Different Head Group Dependence on the Lipid Thermodynamic Property of the Myelin Basic Protein in the Lipid Monolayer
ZHANG Lei, ZHANG Ming, SUN Run-Guang
(1)Department of Experimental Teaching Center for Optoelectronic Science and Information Engineering, Xi’an Aeronautical University, Xi’an 710077, China;2)School of Physics and Information Technology, Biophysics Research Laboratory, Shaanxi Normal University, Xi’an 710062, China)
Abstract The 18.5 kD myelin basic protein (MBP) isoform interacts with phospholipids and its role has been thought to maintain the stability and compactness of the myelin sheath structure. In this study, we describe the statistical thermodynamic theory of certain concentration effects on MBP in the major myelin lipid (1-palmitoyl-2-oleoyl-sn-glycero-3-phosphocholine (POPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphoethano-lamine (POPE), and 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS)) monolayers at the air/subphase interface via the Langmuir-Blodgett (LB) technique. A simple statistical mechanical theory is established that predicts the interaction between proteins and phosphate head groups at low surface pressures and the second virial coefficient dependences for the PC, PE, and PS head groups are illustrated. Two-dimensional virial equation of state (2D-VES) suggested that the interaction in the monolayer structure at the MBP-myelin interface is a repulsive force, and it induces a phase change in the monolayer. This is consistent with atomic force microscope (AFM) observations of domain and aggregate structures as well as with changes in the surface morphology induced by MBP. These analyses pertaining to membrane structures will provide a theoretical and experimental basis for the establishment of the myelin membrane modeling system.
Key words myelin basic protein(MBP); lipid monolayers; isotherm; virial coefficients; atomic force microscopy(AFM)
The myelin basic protein (MBP) is an important component of myelin. The severity and extent of nerve injury as well as its outcome and prognosis of the disease require the detection of MBP concentrations in serum and cerebrospinal fluids. The central nervous system (CNS) and peripheral nervous system compacted myelin proteins are MBP, which accounts for about 30% of the total myelin sheath[1-3]. MBP is one of the major proteins in the myelination maturation period of the CNS. MBP is associated with various neurological diseases such as allergic encephalomyelitis (EAE) and multiple sclerosis, and is a specific biochemical indicator that reflects whether the CNS has substantial damages or demyelination. Specifically, 18.5 kD MBP is the main protein in the mature myelin of the CNS and is the most conserved protein in the MBP family during evolution. It is reported that the 18.5 kD MBP of vertebrates contains 170 amino acid residues that play a key role in the structure and function of the protein[3, 4]. MBP promotes the formation of the major dense line by compacting oligodendrocyte membrane stacks that arise from the spirally wrapped plasma membrane around the axon[5, 6]. The interaction between MBP and myelin membrane phospholipids may be closely related, which confers stability to the myelin structure and function. Moreover, nerve conduction insulation is improved along with conduction speeds, thus playing a very important role in myelin formation as well as brain differentiation and maturation[7-9].
In our previous studies[10-12], we have investigated the adsorption of MBP at the free air/subphase interface and onto different monolayers at the interface. The results showed that the surface phenomena are closely related to the concentration of proteins, surface pressure, subphase and so forth. Additionally, we found that the adsorption of MBP into the various phospholipids was necessary due to the electrostatic and hydrophobic interactions between the penetrating MBP and the negatively charged head and hydrocarbon chains of the phospholipids. However, other studies have shown that the lipid monolayers possessed a lipid composition of myelin, such as dipalmitoyl phosphatidyl cholines (DPPC)[13], dipalmitoyl phosphatidyl serine (DPPS)[14], phosphatidylglycerol (PG)[15], and phosphatidicacid (PA)[16]. Among them, the myelin lipids (neutral POPC and POPE as well as anionic POPS) had interesting thermodynamic properties in the two-dimensional interfacial phase. All of the three lipids contain one palmitoyl chain (16 carbons, fully saturated), and one oleoyl chain (18 carbons with an unsaturated bond in the omega-9 position, which is in a cis conformation)[17](Fig.1). The two fatty acid chains not only have a different amount of carbons, but their thermodynamic properties, spatial dimensions and degrees of freedom are different.

Fig.1 The chemical structure of the phospholipids (A) The chemical structure of POPC (zwitterionic), (B) the chemical structure of POPE (zwitterionic), and (C) the chemical structure of POPS (anionic)
This investigation does not study the transverse structure of myelin such as nerve impulse propagation, conductivity, and membrane order perturbation; it focuses on the lateral organization adopted by the proteins and myelin lipids when the monolayer is formed at the air/subphase interface. Essentially, the influence of MBP on the dynamics and structure of myelin membranes model was explored using LB(Langmuir-Blodgett) technology and AFM (atomic force microscopy). AFM is a powerful tool for the analysis of lipid-protein interactions and the formation of microdomains. Therefore, the study of bionic membranes is conducive to understanding the independent functions of various components of the biofilm, which could contribute to further revealing the mystery of natural bio-membranes. The mechanism of interaction between MBP and the various components of the myelin membrane will help study the complete myelin structure in the later stage. Therefore this study regarding the supramolecular structure of myelin is of biophysical significance and medical value.
1 Materials and Methods
1.1 Chemicals
The protein used in the experiments, MBP, was collected and extracted from the bovine brain and purified in a water-soluble solution according to the procedures described by Deibleretal[18]. It was then dialyzed against pure Millipore water and used at a concentration of 1.0×10-9mol/L. The buffer was 10 mmol/L Tris-HCl, and the pH was adjusted to 7.2. POPC (molecular weight 760.1 g/mol), POPE (sodium salt, molecular weight 718.0 g/mol), and POPS (sodium salt, molecular weight 784.0 g/mol) of high a purity (at least 99%) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, USA) and used without further purification. Working solutions of lipids (1 mg/mL) were dissolved in a chloroform/methanol 3∶1 v/v mixture. The water (with a resistivity higher than 18.2 Mcm) used in the experiment was obtained from a Millipore purication system.
1.2 Isotherms
Langmuir experiments were fabricated with a KSV Minitrough system (Helsinki, Finland) with an operational area of 273 cm2. All experiments were performed using Tris-HCl as the subphase. The Tris-HCl buffer solutions were prepared by Tris (hydroxyethyl) amino-methane (concentration of 10 mmol/L) and titrated with HCl solutions to the desired pH value (pH=7.2). Prior to each measurement, the LB trough and barriers were thoroughly washed and wiped with ethanol and triple distilled water several times. The appropriate amount of lipid solutions (30 μL) was spread with precision microvolume syringes onto the subphase containing the moderate MBP. The solvent was allowed to evaporate for 15 minutes until the surface pressure (π) was stabilized. Subsequently, the monolayer was compressed to obtain theπ-Aisotherms. The constant rate of barrier during compression was 10 mm/min at a fixed temperature of 22±1 ℃ via circulating water bath. Each run was repeated at least three times to obtain reproducible results.
1.3 Langmuir-Blodgett transfer
Transfer of amphiphilic monolayers onto the mica substrate was achieved using the Langmuir-Blodgett technique. Prior to transferring, freshly cleaved mica was immersed into the Tris-HCl subphase. Then, the barriers were compressed up to the desired surface pressure of 10 mN/m. Following 15 minutes of stabilizing the monolayer, the mica sheet was removed from the subphase with a constant dipper rate of 20 mm/min to obtain the transfer ratio of 1.0 ± 0.1.
1.4 Atomic force microscopy
The transferred amphiphilic monolayers were monitored using a SPM-9500-J3 Atomic Force Microscope (AFM, Shimadzu Corporation, Japan). The scans were performed in a contact mode using a Micro V-shaped Cantilever (Olympus Optical Co. Ltd., Japan) with a spring constant = 0.06 N/m, length of 100 μm, and thickness of 400 nm. The scan rate was 1.0 Hz per line with a resolution of 512 pixels per line.
1.5 Theoretical analysis

(1)

1.5.2 Virial coefficients for different lipids and MBP interactions Theπ-Aisotherms in the region starting from the isotherms toπ= 10 mN/m were fitted into the following two-dimensional virial equation of state (2D-VES)[20]:
(2)
whereadenotes the aggregation coefficient. Ifa= 1, there is no aggregation between molecules at a low surface pressure, however, whena< 1, aggregation occurs between molecules, and the lower the value of a, the higher the degree of aggregation.bis the virial coefficient, whereb> 0 andb< 0 characterize the repulsive and attractive properties of the intermolecular interaction, respectively.
2 Results
2.1 π-A isotherm measurements of Tris-HCl subphase containing 1 nmol/L MBP with POPC, POPE, and POPS monolayers
This experiment analyzed the behavior of MBP incorporated into myelin sheath membranes, and it consists of three very important lipids: POPC, POPE, and POPS. From the π-A isotherms observed in Fig.2 at a wide range of surface pressures (0~50 mN/m), the addition of lipids of different head groups to the Tris-HCL subphase containing 1 nmol/L MBP was found to produce higher values in mean molecular areas as the concentration rose, indicating the existence of interactions between the MBP and phospholipids. Pure POPC isotherm embodied a liquid-expanded (LE) phase during compression under the present experimental conditions. The compression of the barriers to surface pressure above 40.6 mN/m (equivalent to A = 54.8 Å2) generally led to the collapse of the film. These results aligned with those of previously reported isotherms[21, 22]. The mixed monolayer curve possessed an identical smooth shape and operated almost in parallel to each other (Fig.2A). POPE is different from POPC in that it exhibited a fluid liquid-expanded (LE) phase up to 36.0 mN/m, where a phase transition between two liquid-condensed entities (namely LC and LC′) occurred[23, 24]. The mixed monolayer, withCMBP=1 nmol/L, demonstrated a LC-LC′ phase transition but atπ= 34.9 mN/m, along with a collapse in surface pressure (πc) of 37.5 mN/m. This implies that MBP adsorbed onto the POPE monolayer to make the monolayer closer, resulting in smaller values of phase transition and collapsing pressures (Fig.2B). In terms of POPS (Fig.2C), the isotherm demonstrated a LE phase state of the monolayer. The collapse pressure of the isotherm appears atπ= 36.5 mN/m[25]. The isotherms with 1 nmol/L MBP show higher mma’s than the isotherm of pure POPS. This outcome was probably due to the adsorption of MBP onto the monolayer via electrostatic interaction.

Fig.2 Compression isotherms and of POPC (A), POPE (B), and POPS(C) on 1 nmol/L MBP subphase (A) Isotherm of POPC surface films before(○) and after (Δ) the subphase contains MBP(1 nmol/L). (B) Comparison of POPE (○) with the MBP (Δ) in the presence of 1 nmol/L. (C) Comparison of POPS (○) with the MBP (Δ) in the presence of 1 nmol/L. Inset: the dependence of on π
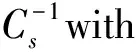
As evidenced in Fig.3, all mixed monolayers demonstrated an increased mean molecular area in all phases compared to the isotherm of pure lipids. In addition, the MBP-POPS mixed monolayer exhibited greater mma’s change in% than that of the MBP-POPC and MBP-POPE monolayers. The results revealed that the stability of myelin structures of the three lipids with MBP (CMBP= 1 nmol/L) mixtures increases in the order of MBP-POPE< MBP-POPC < MBP-POPS. The insertion of the MBP protein in different lipid monolayers at a pressure of 10 mN/m suggests the existence of hydrophobic and electrostatic interactive behaviors. And their electrostatic interactions are stronger than hydrophobic interactions. Table 1 summarizes the mean molecular area (Å2) at lift-off, at aπof 10 mN/m and at collapse, along with the percentage change with MBP = 1 nmol/L in the POPC, POPE, and POPS monolayers. The results show the insertion of the MBP protein in different lipid monolayers at a pressure of 10 mN/m, and it suggests the existence of hydrophobic and electrostatic interactive behaviors.

Fig.3 The mean molecular area (MMA) changes in% upon mixing the MBP with the different head group of phospholipids Histograms of the MMA change of POPC, POPE and POPS in the same MBP at a surface pressure of 10 mN/m. The MMA change in% is: 15.27(MBP-POPC), 8.76(MBP-POPE) and 24.47(MBP-POPS). The subphase was 1 nmol/L MBP at pH 7.2 and 10 mmol/L Tris-HCl

Table 1 Mean molecular area (Å2) at lift-off, π=10 mN/m, collapse and% change along with the compressibility moduli with MBP (CMBP =1 nmol/L) for POPC, POPE and POPS
2.2 Two-dimensional virial equation of state for different lipids and MBP interactions
In order to better explore the characteristics of MBP with phospholipids of different head groups, the π-A curves were analyzed using simple statistical theory (2D-VES) at a low surface pressure (10 mN/m). The results of Eq. (2) according to this experiment’s parameters are exhibited in Fig.4. The second virial coefficients (b> 0) for these interactions may be explained as the interactions between the heads of the molecules are repulsive. The analysis showed that the inclusion of 1 nmol/L MBP into the subphase makes the repulsion coefficient to increase from 0.2624 to 0.3072 (POPC), 0.1322 to 0.1678 (POPE), and 0.2435 to 0.3023 (POPS), respectively. And this is consistent with the increase of the lift off area in the existence of MBP. The aggregation coefficient was obtained for the pure POPC, POPE and POPS monolayers at a surface pressure of 10 mN/m. Beyond that, all monolayers show very a low aggregation coefficients (a< 1). This indicates that the higher aggregation of intermolecular at a surface pressure of 10 mN/m could be due to electrostatic and hydrophobic interactions between MBP and the three types of phospholipid head groups.

Fig.4 2D-VES theoretical study of the pure phosphatides and the mixed MBP-POPC, MBP-POPE, and MBP-POPS surface monolayers Measured values of πA/kT for pure POPC(A) and MBP-POPC(B) monolayers as a function of π, the second virial coefficients is 0.2624 and 0.3072, respectively. Measured values of πA/kT for pure POPE(C) and MBP-POPE(D) monolayers as a function of π, the second virial coefficients is 0.1322 and 0.1678, respectively. Measured values of πA/kT for pure POPS(E) and MBP-POPS(F) monolayers as a function of π. And the second virial coefficients is 0.2435 and 0.3023, respectively
2.3 Morphological monitoring of MBP with the POPC, POPE, and POPS monolayers using AFM
To further evaluate potential microstructure changes within the mixed monolayers upon lipid (POPC, POPE, and POPS)-MBP interaction, AFM was used to characterize the surface topography via the contact mode. AFM topographic images were taken from MBP-POPC, MBP-POPE, and MBP- POPS films following the adsorption of the Tris-HCl subphase, which was seen to increase the concentration of MBP at 1 nmol/L atπ=10 mN/m. MBP molecules were found to possess granular features (Fig.5), and the cross-section images in the height profile demonstrated that the aggregation of MBP molecules. The height of the pure phospholipid monolayer is about 2 nm. The measured protein has a diameter of 30~120 nm and a height of 6~32 nm. This value is much higher than the phospholipid monolayer. Therefore, the white spherical particles we observed are aggregates of proteins, or MBP aggregates. Significant changes occurred in the Tris-HCl subphase containing MBP at a constant concentration. The images seen in Fig.6 show the monolayers’ heterogeneity induced by the presence of MBP. At a surface pressure of 10 mN/m, the monolayers of POPC, POPE, and POPS were observed to be homogeneous until the addition of MBP, suggesting with an MBP concentration of 1 nmol/L in the subphase, the quantity of MBP particle domains as well as topographical changes observed at 10 mN/m become increasingly obvious. This phenomenon indicated that MBP molecules are embedded into the lipid monolayer, making the mean molecular area larger. These results are consistent with similarπ-Aisotherms and the second virial coefficients shown in Fig.2 and Fig.4, which are also due to the strong interactive effects of MBP to POPC, POPE, and POPS, resulting in a high compaction of myelin sheath. Regarding the MBP-POPE monolayer, it appeared that the participation of MBP did not induce the obvious morphological changes on the monolayer as seen in Fig.6 (C, D). The adsorbed amount of MBP was greatly increased when the Tris-HCl buffer solution contains 1 nmol/L MBP. From the MBP concentrations in the subphase of the 1 nmol/L image, more “collapse domain” was formed on the POPC monolayer (Fig.6B and Fig.7A) with a height of 0.44~3.88 nm, indicating that MBP molecules were squeezed into the subphase. In terms of the mixed monolayer systems featuring MBP-POPS, the presence of MBP induced significant changes in organizational structures, which is especially evident at concentrations at 1 nmol/L. In addition, the domain structures changes from “disk-like” to “densely-branched” structures observed on the Tris-HCl subphase (Fig.6 (E, F)). Moreover, it can be seen that the MBP is tightly adsorbed on the POPS monolayer, thus stabilizing the monolayer. In addition, as numerous MBP particles were adsorbed on the surface of the POPE and POPS monolayers, various aggregated protein particles with diameters up to 0.3 μm as well as relative heights of 9.2 nm (MBP-POPE) and 6.5 nm (MBP-POPS) were evident and depended on the lipid surface roughness (Fig.7 B-C). These results are in accordance with the previous analysis of the second virial coefficients, which further illustrates that MBP-lipid (POPC, POPE, and POPS) interactions show a significant dependence on phospholipid head groups and is likely governed by electrostatic and hydrophobic forces. Such interactions are further illustrated by the model in Fig.8.

Fig.5 AFM images (10 μm×10 μm) of MBP Left: The MBP is deposited on the mica. The lighter areas represent the protein particles, the scale bar is 5 μm. The right panel shows the height plot corresponding to the left panel
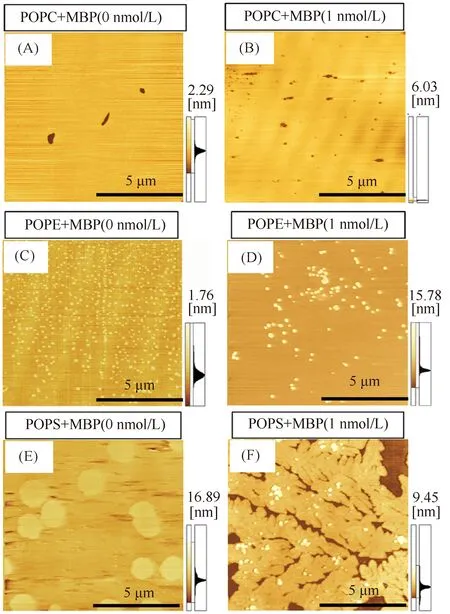
Fig.6 AFM micrographs of the phospholipid monolayer with different head groups (A, B) From the MBP concentrations in the subphase of the 1 nmol/L image, more “collapse domain” was formed on the POPC monolayer. (C, D) Regarding the MBP-POPE monolayer, it appeared that the participation of MBP did not induce the obvious morphological changes on the monolayer. The adsorbed amount of MBP was greatly increased when the Tris-HCl buffer solution contains 1 nmol/L MBP. (E, F) In terms of the mixed monolayer systems featuring MBP-POPS, the presence of MBP induced significant changes in organizational structures, which is especially evident at concentrations at 1 nmol/L. The scale bars are 5 μm

Fig.7 MBP induces changing of conformational of myelin membrane (A) POPC+MBP (1 nmol/L), the lighter areas represent the surface of the mixed monolayer, and the darker areas represent the mica substrate. A color-height scale is shown on the left. The right panel shows the height plot corresponding to the left panel. (B) POPE+MBP (1 nmol/L), AFM image of a monolayer that had been incubated with MBP(1nmol/L). MBP binds to the monolayer. (C) POPS+MBP (1 nmol/L), image of a dense lipid coating that had been incubated with MBP. MBP binds to the mica substrate as well as the lipid membrane. Left: Cross-sectional analysis based on the horizontal line shown. The scan size and height scale are given for each image. Scale bar = 5 μm

Fig.8 Schematic presenting the model of mixed monolayers (A) MBP alone were deposited on the surface of the mica and showed brighter regions in the AFM images. (B) AFM images of the POPC monolayer in the presence of 1 nmol/L, and more “collapse domains” were formed on the POPC monolayer. (C) MBP does not induce POPE monolayer surface changes, when the adsorption amount of MBP increased. (D) MBP induced significant changes in organizational structures of the POPS monolayer, which is evident at concentrations at 1 nmol/L
3 Discussion
The results provide qualitative and quantitative information regarding the thermodynamic mechanism of how MBP is adsorbed to myelin monolayer surfaces. In recent years, the main methods and techniques of the interaction between MBP and myelin membrane have the following aspects: Trgeretal[26]have studied the effect of cholesterol contents on the myelin membrane using fluorescence microscopy; Oliveiraetal[27]can observe myelin membrane heterogeneity by fluorescence microscopy and Brewster angle microscopy. Their results interpret the dynamic process of the interaction between protein and lipid monolayer membranes. However, there are few studies on the quantitative analysis of the interaction between MBP and myelin lipid membrane.
There have been many reports on the interaction between MBP and myelin lipid molecules[28-31], but there are few studies on MBP and different model membrane systems. In the experiment, MBP will be adsorbed to a single component lipid monolayer membrane. The rearrangement and reconstruction of the microdomain structure have been observed, but how to quantitatively analyze the dynamic elasticity remain unresolved. The mechanism of stability and whether these parameters can be used as criteria for multiple sclerosis is still not clear.

The results of our study also raise the questions about interactions of MBP with other lipid models, such as “lipid raft”, “plasma membrane”, and “myelin membrane model”. The myelin lipid bilayers had a lipid conformational characteristic of myelin from “healthy” and “disease-like” in the cytoplasmic leaflets. They exhibit different adsorption mechanisms. These relevant issues in this field warrant further investigations.
Conflictofinterest
The authors declare that they have no conflict of interest.

