances (under standard atmospheric conditions: e−, O, H, OH, O3) that substantially degrade pollutants emitted by diesel engines[9–11].The use of DBD in generating NTP to purify exhaust gas has been recognized as an approach to diesel engine exhaust purification.Babaie et al [2] passed the cooled diesel engine exhaust gas into a DBD reactor for high-voltage discharge, and the generated plasma was used to decompose PM.In the test, the mass removal rate of soot was 73% at 13.5 kV, and the removal rate of particles larger than 30 nanometers exceeded 59%.Similarly, Song et al [12] evaluated the removal efficiency of diesel engine exhaust gas before and after DBD treatment; the results showed that the maximum removal rate of PM,HC,and NOxby NTP exceeded 80%,75%,and 65%,respectively.Fushimi et al [13] conducted PM removal experiments in DBD reactors with different structures and identified O3and NO2as the main active substances exerting oxidative decomposition effects on PM.To assess the potential of NTP to completely regenerate the DPF,Kuwahara et al[14]measured the difference in pressure between the inlet and the outlet of the DPF; the results showed that O3was passed into the DPF at 34.8 g h−1, and the DPF was completely regenerated after 2 h.Shi et al[15]used air as the feed gas of the DBD to regenerate the DPF.The test results indicated that NTP could decompose PM at an ambient temperature of 20 °C–300 °C, and identified CO2and CO as the main products;moreover, superior DPF regeneration effects were observed at 150 °C (with air as the gas source).However, related studies have shown that with an increase in temperature, the decomposition reaction of O3and NO2intensifies, and considerable decomposition occurs when the temperature exceeds 100°C[16].The temperature in the discharge area of the NTP reactor with natural cooling increased quickly, resulting in a short existence time and low concentration of the active substance [17].Therefore, in the previous experiment, we used water cooling to effectively control the surface temperature of the NTP discharge area by adjusting the cooling water flow rate and keeping the surface temperature below 90 °C [18].Existing studies have shown that NTP can more efficiently oxidize and remove PM.Compared with traditional regeneration technologies, such as thermal regeneration [19] and catalytic regeneration [20], NTP regeneration can avoid high temperatures in the DPF and requires no additional catalysts,showing potential for application.
To enhance the efficiency of NTP technology in purifying pollutants, numerous studies have optimized the DBD reactor.This approach can effectively introduce solid dielectric particles in the discharge area to improve the discharge[21].Jodzis et al[22]assessed the influence of a DBD reactor on O3generation under an O2/N2gas mixture.The results showed that packing SiO2particles could increase the O3concentration by 20% to 40%, with the maximum increase observed when the O2content was 20%.Liang et al[23]concluded that packing dielectric particles would reduce the breakdown voltage, resulting in an increased energy density given the same amount of applied power, which was conducive to the generation of high-concentration O3.Ray et al[24]used a packed-bed DBD reactor to convert CO2to CO.The efficiency of the CO2conversion was significantly improved with the use of glass beads,TiO2,Al2O3,CeO2, and other dielectric particles as packing materials;moreover, CeO2could stabilize O atoms to prevent CO oxidation, achieving the highest conversion rate.Liu et al [25]introduced MnOx/CeO2pellets into the discharge gap and examined the synergistic removal of PM by catalysts and NTP.The results showed that the PM removal rate could reach 85.2%at 20 °C.Packing dielectric particles enhance the ability of the DBD reactor to generate NTP active substances, which contributes to improving the conversion efficiency of pollutants.
The generation of the NTP active substance is affected by various factors, and the concentration of active substances directly influences the effect of DPF on regeneration.Previous studies have focused on the influence of a single operating parameter of the NTP reactor.A packed-bed DBD reactor was designed in this study.By adjusting the feed gas, packing particles (material or size), and cooling water temperature,the working performance of the reactor was optimized, with the NTP active substance as the index.A set of improved working parameters was selected to conduct a DPF regeneration test in the reactor to assess the effects of different O3concentrations on PM oxidation decomposition and DPF regeneration.This study aims to improve the energy efficiency of low-temperature DPF regeneration by a DBD reactor and to provide a basis for the practical application evaluation of DPF regeneration using the packed-bed DBD technology.
2.Experimental setup and method
Figure 1 presents the DPF regeneration test system using a packed-bed DBD reactor.The reactor power supply served as the intelligent electronic impact machine (CTP-2000 K).The discharge frequency was continuously adjusted in the 7–20 kHz range, and the output voltage was 0–25 kV.The capacitor voltage divider was used to divide the input voltage of the reactor and convert the input voltage into a voltage signal that could be monitored by an oscilloscope(Tektronix TDS 3034C).The P2220 passive voltage probe was used to input the voltage signal into the oscilloscope,and the P6200 probe was used to monitor the voltage signal across the measuring capacitor.The capacitive voltage divider consisted of two capacitors in series of 47 pF and 47 nF.The voltage division ratio was set to 1000:1.The sampling interval of the oscilloscope was 400 ps; that is, the sampling frequency was 2.5 GHz.The surface temperature of quartz glass in the discharge area was monitored using an infrared thermometer (TASI8606).Water cooling was used to dissipate heat in the reactor, and the cooling water circulated through the water pump, water tank,and water pipe.The gas source used in the test was divided into an oxygen source and an air source.The oxygen was provided by an oxygen cylinder with a purity of 99.99%,and the air was provided by an air compressor.The feed gas of the DBD reactor was controlled by a mass flow controller.O3was the main active product of DBD,which characterized the concentration of the NTP active gas[26].A Mini-HiCon ozone analyzer was used to monitor the O3concentration (Interscan4480).Before the start of the DPF regeneration test, valve 2 was opened,whereas valves 1 and 3 were closed.The ozone analyzer monitored the O3concentration produced by the DBD reactor(the front end of the DPF).When valve 2 was closed and valves 1 and 3 were opened,the active substance produced by the DBD reactor entered the DPF via the pipeline and reacted with the deposited particles.The ozone analyzer then monitored the O3concentration at the back end of the DPF.The PM capture test was conducted on four green DPFs (Jiangsu Province Yixing Nonmetallic Chemical Machinery Factory Co.,Ltd).The diesel engine model YD480 was used under the following conditions: torque, 69 N m−1; speed, 2000 r min−1;and trapping time, 120 min.The DPF to be regenerated was placed in a temperature control device,the temperature of which was maintained at 80 °C.The NTP active gas was passed though the DPF for DPF regeneration.The gas analyzer was used to monitor changes in the concentrations of CO and CO2,the DPF regeneration products.

Figure 1.DPF regeneration test system using a packed-bed DBD reactor.
Figure 2 presents a schematic of the cross-sectional structure of a packed-bed DBD reactor.The quartz glass acts as the discharge medium and has a quartz tube with a 56 mm outer diameter and a 2 mm thick wall.The stainless steel tube is used as a high-voltage electrode,with a 48 mm outer diameter and a 4 mm thick wall.Both the quartz tube and the stainless steel tube are 400 mm in length and are arranged in a coaxial cylindrical structure.The discharge gap is 2 mm,which can be packed with microparticles.The grounding electrode is a stainless steel mesh covering the outside of the quartz tube,which is fixed by a copper wire and connected to a grounding wire.In the experiment,the waveform of the applied voltage to the reactor is a sine wave,and the frequency is 8 kHz.To ensure that the particles can be tightly filled in the discharge gap of the reactor, particles with a diameter of less than 1.5 mm are used.Table 1 lists the relevant parameters of the packing particles.

Figure 2.Schematic of the cross-section of a packed-bed DBD reactor.
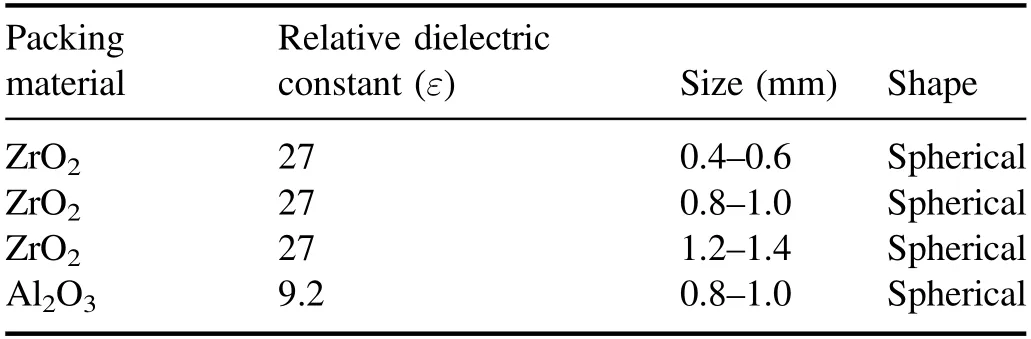
Table 1.Packing particle parameters.
Table 2 lists the structural parameters of the DPF used in the test.During DPF regeneration, the internal temperature is monitored by a thermocouple (TT-K-30-SLE).The preset 12 temperature measurement points are distributed as shown in figure 3, which are equally spaced in the axial and radial directions.The outer cross-sectional area of the thermocouple is 0.6 mm2, which is considerably smaller than that of the filter hole(4 mm2);thus,the influence of the thermocouple on the gas flow inside the DPF can be ignored [18].
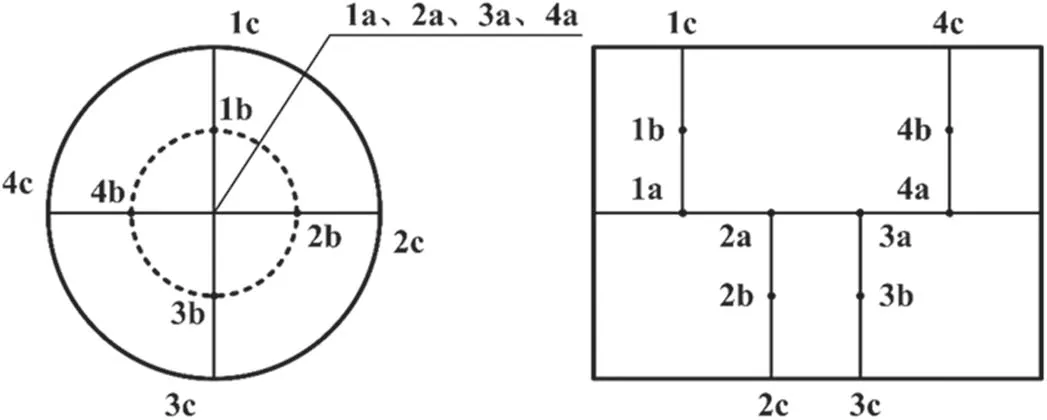
Figure 3.Location of temperature measurement points in DPF.
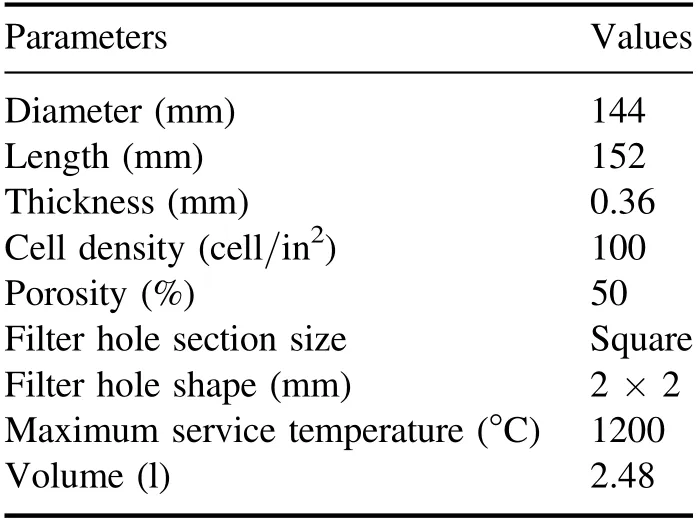
Table 2.DPF structural parameters.
2.1.Influence of reactor parameters on O3 production
2.1.1.Influence of feed gas and packing materials on DBD.Oxygen and air were introduced as feed gases into the DBD reactor with a gas flow rate of 5 l min−1.The cooling water temperature was 20 °C, the discharge voltage was adjusted in the 12.5–15.5 kV range, and the discharge frequency was 8 kHz.The power calculation formula is shown in equation(1)[27].

where P is the discharge power, f is the discharge frequency,CMis the measured capacitance value,k is the voltage division ratio, and S is the area of the Lissajous figure.
The reactor was packed with ZrO2and Al2O3particles measuring 0.8–1.0 mm for the discharge test.Figure 4 shows the different O3concentrations produced by the DBD reactor with power applied when different feed gases are used.When oxygen was used as the feed gas, the O3concentration was significantly higher than that of air, and the highest O3concentration was 33.2 g m−3.When air was used as the feed gas, the highest O3concentration was only 9.3 g m−3.In the test, as the discharge voltage was increased from 12.5 kV to 15.5 kV, the power of the reactor was 0–90 W when the feed gas used was oxygen and 0–100 W when the feed gas used was air.When the voltage remained constant, the power of the reactor was lower when the feed gas used was oxygen rather than air.Air consists of 21% O2and 78% N2.The ionization energy of O2molecules is 1165.9 kJ mol−1, and that of N2molecules is considerably higher at 1501 kJ mol−1.When air was used as the feed gas, the presence of numerous N2molecules with high ionization energy in the air inhibited the discharge[22].Ionization, excitation, recombination, and other chemical reactions of N2molecules in the reactor consume energy in a discharge reaction;the higher the proportion of N2in the feed gas,the greater the energy consumption.Therefore,the O3concentration is lower when air, rather than O2, is used as the feed gas, and the reactor power is higher.The O3concentration rises as the discharge power of the reactor increases.The reason is that the increase in power increases the number of high-energy particles, leading to the dissociation of more O2molecules to form O3[28, 29].
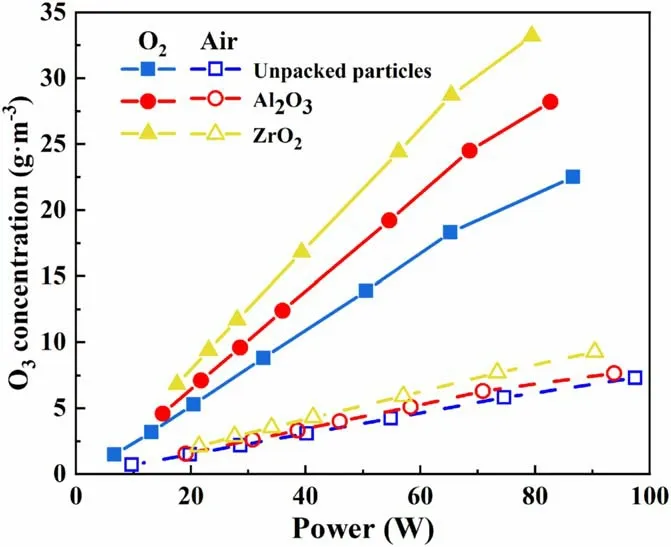
Figure 4.Variation in the O3 concentration generated by the DBD reactor with different power intensities applied and different feed gases.
When the ZrO2particles measuring 0.8–1.0 mm were packed under the oxygen source,the O3concentration reached 33.2 g m−3.When the Al2O3particles measuring 0.8–1.0 mm were used as the packing particles, the O3concentration reached 28.2 g m−3.However, the O3concentration in the empty-bed reactor (with unpacked particles) was only 22.5 gm−3.Relative to that in the empty-bed reactor, the‘average width’ of the discharge gap in the DBD reactor was reduced by packing particles; that is, the packing particles occupied part of the discharge gap volume, increasing the surface area of the medium and the pore structure complex;moreover,the formation of more micro-discharges contributed to the efficiency of ozone synthesis [22, 29].The O3concentration also increased, consistent with the results reported in the literature [30, 31].When particles were added into the DBD gap, the electric field intensity of the discharge area was altered, enhancing the discharge effect; meanwhile,the original discharge electric field was distorted [32].The difference in the degree of distortion was attributable to the difference in packing materials[31,33].The relative dielectric constant of ZrO2is 27, and that of Al2O3is 9.2.Both the relative dielectric constant and the electric field intensity near the particles gradually increased.The O3concentration was positively correlated with the electric field intensity.Therefore,using ZrO2rather than Al2O3particles as packing materials achieved a higher O3concentration.
2.1.2.Effects of packing particle materials and cooling water temperature on DBD.The reactor was packed with ZrO2and Al2O3particles measuring 0.8–1.0 mm, the cooling water temperature was 5 °C–35 °C, O2was introduced as the feed gas with a flow rate of 5 l min−1, the discharge voltage was 15.5 kV, and the discharge frequency was 8 kHz.Figure 5 presents the variations in the O3concentration produced by the DBD reactor with different cooling water temperatures and different packing materials.

Figure 5.Variations in the O3 concentration generated by the DBD reactor with different cooling water temperatures and different packing particles.
As shown in figure 5, at different cooling water temperatures, the sequence (in descending order) of O3concentration generation in the packed-bed DBD reactor follows the order ZrO2particles > Al2O3particles > empty.This sequence is consistent with the conclusions presented in figure 4.Compared with that in the empty-bed reactor, the incremental rate of O3increased first and then decreased with an increase in temperature.Packing particles can improve the cooling environment for the gas–solid two-phase heat exchange of the reaction gas,enhance the cooling effect of the discharge area[34],delay the pyrolysis reaction of O3,and increase the O3concentration.After the DBD reactor was packed with dielectric particles,the electric field in the discharge area was intensified,contributing to the increase in O3concentration.When the cooling water temperature was 5°C,the O3concentration in the empty-bed reactor was 20 g m−3.When ZrO2particles were used as the packing materials, the O3concentration was 30.6 g m−3;that is, 10.6 g m−3higher than that in the emptybed reactor,with an increase rate of 53%.When Al2O3particles were used as the packing materials, the O3concentration was 26.2 g m−3;that is,6.2 g m−3higher than that in the empty-bed reactor, reflecting an increase rate of 31%.As the temperature of the cooling water was increased, the O3concentration gradually decreased.When the cooling water temperature reached 35 °C, the O3concentration in the empty-bed reactor was 16.5 g m−3.When ZrO2particles were used as the packing materials,the O3concentration was 25.6 g m−3.Relative to that in the empty-bed reactor, the increase rate was 55.2%.When Al2O3particles were used as the packing materials, the O3concentration was 21 g m−3.The increase rate was 27.3%relative to that in the empty-bed reactor.The larger the difference in temperature between the cooling medium and the heat dissipation surface, the greater the amount of heat transferred[35].Therefore,when the cooling water temperature was 5 °C, the heat transferred was markedly greater than that when the cooling water was set to 35 °C, exerting a better cooling effect on the temperature in the discharge area of the reactor.As the temperature of the cooling water increases, the heat transferred to the cooling water decreases, which accelerates the pyrolysis of O3[36], gradually reducing the O3concentration.
2.1.3.Influence of packing particle size on DBD.Oxygen at a flow rate of 5 l min−1was used as the air source, and ZrO2particles with different particle sizes were packed in the DBD reactor.The reactor discharge voltage was adjusted within the 12.5–15.5 kV range, and the discharge frequency was 8 kHz.The cooling water temperature was set to 20 °C.Figure 6 presents the variations in the O3concentration produced by the DBD reactor using ZrO2particles with different sizes as packing materials.

Figure 6.Variations in the O3 concentration generated by the DBD reactor using ZrO2 particles with different sizes as packing materials.
As shown in figure 6, under the same power, the emptybed DBD reactor has the lowest O3concentration; the larger the particles, the higher the O3concentration.As the reactor power increased, the O3concentration showed an upward trend.The O3concentration was 41.8 g m−3(the highest) in the DBD reactor with particles measuring 1.2–1.4 mm but only 22.5 g m−3in the empty-bed DBD reactor, reflecting a difference of 19.3 g m−3.Moreover, the maximum O3concentration was 33.2 g m−3in the DBD reactor with particles measuring 0.8–1.0 mm but only 26.9 g m−3in the DBD reactor with particles measuring 0.4–0.6 mm.When particles were added into the discharge area, the electric field near the particles was locally enhanced[37–39];the larger the particle size, the greater the enhancing effect on the electric field.As the particle size increased,the range of electric field intensity in the discharge area widened,the enhancement area also increased, and the attenuation range of electric field intensity decreased.Therefore,the O3concentration increases as the particle size increases.
2.2.Influence of O3 concentration on DPF regeneration
2.2.1.Theoretical basis of NTP regeneration DPF.In the experiment, a large number of active substances produced by the DBD reactor were used as the source of oxygen to react with the PM in DPF to realize DPF regeneration.The chemical reaction formulas for the ionization, formation, and decomposition of oxygen and ozone molecules are shown in equations (2)–(6) [40–42]:
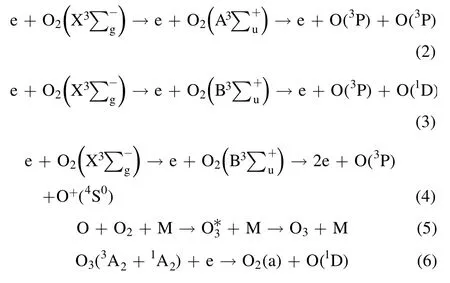
Discharge-generated O ions combine with the O2molecules and neutral particles of the third body to produce O3.However,the discharge-generated O3is still decomposed into O2molecules and O atoms after collision with electrons.Therefore,O3generation by DBD is a reversible reaction with dynamic equilibrium.The PM trapped in DPF is not a single carbon but a mixture of soot, soluble organic fraction (SOF),inorganic salt, and metal particles.The SOF and soot in the PM can react with active substances, rendering the regeneration reaction mechanism complex.The reaction process is shown in equations (7)–(13) [14, 43, 44]:
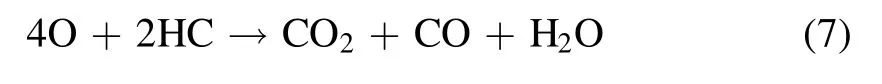
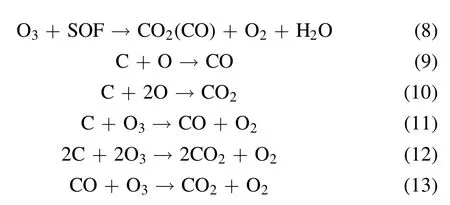
The oxidation of PM with oxygen as a reaction gas is described by equations(7)–(13).The O3and excited O atoms generated by the packed-bed DBD reactor first react with the SOF on the surface of the PM.When the SOF reacts with O3and the excited O atoms are exhausted, the soot reacts with the O3and the excited O atoms.As shown in equations (7)–(13), CO and CO2are the main final oxidation products, and O3plays an important role in PM oxidation [45].
2.2.2.Influence of O3concentration on PM oxidation products.The captured DPF was placed in a temperature control device to keep the ambient temperature of DPF regeneration at 80 °C [18].O2and ZrO2particles with particles measuring 1.2–1.4 mm were selected based on the results of the DBD reactor parameter optimization.The cooling water temperature was set to 20°C(which is close to room temperature), and the energy of heat exchange without additional cooling water could be obtained directly, ensuring a higher O3concentration.After O2was discharged via the DBD reactor, the NTP active materials were generated.The discharge frequency was 8 kHz, the discharge voltage was changed to produce different concentrations of O3, and the NTP active gas passed though the DPF for regeneration.
Figures 7 and 8 show the changes in CO and CO2volume fractions under different O3concentrations.As shown in figure 7, the CO volume fraction increases with the increase in regeneration time and then becomes stable; with the increase in O3concentration, the CO volume fraction exhibits an upward trend.When the O3concentration was 45 g m−3, the maximum CO volume fraction could be stabilized at about 0.2%.When the O3concentrations were 35, 25, and 15 g m−3, the CO volume fractions were 0.15%,0.1%, and 0.03%, respectively.As shown in figure 8, when the O3concentration ranges from 25 g m−3to 45 g m−3, the CO2volume fraction increases initially and then decreases with the regeneration time and ultimately becomes stable.The peak CO2volume fraction reached 1.05%.However,the peak CO2volume fractions under other O3concentrations decreased relative to that under an O3concentration of 45 g m−3, decreasing by 0.93% and 0.59%, respectively.When the O3concentration was 15 g m−3, the CO2volume fraction increased slowly with the increase in regeneration time;however,after the peak value of 0.28%was reached,the CO2volume fraction gradually decreased.
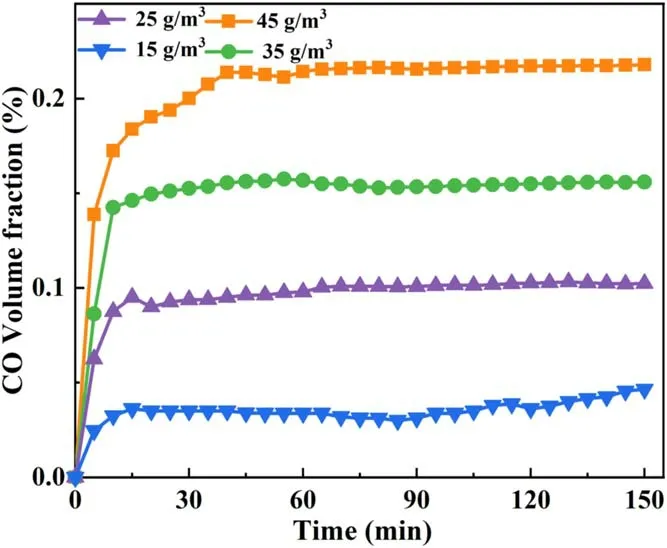
Figure 7.Relationship between CO volume fraction and O3 concentration.

Figure 8.Relationship between CO2 volume fraction and O3 concentration.
When NTP was introduced into the DPF, the PM captured in the DPF reacted with O3; the higher the O3concentration, the more violent the reaction with PM.Thus,the oxidation reaction was more intense when the O3concentration was 45 g m−3than when the O3concentration was 15 g m−3.The reaction with PM intensified, producing more CO, resulting in an increase in the CO volume fraction in the reaction product.The CO volume fraction increased with an increase in the regeneration time and then stabilized.In the initial stages of regeneration,O3came into contact with the PM surface.The SOF on the surface was easy to oxidize[46], inducing a violent reaction.Therefore, the CO volume fraction in the initial stages of regeneration increased with the increase in regeneration time [15].As the regeneration time increased, the soot inside the PM participated in the reaction.Compared with the SOF,the soot did not easily react,and the reaction environment in the reaction zone tended to be stable[47].Consequently, the CO volume fraction increased with the regeneration time and then stabilized.The CO2volume fraction also initially increased and then decreased with the increase in regeneration time.The reason is that O3removed the PM and regenerated the DPF.The evolution law of DPF regeneration is to gradually break down and decompose carbon deposits in the DPF pores,and the order of removal is from the surface to the inside of the pore [48].At the beginning of the regeneration, PM and O3reacted violently and generated a large amount of CO2, leading to an increase in the CO2volume fraction.In the late stages of regeneration,the carbon deposits in the DPF channels were oxidized, and the surface PM reacted completely.PM in the deep filtration layer of DPF reacted poorly with O3, significantly lowering the reaction intensity.This reduction led to a decrease in the complete oxidation product CO2and a rapid decrease in the CO2volume fraction; the higher the O3concentration, the larger the amount by which the CO2volume fraction is expected to increase and then decrease.In this study, when the O3concentration was 15 g m−3,the O3concentration was considerably low, resulting in a low reaction intensity.This result delayed the reaction process and prolonged the reaction time.Therefore, the CO2volume fraction did not decrease rapidly in the late stages of regeneration.
The change in energy density in the packed-bed DBD reactor under different O3concentrations is shown in table 3.As shown in the table, the corresponding energy density ranges from 340.8 J l−1to 1065.6 J l−1under different O3concentrations, and the energy density of the DBD reactor increases with the increase in O3concentration.At the O3concentration of 45 g m−3, the energy density of the NTP reactor was 1065.6 J l−1.
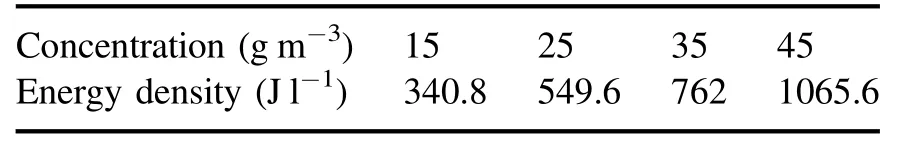
Table 3.Energy density under different O3 concentrations.
2.2.3.Influence of O3concentration on the removal of carbon deposits.The total carbon deposit removal can be calculated from the mass of the carbon elements in CO and CO2,and the calculation formulas are shown in equations (14) and (15)[13, 22]:

wherem1is the mass of carbon in CO,m2is the mass of carbon in CO2,mcis the mass of carbon removal,Mcis the molar mass of C which is 12 g mol−1, Vmolis the molar volume of gas which is 22.4 l mol−1, q is the gas flow which is 5 l min−1,φCOis the CO volume fraction,φCO2is the CO2volume fraction, and t is the regeneration time.
The quality of the carbon deposit removed by a unit volume of O3is used as the evaluation index of the O3utilization rate, and its calculation formulas are shown in equations (17)–(19):


whereMO3is the molar mass of O3which is 48 g mol−1, n(mol) is the amount of substance, c (g m−3) is the ozone concentration,q1andq2are the mass of the carbon element in CO and CO2generated per unit volume of O3, respectively,andqcis the total mass of carbon consumed per unit volume of O3.
Figure 9 shows the variation in the amount of carbon removal and carbon deposition removed per unit O3volume with O3concentration.As shown in the figure, bothm1andm2increase with an increase in O3concentration, indicating that the removal of carbon deposited in the DPF increases and is mainly oxidized and removed by O3in the form of CO2.When the O3concentration was 45 g m−3, the amount of carbon removedm1in CO was 0.99 g, and the amount of carbon removedm2in CO2was 2.22 g.The increases were 0.82 g and 1.21 g, respectively, relative to the O3concentration of 15 g m−3; that is, the amount of carbon removedmcincreased by 2.03 g.Therefore, increasing the O3concentration improves the total quality of the amount of carbon deposit removed.However, with the increase in O3concentration, the mass of the carbon elementq1in the generated CO per unit volume of O3slowly increased,whereasq2decreased continuously.The mass of carbon elements removed per unit volume of O3(qc) and O3concentrations increased first and then decreased.When the O3concentration was 15 g m−3, the masses of the carbon elements removed wereq1= 0.053 g l−1,q2= 0.32 g l−1,andqc= 0.37 g l−1.When the O3concentration was 45 g m−3,q1= 0.104 g l−1,q2= 0.235 g l−1, andqc= 0.339 g l−1.When the O3concentration was 25 g m−3,qc= 0.39 g l−1, the maximum value, indicating O3exhibited the highest utilization rate at the time.During PM oxidation and DPF regeneration, the higher the O3concentration, the more violent the oxidation decomposition reaction of PM.An increase in O3concentration led to an increase in the amount of total carbon deposit removed.However,with regard to the utilization rate of O3per unit volume, a higher O3concentration is not necessarily favorable, as shown in figure 9.With an increase in O3concentration, the mass of carbon removed by O3per unit volume first increases and then decreases.

Figure 9.Relationship between carbon removal and O3 concentration.
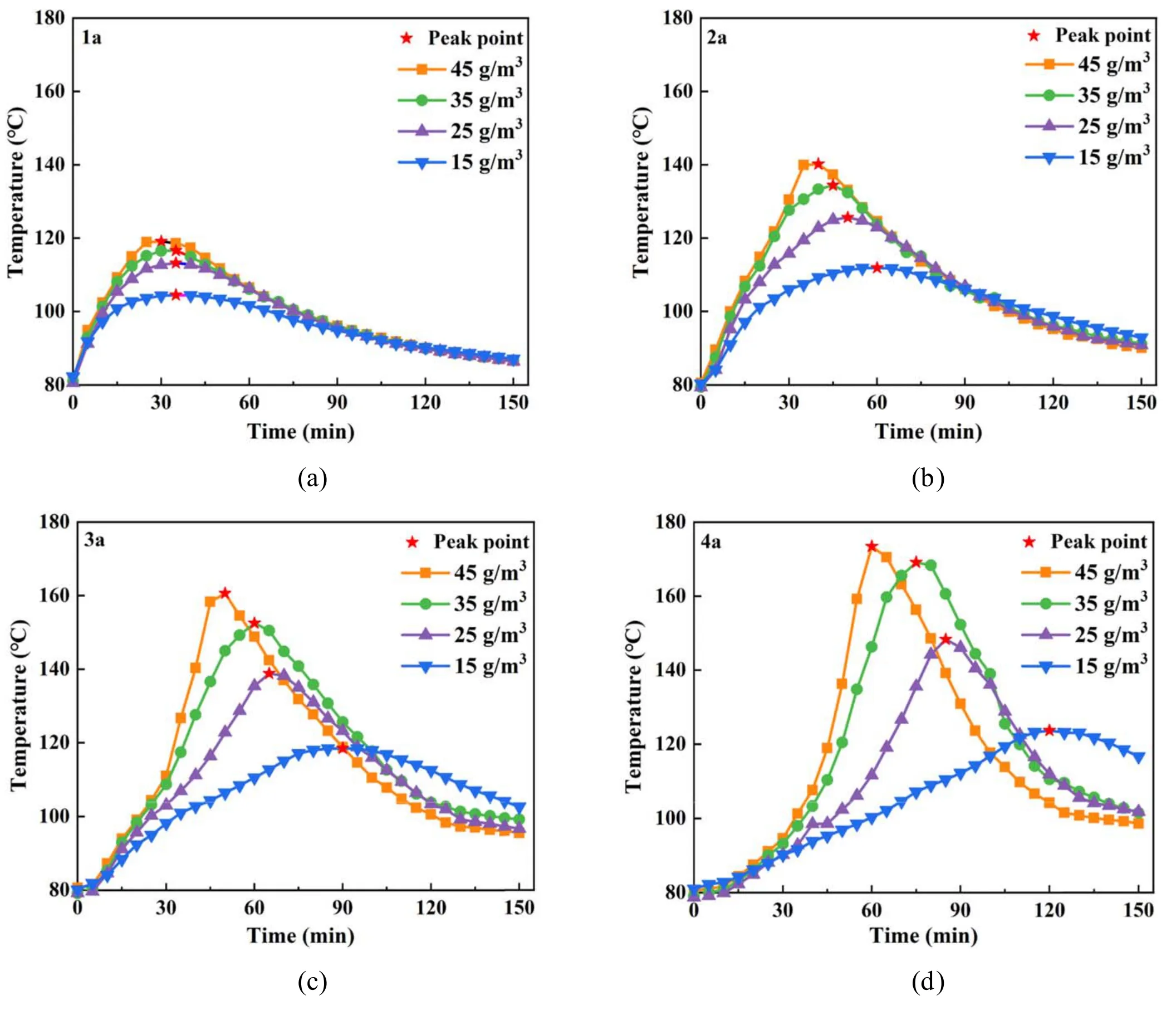
Figure 10.Effects of O3 concentration on axial regeneration temperature.

Table 4.Axial peak temperature and its arrival time of DPF.
2.2.4.Influence of O3concentration on axial temperature.Figure 10 shows the influence of O3concentration on the axial regeneration temperature.The curves in figures 10(a)–(d)depict the changes in the temperature of DPF at 1a, 2a, 3a, and 4a,respectively.Table 4 shows the axial peak temperature and the time required to reach the peak temperature during DPF regeneration.As shown in figure 10, when the temperature measurement point is fixed,the regeneration temperature is first increased and then decreased with the increase in the regeneration time; the peak regeneration temperature also gradually increases as the O3concentration rises.To clarify the effect of the O3concentration on the regeneration temperature,the change in regeneration temperature peak was used to refer to the change in regeneration temperature [49].The regeneration and peak regeneration temperatures exhibited similar characteristics.The regeneration temperature increased first and then decreased with the increase in regeneration time and had a peak value.Second,both the regeneration and peak regeneration temperatures increased with the increase in O3concentration.Third, the rate of increase of the regeneration temperature escalated as the O3concentration rose, and the peak time of regeneration temperature gradually advanced.Fourth, both the regeneration temperature and the peak regeneration temperature increased gradually from 1a to 4a as the temperature measurement point moved backward.Fifth,the increase rate of the regeneration temperature gradually decreased with a shift in the temperature measurement point,indicating that the peak arrival time of the regeneration temperature was gradually delayed.When the O3concentration was 45 g m−3, the peak regeneration temperature at 4a was 173.4°C;this temperature was higher than those at 3a,2a,and 1a(12.8 °C, 33.2 °C, and 54.3 °C, respectively).At 4a, when the O3concentration was 15 g m−3, the peak regeneration temperature was 123.7°C,and the peak time was 121 min.The peak regeneration temperature was higher than those at 25,35,and 45 g m−3, respectively.The reductions were 24.6 °C,45.4°C,and 49.7°C,and the lags were 35,47,and 61 min.In the initial stage of DPF regeneration, the O3reacted with the PM, causing the temperature to rise.As the regeneration time increased, the reaction further continued, releasing a large amount of heat to reach the peak regeneration temperature[50].The subsequent decrease in the intensity of the reaction reduced the regeneration temperature, which then fluctuated with the regeneration time.Increasing the O3concentration can increase the intensity of the oxidation reaction, gradually increasing the reaction rate and allowing the peak regeneration temperature to slowly rise and surge with an increase in O3concentration.In the DPF axial direction, gas flowed from 1a to 4a.The heat generated by the oxidative decomposition reaction of PM with O3from the front area of the DPF accumulated and transferred backward with airflow as the conduction medium.The peak regeneration temperature increased from 1a to 4a.
3.Conclusions
This study evaluated the effects of feed gas,packing particles(material or size),and cooling water temperature in a packedbed DBD reactor on the generation of an active substance(O3) under different generator operating voltages.A set of optimized parameters was used to generate NTP active gas for DPF regeneration at low temperatures.The research results were as follows.
(1) The power of the DBD reactor was less than 100 W.Packing particles into the discharge gap of the DBD reactor significantly increased the O3concentration, and the enhancing effect was amplified as the particle size increased.The larger the relative dielectric constant of the packing particles,the stronger the enhancing effect of the electric field distortion and the higher the O3concentration.Reducing the temperature of the cooling water improved the heat dissipation effect in the NTP discharge area and helped reduce the thermal decomposition of O3.Therefore, the oxygen source,1.2–1.4 mm ZrO2, and 20 °C cooling water temperature were used as the optimal parameters of the DBD reactor.
(2) The concentration of O3, the main active substance of NTP, directly affected the intensity of the oxidation reaction of carbon deposits in the DPF.As the O3concentration was increased, the volume fractions of CO and CO2produced by the reaction also increased,and carbon deposit removal increased.The amount of carbon deposit removed per unit volume of O3initially increased and then decreased.The largest amount of carbon deposit removed per unit volume of O3and the highest O3utilization rate were realized when the O3concentration was 25 g m−3.
(3) Increasing the O3concentration increased the peak regeneration temperature of the DPF,reaching the peak regeneration temperature at the same interface earlier.Along the DPF axis, the peak regeneration temperature gradually increased as the temperature measurement point shifted (from 1a to 4a), and the peak temperature of DPF regeneration was observed in the middle and back sections (4a).The peak temperature (173.4 °C) of DPF regeneration in the packed-bed DBD reactor was considerably lower than the service temperature of DPF(<1200 °C), the PM ignition temperature required for thermal regeneration (>600 °C) and catalytic regeneration (>450 °C).This result indicates that NTP regeneration of DPF can be used at significantly low temperatures, thus presenting an advantage.
Acknowledgments
This work is currently supported by National Natural Science Foundation of China (No.51806085); China Postdoctoral Science Foundation (No.2018M642175); Jiangsu Planned Projects for Postdoctoral Research Fund (No.2018K101C);Open Research Subject of Key Laboratory of Automotive Measurement, Control and Safety (Xihua University) (No.QCCK2021-007),and the Graduate Student Innovation Fund Project of Jiangsu Province (No.KYCX21_3354).
 Plasma Science and Technology2021年11期
Plasma Science and Technology2021年11期
- Plasma Science and Technology的其它文章
- Spatial and temporal evolution of electromagnetic pulses generated at Shenguang-II series laser facilities
- Numerical study on the loss of fast ions produced by minority ion cyclotron resonance heating in EAST
- Machine learning of turbulent transport in fusion plasmas with neural network
- Observation of coherent mode induced by a molybdenum dust on EAST
- Investigation of stimulated Raman scattering in longitudinal magnetized plasma by theory and kinetic simulation
- The influence of magnetic field on the beam quality of relativistic electron beam long-range propagation in near-Earth environment
