胃癌患者外周血T淋巴细胞亚群及NK细胞受体表达水平分析
雷泽洪 林忠顺 李荣岗 区卫林 梁萍娟
【摘要】 目的:研究胃癌患者外周血T淋巴細胞亚群及NK细胞受体表达水平。方法:将2018年1月-2020年12月江门市中心医院收治的142例胃癌患者纳入本研究,另纳入58例健康体检者作为对照组,使用流式细胞仪检测外周血T淋巴细胞亚群(CD8+、CD4+、CD3+、CD4+/CD8+)分布情况和外周血NK细胞受体(NKG2D、NKp30、NKG2A、CD158a)表达情况。结果:胃癌组患者外周血CD3+T细胞、CD4+T细胞、CD4+/CD8+比值较对照组均明显下降(P<0.05),CD8+T细胞比例较对照组则明显升高(P<0.05);NK细胞活化性受体NKG2D和NKp30表达较对照组均明显下降(P<0.05),抑制性受体NKG2A和CD158a表达较对照组均明显升高(P<0.05)。肿瘤直径≥5 cm、低分化、Ⅲ~Ⅳ期、淋巴结转移、深层浸润的胃癌患者外周血CD3+T细胞、CD4+T细胞、CD4+/CD8+比值、NK细胞活化性受体NKG2D和NKp30表达较肿瘤直径<5 cm、中高分化、Ⅰ~Ⅱ期、无淋巴结转移、无深层浸润均明显下降(P<0.05),CD8+T细胞比例、NK细胞抑制性受体NKG2A和CD158a表达较肿瘤直径<5 cm、中高分化、Ⅰ~Ⅱ期、无淋巴结转移、无深层浸润均明显升高(P<0.05)。结论:胃癌患者外周血T淋巴细胞亚群分布紊乱,外周血NK细胞活化性受体表达降低,抑制性受体表达升高,且与胃癌的发生发展有关,检测胃癌患者外周血T淋巴细胞亚群和NK细胞受体表达有助于评估细胞免疫功能、指导临床治疗和评价预后。
【关键词】 胃癌 T淋巴细胞亚群 NK细胞受体
Expression of T Lymphocyte Subsets and NK Cell Receptors in Peripheral Blood of Patients with Gastric Cancer/LEI Zehong, LIN Zhongshun, LI Ronggang, OU Weilin, LIANG Pingjuan. //Medical Innovation of China, 2022, 19(08): 019-023
[Abstract] Objective: To study the expression of T lymphocyte subsets and NK cell receptors in peripheral blood of patients with gastric cancer. Method: A total of 142 patients with gastric cancer treated in Jiangmen Central Hospital from January 2018 to December 2020 were included in this study, and 58 healthy people were included as the control group. The distribution of T lymphocyte subsets (CD8+, CD4+, CD3+, CD4+/CD8+) and the expression of NK cell receptor (NKG2D, NKp30, NKG2A, CD158a) in peripheral blood were detected by flow cytometry. Result: The peripheral blood CD3+ T cells, CD4+ T cells and CD4+/CD8+ ratio in the gastric cancer group were significantly lower than those in the control group (P<0.05), and the proportion of CD8+ T cells was significantly higher than that in the control group (P<0.05); the expressions of NK cell activating receptors NKG2D and NKp30 in peripheral blood of patients with gastric cancer were significantly lower than those in the control group (P<0.05), and the expressions of inhibitory receptors NKG2A and CD158a were significantly higher than those in the control group (P<0.05). The expression of CD3+ T cells, CD4+ T cells, CD4+/CD8+ ratio, NKG2D and NKp30 in peripheral blood of gastric cancer patients with tumor diameter ≥5 cm, low differentiation, stage Ⅲ-Ⅳ, lymph node metastasis and deep infiltration were higher than those of tumor diameter <5 cm, medium and high differentiation, stage Ⅰ-Ⅱ, no lymph node metastasis, no deep infiltration were significantly decreased (P<0.05); the proportion of CD8+ T cells and the expression of NK cell suppressor receptor NKG2A and CD158a in peripheral blood of gastric cancer patients with tumor diameter ≥5 cm, low differentiation, stage Ⅲ-Ⅳ, lymph node metastasis and deep infiltration were higher than those of tumor diameter <5 cm, medium and high differentiation, stage Ⅰ-Ⅱ, no lymph node metastasis, no deep infiltration were significantly increased (P<0.05). Conclusion: The distribution of T lymphocyte subsets in peripheral blood of patients with gastric cancer is disordered, the expression of activated receptor and inhibitory receptor of NK cells in peripheral blood is decreased and increased, which is related to the occurrence and development of gastric cancer, the detection of T lymphocyte subsets and NK cell receptor expression in peripheral blood of patients with gastric cancer is helpful to evaluate cellular immune function, guide clinical treatment and evaluate prognosis.
[Key words] Gastric cancer T lymphocyte subsets NK cell receptor
First-author’s address: Jiangmen Central Hospital, Guangdong Province, Jiangmen 529000, China
doi:10.3969/j.issn.1674-4985.2022.08.005
胃癌是常见的恶性肿瘤之一,发病率和死亡率呈上升趋势,预后较差[1-2]。近年来肿瘤免疫学在肿瘤研究中获得了越来越多的关注,肿瘤的发生发展与患者免疫功能关系密切[3-4]。T淋巴细胞可分为免疫功能不同的亚群,其中CD4+、CD8+在生理状态下保持动态的平衡,共同作用促使机体免疫功能处于稳定状态[5-6]。自然杀伤(NK)细胞是机体重要的免疫细胞可直接杀伤肿瘤细胞,其细胞表面表达活化性受体和抑制性受體[7]。肿瘤细胞可通过调控NK细胞受体表达,而影响NK细胞对肿瘤细胞的免疫监视及清除作用[8-9]。因此,本文拟研究胃癌患者外周血T淋巴细胞亚群及NK细胞受体表达水平,以分析胃癌患者的细胞免疫功能状况及临床意义。现报道如下。
1 资料与方法
1.1 一般资料 将2018年1月-2020年12月江门市中心医院收治的142例原发性胃癌患者纳入本研究作为胃癌组。纳入标准:(1)年龄18~75岁,性别不限;(2)符合胃癌诊断标准;(3)均接受手术治疗,且术前均未接受放化疗治疗;(4)临床资料完整。排除标准:(1)合并其他消化系统相关疾病者;(2)合并肺、肝、肾等脏器功能障碍者;(3)合并其他部位恶性肿瘤者;(4)合并精神疾病者。另纳入58例健康体检者作为对照组,其中男32例,女26例,年龄30~72岁,平均(58.25±8.72)岁。纳入标准:(1)年龄18~75岁,性别不限;(2)健康体检结果均显示正常。排除标准:依从性差。本研究经医院医学伦理委员会批准,所有入选者均对本研究知情同意并签署了知情同意书。
1.2 方法
1.2.1 样本采集 采集两组空腹外周静脉血各两管,每管采集5 mL,其中一管用于外周血T淋巴细胞亚群检测,另一管用于NK细胞受体表达检测,均需在采集后2 h内检测完毕。
1.2.2 外周血T淋巴细胞亚群检测 采用美国BDFacscalibur生产的流式细胞仪对两组血液样本进行T淋巴细胞亚群检测,包括CD8+、CD4+、CD3+,并对CD4+/CD8+进行计算,相关试剂盒均购自北京中杉金桥有限公司。
1.2.3 外周血NK细胞受体表达检测 每个流式管中加入50 μL全血,再加入5 μL anti-CD3-FITC、anti-CD56-APC、anti-CD16-Per CP,然后每管再分别加入5 μL单克隆抗体(anti-NKG2D-PE、anti-NKp30-PE、anti-NKG2A-PE、anti-CD158a-PE),同时设置的阴性对照组。各组样本经充分混匀后,在室温下放置20 min,再加入细胞裂解液室温静置10 min,再加入PBS震荡混匀,再用PBS洗涤2次,然后使用流式细胞仪进行上机检测。
1.3 观察指标 比较两组外周血T淋巴细胞亚群、NK细胞受体表达差异性,以及不同病理参数胃癌患者外周血T淋巴细胞亚群、NK细胞受体表达的差异性。
1.4 统计学处理 使用SPSS 20.00处理数据,符合正态分布的计量资料以(x±s)表示,两组比较采用t检验;计数资料以率(%)表示,采用字2检验。以P<0.05为差异有统计学意义。
2 结果
2.1 两组一般资料比较 胃癌组,其中男77例,女65例;年龄36~75岁,平均(59.04±9.36)岁;分化程度:中高分化81例,低分化61例;TNM分期:Ⅰ~Ⅱ期57例,Ⅲ~Ⅳ期85例;肿瘤直径1.9~9.8 cm,平均(5.89±1.63)cm;有淋巴结转移者27例;有深层浸润者39例。对照组,其中男32例,女26例,年龄30~72岁,平均(58.25±8.72)岁。两组的年龄、性别相比,差异均无统计学意义(P>0.05),具有可比性。
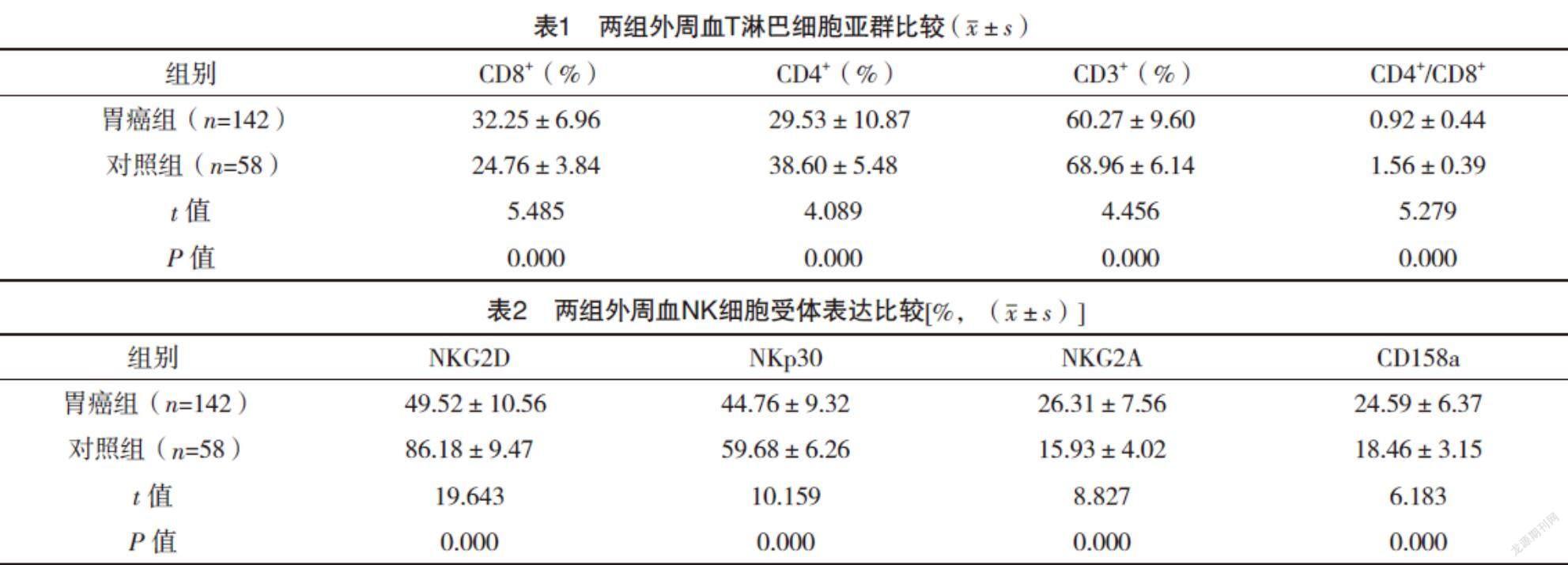
2.2 两组外周血T淋巴细胞亚群比较 胃癌组患者外周血CD3+T细胞、CD4+T细胞、CD4+/CD8+较对照组均明显下降,CD8+T细胞比例较对照组则明显升高,差异均有统计学意义(P<0.05),见表1。
2.3 两组外周血NK细胞受体表达比较 胃癌组患者外周血NK细胞活化性受体NKG2D和NKp30表达较对照组均明显下降,抑制性受体NKG2A和CD158a表达较对照组均明显升高,差异均有统计学意义(P<0.05),见表2。
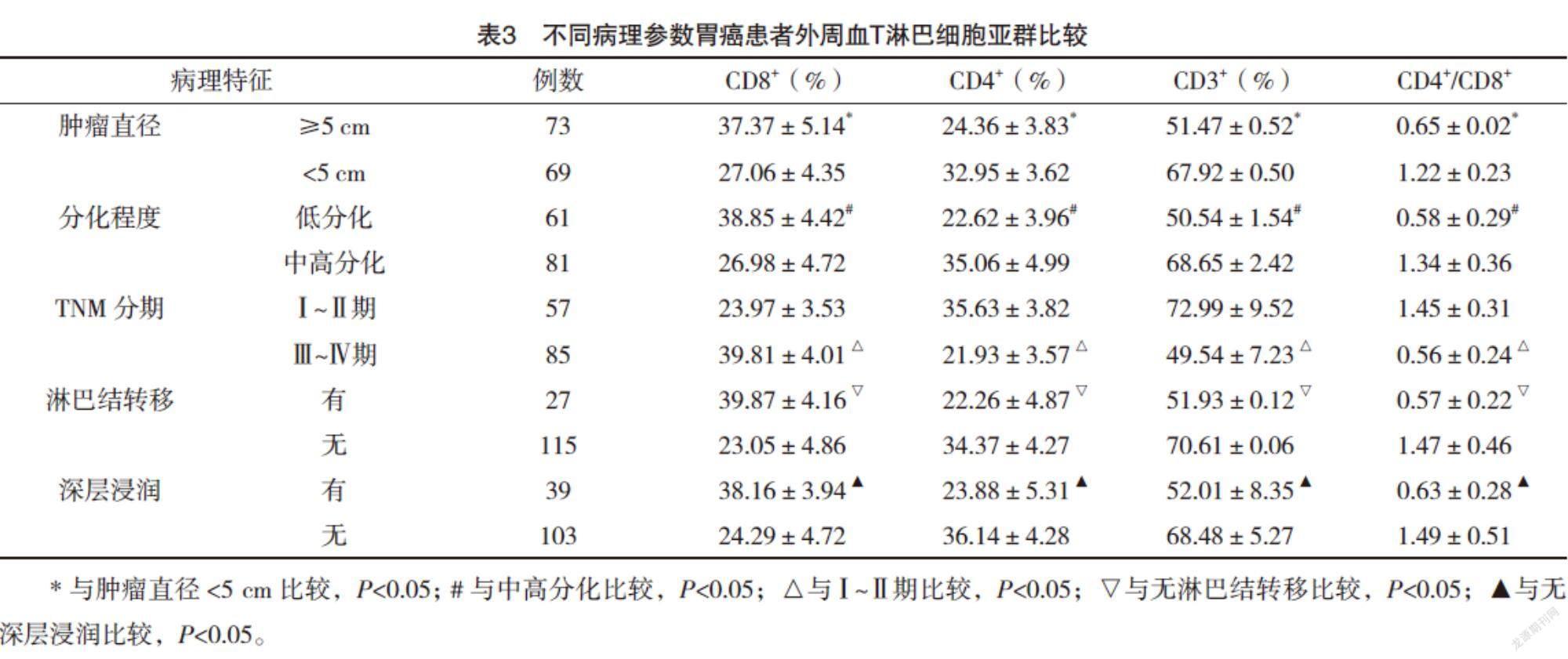
2.4 不同病理参数胃癌患者外周血T淋巴细胞亚群比较 肿瘤直径≥5 cm、低分化、Ⅲ~Ⅳ期、淋巴结转移、深层浸润的胃癌患者外周血CD3+T细胞、CD4+T细胞、CD4+/CD8+比值较肿瘤直径<5 cm、中高分化、Ⅰ~Ⅱ期、无淋巴结转移、无深层浸润均明显下降(P<0.05),CD8+T细胞比例较肿瘤直径<5 cm、中高分化、Ⅰ~Ⅱ期、无淋巴结转移、无深层浸润均明显升高(P<0.05),见表3。
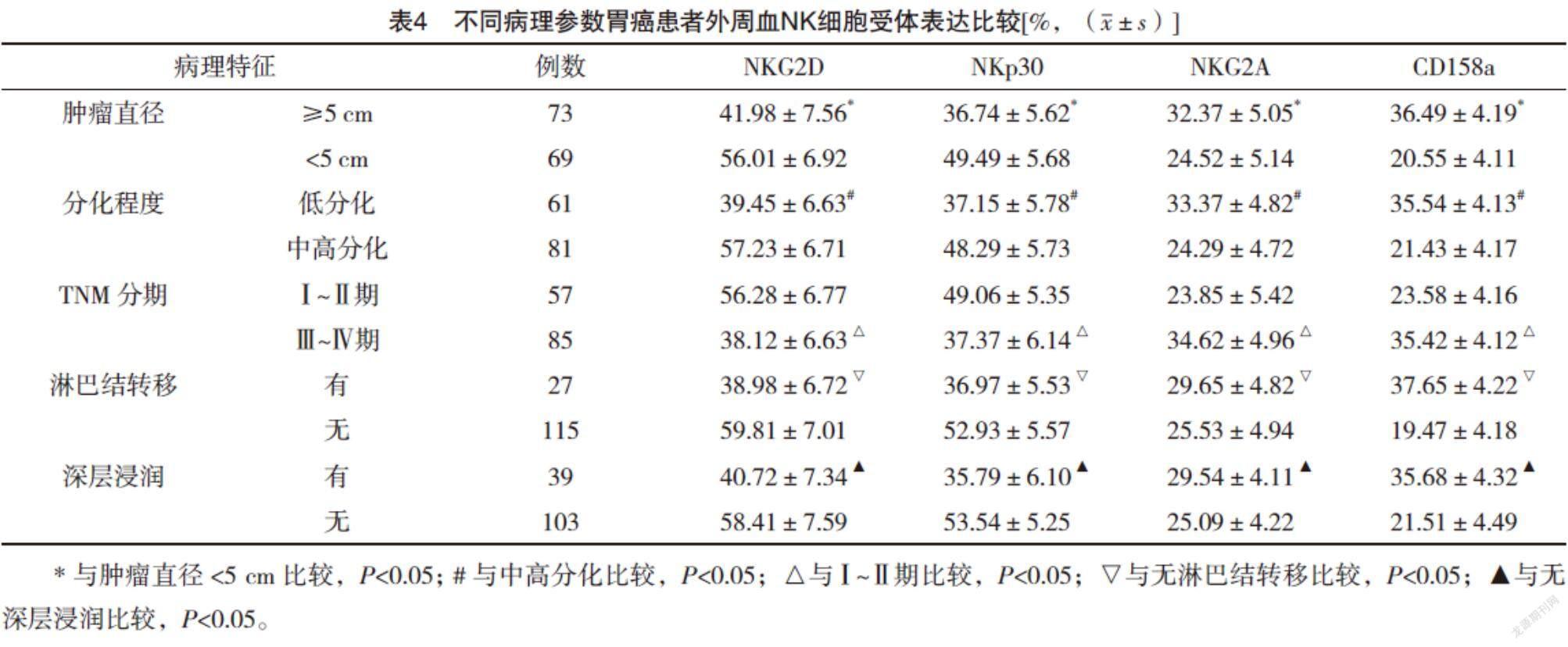
2.5 不同病理参数胃癌患者外周血NK细胞受体表达比较 肿瘤直径≥5 cm、低分化、Ⅲ~Ⅳ期、淋巴结转移、深层浸润的胃癌患者外周血NK细胞活化性受体NKG2D和NKp30表达较肿瘤直径<5 cm、中高分化、Ⅰ~Ⅱ期、无淋巴结转移、无深层浸润均明显下降(P<0.05),抑制性受体NKG2A和CD158a表达较肿瘤直径<5 cm、中高分化、Ⅰ~Ⅱ期、无淋巴结转移、无深层浸润均明显升高(P<0.05)。见表4。
3 讨论
胃癌是一种起源于胃黏膜上皮的恶性肿瘤,目前我国胃癌致死率居恶性肿瘤第三位[10-11]。近年来的肿瘤免疫学研究显示,包括胃癌在内的恶性肿瘤的发生和发展与机体免疫功能异常密切相关,而T淋巴细胞及NK细胞在肿瘤免疫中发挥重要作用[12-14]。
T淋巴细胞可分为CD4+和CD8+T淋巴细胞两个亚群[15]。在正常生理状态下,CD4+和CD8+T淋巴细胞亚群保持动态平衡以维持机体正常免疫功能[16]。最近的研究表明,T淋巴细胞亚群比例不仅可以反映机体的免疫功能状态,还可以反映肿瘤微环境的变化[17-18],因此具有重要的临床应用价值。本研究结果显示,胃癌组患者外周血CD3+T细胞、CD4+T细胞、CD4+/CD8+比值较对照组均明显下降(P<0.05),CD8+T细胞比例较对照组则明显升高(P<0.05),提示胃癌患者外周血T淋巴细胞亚群异常/比例失调,导致患者细胞免疫功能明显下降而有助于肿瘤的发生。进一步分析不同病理参数胃癌患者外周血T淋巴细胞亚群分布发现,肿瘤越大、分化程度越低、分期越晚、发生淋巴结转移及深层浸润的胃癌患者外周血CD3+T细胞、CD4+T细胞、CD4+/CD8+比值越低,CD8+T细胞比例越高,提示胃癌患者恶性程度越高,T淋巴细胞亚群分布越紊乱。
NK细胞对肿瘤细胞具有直接杀伤作用,而肿瘤细胞可通过影响受体表达而影响NK细胞的杀伤作用[19-20]。本研究结果显示,胃癌组患者NK细胞活化性受体NKG2D和NKp30表达较对照组均明显下降(P<0.05),抑制性受体NKG2A和CD158a表达较对照组均明显升高(P<0.05),这与文献[21]的报道结果相符合。本研究进一步分析了不同病理参数胃癌患者NK细胞受体表达情况,结果显示肿瘤体积越大、分化程度越低、分期越晚、发生淋巴结转移及深层浸润的胃癌患者NK细胞活化性受体NKG2D和NKp30表达越低,抑制性受体NKG2A和CD158a表达越高,提示胃癌患者惡性程度越高,NK细胞的活性降低,免疫监视功能越弱。
综上所述,胃癌患者外周血T淋巴细胞亚群分布紊乱,NK细胞活化性受体表达降低,抑制性受体表达升高,且与胃癌的发生发展有关,检测胃癌患者外周血T淋巴细胞亚群和NK细胞受体表达有助于评估细胞免疫功能、指导临床治疗和评价预后。
参考文献
[1] ITO A,KAGAWA S,SAKAMOTO S,et al.Extracellular vesicles shed from gastric cancer mediate protumor macrophage differentiation[J].BMC Cancer,2021,21(11):583-589.
[2] JEPSEN P,VILSTRUP H,ANDERSEN P K,et al.Pressurized intraperitoneal aerosol chemotherapy (PIPAC) of peritoneal metastasis from gastric cancer: a descriptive cohort study[J].Clin Exp Metastasis,2020,48(12):214-220.
[3] NAIK A,MONJAZEB A M,DECOCK J.The Obesity Paradox in Cancer, Tumor Immunology, and Immunotherapy: Potential Therapeutic Implications in Triple Negative Breast Cancer[J].Frontiers in Immunology,2019,10(6):1940-1947.
[4] MA J,ZHANG H,TANG K,et al.Tumor-derived microparticles in tumor immunology and immunotherapy[J].European Journal of Immunology,2020,50(11):921-928.
[5] ZAHRAN A M,SAYED M M,SHAFIK E A,et al.The Frequency and clinical Implications of Lymphocyte Subsets and Circulating Plasma Cells in Newly Diagnosed Multiple Myeloma Patients[J].The Egyptian Journal of Immunology/Egyptian Association of Immunologists,2019,26(2):117-131.
[6] LV Y,SONG M,TIAN X,et al.Impact of radiotherapy on circulating lymphocyte subsets in patients with esophageal cancer[J].Medicine,2020,9(5):829-835.
[7] VITO C D,MIKULAK J,ZAGHI E,et al.NK cells to cure cancer[J].Seminars in Immunology,2019,41(7):101-108.
[8] KAUSHIK N K,KAUSHIK N,BHARTIYA P,et al.Glycolytic inhibitor induces metabolic crisis in solid cancer cells to enhance cold plasma-nduced cell death[J].Plasma Processes and Polymers,2021,15(8):357-362.
[9] OH J H,KIM M J,CHOI S J,et al.Sustained Type I Interferon Reinforces NK Cell-Mediated Cancer Immunosurveillance During Chronic Virus Infection[J].Cancer Immunology Research,2019,15(8):229-235.
[10] SPOLVERATO G,PAWLIK T M.Clinicopathological evaluation of recurrence in early gastric cancer[J].American Journal of Surgery,2019,157(3):202-207.
[11] ASPIRIN A P,AURELIO A,KIM Y.Polytherapeutic strategies with oncolytic virus–bortezomib and adjuvant NK cells in cancer treatment[J].Journal of The Royal Society Interface,2021,18(174):20200669.
[12] CAO W,YAO X,CEN D,et al.The prognostic role of platelet-to-lymphocyte ratio on overall survival in gastric cancer: a systematic review and meta-analysis[J].BMC Gastroenterology,2020,20(12):1126-1131.
[13] LAUDER S N,MILUTINOVIC S,PIRES A,et al.Using methylcholanthrene-induced fibrosarcomas to study tumor immunology[J].Methods in Cell Biology,2020,26(9):853-858.
[14] RAHMAN M A,MURATA K,BURT B D,et al.Changing the landscape of tumor immunology: novel tools to examine T cell specificity[J].Current Opinion in Immunology,2021,69(27):1-9.
[15] WANG Y Y,ZHOU N,LIU H S,et al.Circulating activated lymphocyte subsets as potential blood biomarkers of cancer progression[J].Cancer Medicine,2020,15(3):227-234.
[16] QIU J,ZHOU F,LI X,et al.Changes and Clinical Significance of Detailed Peripheral Lymphocyte Subsets in Evaluating the Immunity for Cancer Patients[J].Cancer Management and Research,2020,12(8):209-219.
[17] CHEN T,KONG F,SONG Y,et al.The effect of acupoint stimulation on T lymphocyte subsets and NK cells in cancer patients: a systematic review and meta-analysis[J].European Journal of Integrative Medicine,2021,12(7):101309.
[18] HOFER T P,KSMANN L,PELIKAN C,et al.Dynamic changes of lymphocyte subsets during multimodal treatment of patients with inoperable stage Ⅲ NSCLC[J/OL].Journal of Clinical Oncology,2020,38(15 suppl):e21011.
[19] KANG S,GAO X,ZHANG L,et al.The Advances and Challenges of NK Cell-Based Cancer Immunotherapy[J].Current Oncology,2021,28(2):1077-1093.
[20] TARAZONA R,LOPEZ-SEJAS N,GUERRERO B,et al.Current progress in NK cell biology and NK cell-based cancer immunotherapy[J].Cancer Immunology and Immunotherapy,2020,69(Suppl 1):992-998.
[21] ZHANG R,QI F,ZHAO F,et al.Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer[J].Cell Death & Disease,2019,10(4):426-433.
(收稿日期:2021-09-02) (本文編辑:张爽)

