油茶新害虫南方锦天牛形态特征和生物学特性研究(英文)
赵丹阳,黄焕华,黄华毅,黄咏槐,扈丽丽,陈刘生
(广东省林业科学研究院,广东省森林培育与保护利用重点实验室,广州 510520)
The longhorn beetles (Coleoptera: Cerambycidae), one of the most species-rich families in the tropics, have been an important focal group in the forest ecosystems. Their adults feed on leaves, flowers, sugary substances and plant exudates, pollen, or fruit pulp (Linsley, 1959; Marlinetal., 2017), while most of their larvae burrow into plant tissues and so some species are considered pests (Noerdjito, 2010; Sakeninetal., 2011). Some species live only in specific host plants, while other species can inhabit a variety of plants (Waqa-Sakitietal., 2014). Damage by cerambycid larvae vary with the species, i.e., causing a swollen region surrounding the trunk, enlarged holes, or longitudinal and expanded elliptical galleries within trunk of the host plants.
TheCamelliatree, an important and high value edible oil plant, is native to the southern subtropical region of China and has been cultivated for more than 2 000 years. China is the largest country in terms ofCamelliatrees growing, with three million hectares in area and yielding, 2.7 million tons of oil per year that is more than 90% of the globalCamelliaseed oil production (He and Yao, 2013). In South China, two species of longhorn beetles,ChreonomaatritarsisPic andAeolesthesindutaNewman, have been reproted as important pests ofCamelliatrees which are significant impediments affecting the production and productivity ofCamelliaseed (Chenetal., 2002; Shu and Zhang, 2009; Zhao and Qin, 2015).
Recently, another longhorn beetle,Acaloleptaspeciosa(Gahan 1888), was observed as a stem-boring pest, which has severely damaged hundreds ofCamelliatrees in Guangdong Province. This longhorn beetle was briefly described in 1888 by Gahan under the genusHaplohammus, then was moved by Breuning (1961) to genusAcalolepta. Though Huaetal.(2009) reported that five plant species are its hosts includingCamelliaoleifera, there is no more available information, on both damage and its biology. Hence,A.speciosais reported for the first time as an important boring pest ofCamelliatrees in this work. The main aims of this study are to provide an updated description of the external characters of the longhorn beetle, and to note on its biology, in particular, feeding habits on theCamilliatrees.
1 Materials and methods
1.1 Location of survey
Surveys were conducted to access the incidence ofA.speciosainCamelliaplantations from April 2015 to December 2020 in Xiaokeng Forest Farm, with the following coordinates: 24°40′10″~24°46′12″N;113°47′05″~113°56′45″E, and altitudes of 150~200 m, in the District Qujiang, Shaoguan City, Guangdong Province, China.
1.2 Taxonomy
Specimens were examined using a Zeiss Stereoscopic Microscopes. Genitalia were prepared following the methods outlined in Miller and Bergsten (2012) and were illustrated in water. Smaller anatomical structures such as the metacoxa and genitalia were firstly cleared using KOH then examined using a Keyence VHX-5000 digital microscope. Habitus and other color images were taken on a Visionary Digital BK1 light imaging system. All photographs were edited using Adobe Photoshop CS5 software. Specimens were deposited in Guangdong Provincial Key Laboratory of Forest Culture, Protection and Utilization, Guangdong Academy of Forestry.
1.3 Biology
During April 2016 to June 2020, the damaged portions of the trunk byA.speciosalarvae and pupae were cut off inCamelliaplantations in Xiaokeng Forest Farm and reared to the laboratory in an incubator at 25℃±1℃, 70%±10% RH, and 12-hour photophase. Thirty pairs of newly emerged adults were released to 60 healthy potted plants, and pupation and pupal period duration were checked by splitting the branches. After observation, the plants were tied closed to avoid disturbance to the larvae and pupae. The samples were maintained until adults emerged in June.
2 Results
2.1 Morphology and taxonomy
Material examined. 5 ♂, 6 ♀, collected on host plantCamelliaoleiferatree, 4-25. V. 2018, Qujiang District (24°40′10″~24°46′12″N; 113°47′05″~113°56′45″E, average altitude 306.5 metre), Xiaokeng Forest Farm, Shaoguan City, Guangdong Province, Coll. Danyang Zhao.
2.1.1Diagnosis
Acaloleptaspeciosa(Gahan) can be recognized from other two kinds of longhorn beetles ofCamelliain northern Guangdong Province by the following key.
AkeytospeciesofCamellialonghornbeetles
1 Head and pronotum reddish brown, elytra bluish-purple
ChreonomaatritarsisPic
Body dark or golden brown
2
2 Pronotum with two raised dorsal tubercles
Acaloleptaspeciosa(Gahan)
Pronotum without raised dorsal tubercle
AeolesthesindutaNewman
2.1.2Description of adult
Body length: 17.0~24.8 mm; width: 5.0 mm~8.0 mm (at base of elytra). Golden yellowish, covered with thick reddish brown, golden amber, and silvery grey pubescence.
Head (Fig.1-a, b). Eyes black, crescent-shaped; clypeus glabrous; labrum rectangular, thickly covered with blonde long pubescent; mandibles glabrous towards apex. Palpi with sparsely long setae, pale yellow at basal and apical portion; ligula butterfly-shaped, dense long setae along apical margin which is deeply depressed in middle; mentum almost rectangular, convex in middle of apical margin, glabrous on ventral surface, with single transverse groove. Antennae have 11 antennomeres, length 35.0~40.0 mm and ca. 2.05× the body length in female, 2.2× in male; antennae much longer than body, covered with dense, golden brown pubescence, interrupted by whitish pubescence at base to 3/4 on antennomere II, 2/3 on antennomere III, about 1/2 from IV to XI. Ground colour on basal part of all antennomeres light brownish, and brown towards apex. Antennomere II 2 x longer than antennomere I. Neck black and glabrous at lateral area.

Fig.1 Head of Acalolepta speciosa adultNote: a, Male; b, Female. Scale bar: 5.0 mm.
Pronotum. Cylinder-shaped, slightly wider than long, length 2.82 mm, width 3.40 mm; with two weakly raised dorsal tubercles, surrounded dark brown pubescence. Pronotum covered with appressed amber to yellowish brown pubescence, interspersed with irregular yellowish pubescence; lateral spines short, pointing upwards. Scutellum prominent, shovel-shaped with rounded apex, covered with greyish to yellowish brown pubescence. Prosternum, mesosternum, and metasternum covered with yellowish brown pubescence, with striking silvery grey pubescence laterally.
Elytra (Fig. 2-a, c, d, f). Length 18.7 mm, width 6.8 mm; elongate-ovate, lateral side almost parallel in female, while in male gradually narrowed; covered with appressed greyish to yellowish pubescence; inner half region along suture pale reddish golden yellowish, outer region brownish grey, between them a pale longitudinal band from humera to apex; humeral angle rectangular, a prominent on middle of basal margin; large punctuations present on basal 1/2, those in middle area smaller than those in anterior region.
Legs. Covered with thickly greyish (female) to fulvous (male) pubescence dorsally, light grey ventrally; femora expanded and incrassate centrally; tibia gradually thickening to apex; tarsomere III deeply concave; claws diverging 90°.
Genitalia (Fig.3-d, f). Tegmen ca. 4.7 mm long; lateral lobes divided into two parts, basal 1/3 wider than apical 2/3, with apex acutely rounded with fine long setae along lateral and apical margins; ringed part elbowed in widest portion, without any lateral spine; basal piece distally bifurcated; median lobe plus median struts slightly curved, rounded at apex, almost as long as tegmen; median struts ca. 1/2 total length of median lobe and aedeagus; roof and median foramen slightly curved and rounded; aedeagus stout, curved upward, rounded at apex; tergite VIII (Fig.3-a) broader than long, apical margin slightly concave; setae around sides slightly longer than middle ones. Spiculum ventrale long, slightly curved apically, ca.3.1 mm in length.
Female genitalia (Fig.3-b, c). Spermathecal duct very longer than spermathecal capsule. Spermathecal capsule strongly sclerotized.

Fig.2 Adults of Acalolepta speciosaNote: a~c, Female; a, Dorsal view; b, Ventral view; c, Lateral view; d~f, Male; d, Dorsal view; e, Ventral view; f, Lateral view. Scale bar: 10.0 mm.

Fig.3 Female and male genitalia of Acalolepta speciosaNote: a~c, Female genitalia; a, Tergite VIII; b, Spermatheca and vaginal plate; c, Spermatheca; d~f, Male genitalia; d, Lateral view; e, Ventral view; f, Dorsal view. Scale bar: 1.0 mm.
Sexaul dimorphism (Fig.2). Male and female adults are similar to colour and form. Male elytra narrower than female. And male antennae much longer than female, extending beyond elytral apices by 6.5 antennomeres, while extending beyond elytral apices by 5 antennomeres in female.
2.1.3Distribution
China (Zhejiang, Jiangsu, Anhui, Jiangxi, Fujian, Guizhou, Sichuan, Guangdong, Guangxi, Hainan, Hongkong, Taiwan); Vietnam; Laos (Chiangetal., 1985; Huaetal., 2009).
2.2 Biology
2.2.1Incidence and damage
The larvae of the longhorn beetle prefer to girdle under the bark theCamelliatrunks or branches of 30.0~70.0 mm in diameter, and then move down into sapwood. The injured tissues were stimulated and swelled into nodules (Fig.4-a, d), resulting in weak growth and poor seeds yields. These damaged trees showed the following symptoms: emergence holes, several swollen nodules surrounding the trunk, and ejected frass along the surrounding nodules. The newly emerged adults chewed the bark ofCamelliafor complementary nutrition before mating and reproductive activities (Fig.5).
A total of 238Camelliatrees with ages of 4~20 years old was examined by counting of damaged trunks and branches with swollen nodules, emergence holes, and damaged bark recorded. Age and diameter of damaged trunks or branches of each tree were also recorded. The result indicated that among 238Camelliatrees, 88 trees were damaged by theA.speciosain 2015, 159 trees were damaged in 2016, 211 trees were damaged in 2017, respectively. In 2017, among the affected trees, 36.7% ones were heavily infested and each tree with more than three girdling nodules or even 10~15 girdling nodules can be found on a single trunk or branch ofCamellia. There was no significant difference in hazards between young and old trees.

Fig.4 Camellia trees injured by larvae of Acalolepta speciosa

Fig.5 Newly emerged adults, left one is chewing on bark of Camellia branch
2.2.2Biology
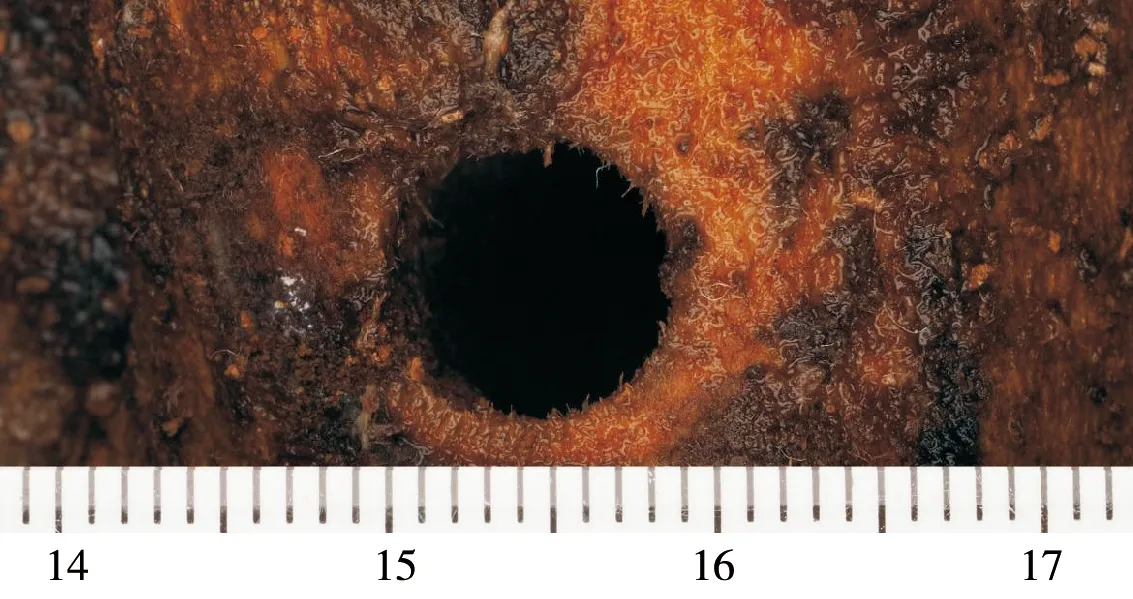
Fig.6 An adult emergence hole of Acalolepta speciosaNote: Scale was in centimeters.
Each generation ofAcaloleptaspeciosalasts two years, with larva as the overwintering stage. Young adults stay in the pupal chamber until their exoskeleton is fully sclerotized. The adult emerges through a roundish exit hole in the period of June and July, but the peak of emergence observed in middle and late July. The diameter of emerging hole (Fig.6) is from 5.0 mm to 9.0 mm. The newly emerged adults feed on the bark of tender branches or shoots, or sometimes on leaves ofCamellia. Adults are photophobic, hided in bushes near the host plants during the daytime; they are not good at flying and active only near the host plants. After 3~5 days of emergence, they begin to mate. Male and female individuals can mate many times, each lasted 9~12 minutes. Before mating, male brushesits right antenna by right fore leg. After 10~13 days of mating, the females makes a spawn scar (Fig.7)(length ca. 10.0 mm, width ca. 15.0 mm) in the bark with its mandibles, then lay an egg under the bark. Femles preferred to lay eggs on branches and trunks with diameters>30.0 mm.
The egg (Fig.8) length is 4.0 mm, and the width 1.0 mm, surrounded by colloidal liquid, which is pale yellow and long oval. Apical part is translucent but gradually deepens in color with time. Eggs last 12~16 days to hatch.
Neonate larvae are yellow. They feed under the bark, and through the crack of the bark they ejected frasses of wood outside. About three weeks later, they move down to the phloem, xylem, and excavated transverse, spiral, and flat tunnels which were blocked with rough wood fiber. Due to the invasion of larvae, the tissues of the host plant proliferate around the injured parts and form tumor like nodes. Mature larvae are elongate (Fig.9-b), cylindrical and almost parallel-sided. They are creamy yellow, the head and pronotum reddish brown and maxillary palpi darker, while the mandibles are dark reddish (Fig.9-a). Before pupation, the mature larva excavated transverse gallery, which blocked with rough wood fiber, then formed a pupal cell inside which the larva pupated (Fig.9-c).

Fig.7 Spawning mark in Camellia trunkNote: Scale was in centimeters.

Fig.8 A fresh laid egg of Acalolepta speciosaNote: Scale was in centimetres.
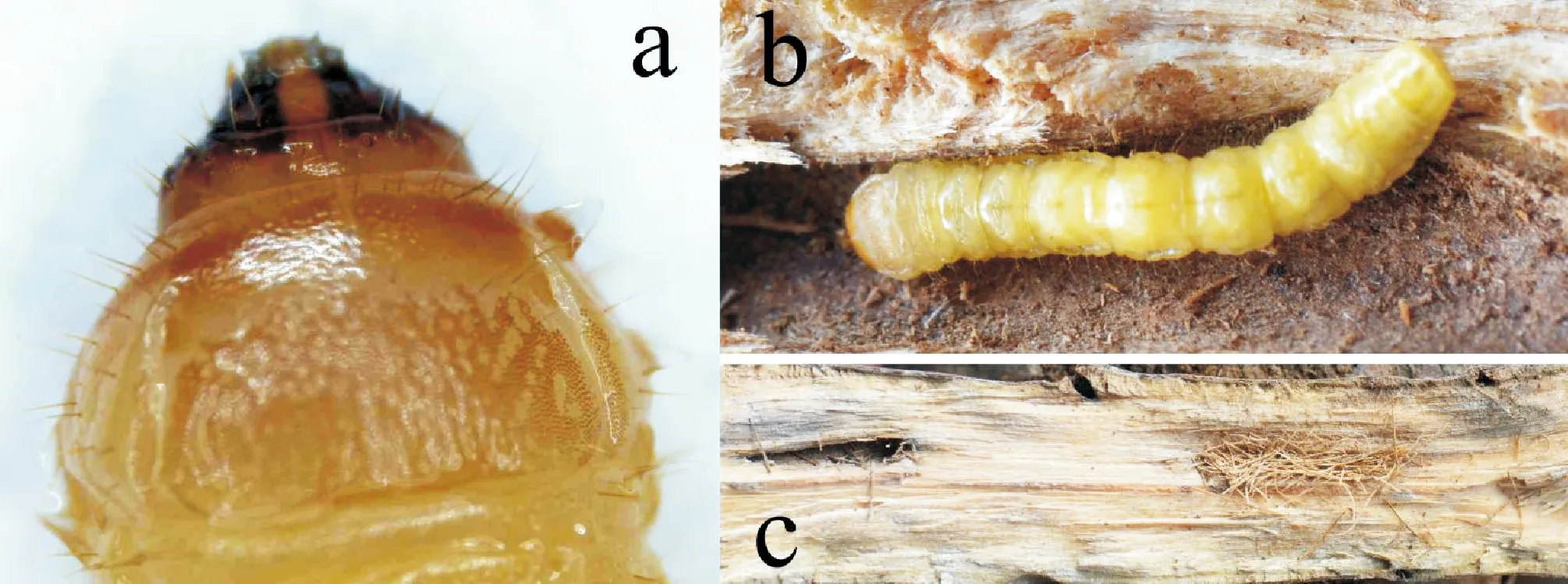
Fig.9 Larva and pupal chamber of Acalolepta speciosaNote: a, Head and prothorax; b, Larva in tunnel; c, Pupal chamber.
3 Discussions
Acaloleptais one species-rich genus of Lamiinae, mainly distributed in Asia, Australia, and New Guinea. The humid climate and rich vegetation offer favorable conditions for a large diversity of these beetles. The larvae ofAcaloleptaare almost all woodbores, damaging cultivated and native forest plants. Approximately 20 species of the genus are reported in China, but hosts information is limited to only a few species:A.cervinusHope has a wide range of host plants, i.e.Anthocephaluschinensis,Coffeaarabica,Neolamarckiacadamba,Murrayaexotica,Tectonagrandis,Camptothecaacuminataand other several species of plants (Xu and Li, 1982; Huang, 2000; Yu and Kuang, 2001);A.sublusca(Thomson) feeds onEuonymusjaponicas,Buxusmegistophylla, andGlochidionpuberum(Anetal., 2000; Yuetal., 2007);A.floculata(Gressitt) damagesZanthoxylumbungeanumandCitrusreticulata(Renetal., 2017). The reported hosts ofA.speciosawere associated with five species of plants, but lacking of detail information. Aside fromSchimasuperbaandCamelliaoleifera, both belong to Theaceae, the other species belonged to different three families, and there is no genetic relationship among the four families. Therefore, evidence of other hosts remains to be studied.
The damage symptoms ofA.speciosaare likely to be confused with those ofChreonomaatritarsisPic, which is also a severe borer inCamelliatrees. The damage was thought previously to be caused by the latter beetle, but the adults are actuallyA.speciosa. Field investigation confirmed thatA.speciosamainly infestsCamelliatrunks, whereasC.atritarsismainly damages branches. Apart fromCamelliaoleifera, the host plants ofA.speciosaare alsoCamelliagauchowensisChang andCamelliasemiserrataChi, which are widely cultivated in South China region too. But in the condition of laboratory, females ofA.speciosafavor to lay eggs onC.gauchowensis. So, host selection ofA.speciosais worthy of further studied.
Currently, the only control method available for the longhorn beetle pest is collection, burning of girdled branches, and the application of chemical pesticides. But in view ofC.oleiferaas an edible oil plant, chemical pesticides should be avoided so it is important to devise forestry techniques that promote biodiversity, and enhance natural control ability of predators and parasitoids in theCamelliaplantations.

