Assembling Fe2O3/BiOCl Composite for Highly Effective Degradation of Water Pollutants under Visible-Light Irradiation
Liu Xiaoqing; Wang Zisha,2; Hu Yuxing; Wang Haifang
(1. School of Environment and Safety Engineering, North University of China, Taiyuan 030051;2. Beijing Aerospace Institute for Metrology and Measurement Technology, Beijing 100076)
Abstract: Fe2O3 was synthesized by the solvothermal method, and the synthesized Fe2O3 was added in the process of preparing BiOCl by hydrolysis, and then Fe2O3/BiOCl photocatalytic materials with different composite ratios were prepared. The optimal Fe2O3/BiOCl (1Fe/50Bi) sample showed a highest photocatalytic efficiency for cationic dyes(Rhodamine B) and anionic dye (methyl orange) degradation irradiated with visible light, as compared with that of a bare BiOCl catalyst. Meanwhile, radical capturing experiments indicated that the photo-induced holes (h+) is the main active species. X-ray powder diffraction and ultraviolet-visible diffuse reflectance spectroscopy were used to characterize the structural and optical properties, which proved that Fe2O3 was successfully composited to the BiOCl surface and effectively reduced the bandgap of BiOCl. More importantly, the optimal 1Fe/50Bi sample shows the highest photocatalytic efficiency for tetracycline (TC) degradation (98%) irradiated with visible light, as compared with that of a bare BiOCl catalyst.Consequently, the Fe2O3/BiOCl photocatalyst have potential applications in environmental purification.
Key words: Fe2O3; BiOCl; composite; photocatalytic
1 Introduction
In recent years, environmental problems have become one of the most major challenges due to the economic development and rapid expansion of industrialization.Water, as the source of human lives, has become a disaster area of environmental pollution[1-3]. Furthermore,water pollution seriously affects human health, threatens the human living environment and restricts economic progress and sustainable development. Therefore,exploring new efficient treatment methods of pollutants and solving the problems of environmental pollution have become an important strategic goal of our country,which are beneficial to the improvement of our living quality and the sustainable development of society[4-6].Solar energy, as the green renewable energy, is best known for its inexhaustible, pollution-free, and widely available advantages. How to efficiently utilize solar energy is one of the research hotspots. At present, the use of solar energy through photocatalysis mainly focuses on solar hydrogen production, solar cells, photocatalytic degradation of pollutants, etc.[7]Photocatalytic technology is considered to be the most promising technology solution for global energy and environmental problems to degrade or even completely mineralize organic pollutants, based on the direct conversion of solar energy into chemical energy and electrical energy through photocatalytic semiconductors[8]. In order to promote and expand the application of photocatalytic materials in clean energy and environmental purification, the development of new photocatalytic materials is one of the most feasible methods. The exploration of novel photocatalytic materials is one of the forefronts of science, and has great significance in the field of environmental protection[9].
As a class of layered semiconductor materials, BiOCl(bismuth oxychloride), has notable UV photoresponse[10],which has attracted much attention due to its special structure and good chemical stability[11-14]. However, it also has some disadvantages, including wide bandgap,and rapid recombination of photogenerated electrons and hole pairs. Semiconductor recombination can improve photocatalytic performance effectively. Fe2O3has many advantages such as no pollution to the environment,low production cost, stable structure, and wide sources availability compared with other oxides[7,15]. In this work,Fe2O3/BiOCl composite was prepared and its photocatalytic performance was analyzed by degrading the cationic dye Rhodamine B (RhB), anionic dye methyl orange (MO),and the antibiotic tetracycline in wastewater.
2 Experimental
2.1 Materials and reagents
Ferric nitrate nonahydrate (Fe(NO3)3·9H2O), bismuth nitrate pentahydrate (Bi(NO3)3·5H2O), ammonia water(NH3·H2O), ethylene glycol (C2H6O2), acetic acid (C2H4O2),potassium chloride (KCl), anhydrous ethanol (C6H5O),tert-butanol (C4H10O), p-benzoquinone (C6H4O2), silver nitrate (AgNO3), sodium oxalate (Na2C2O4), Rhodamine B (C28H31ClN2O3) , methyl orange (C14H14N3NaO3S), and tetracycline hydrochloride (C22H24N2O8), were bought from Aladdin (Shanghai, China). All the chemical reagents,which were of analytically pure grade, were used without further purification.
2.2 Preparation of Fe2O3/BiOCl composite
2.2.1 Preparation of Fe2O3nanoparticles 0.323 g of Fe(NO3)3·9H2O was added into 25 mL of ethylene glycol, and then 5 mL of NH3·H2O were dropped into the Fe(NO3)3solution. After completely stirring,the clarified solution was obtained. The solution was transferred into 50-mL autoclave to enter into reaction at 210 °C for 40 h. After being cooled down to room temperature, the reaction product was centrifuged with the distilled water and anhydrous ethanol alternately for 6 times. Then the solid was dried at 60 °C for 24 h to yield pure Fe2O3. The product Fe2O3was prepared as shown in Figure 1.
2.2.2 Preparation of BiOCl nanoparticles
0.182 g of Bi(NO3)3·5H2O was added into acetic acid solution (H2O: acetic acid =100 mL: 5 mL), then the mixed solution was stirred at room temperature, after Bi(NO3)3·5H2O was completely dissolved in the solution.30 mL of KCl solution (0.0125 mol/L) were quickly added dropwise to the mixted solution. After being stirred for 30 min at room temperature, the suspension should be left to stand for 3 h. The obtained precipitates were washed alternatively with deionized water and anhydrous ethanol for three times. The obtained solids after washing were dried at 60 °C for 8 h. They were encapsulated for later use after grinding. The process for synthesis of pure BiOCl is shown in Figure 2.
2.2.3 Preparation of Fe2O3/BiOCl nanocomposite
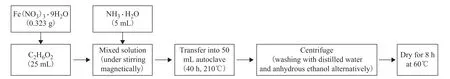
Figure 1 Schematic illustration of the synthetic processes of single Fe2O3

Figure 2 Schematic illustration of the synthetic processes of single BiOCl
0.182 g of Bi(NO3)3·5H2O was added to acetic acid solution (H2O: acetic acid =100 mL:5 mL). A certain amount of Fe2O3powder was added to the above solution,and was dispersed ultrasonically for 30 min at room temperature, followed by being stirred magnetically for 30 min, until no more Fe2O3nano-powder was precipitated. Then 30 mL of KCl solution (0.0125 mol/L) were quickly added to the mixted solution. After being stirred for 30 min at room temperature, the suspension was left to stand for 3 h. The obtained precipitates were alternatively washed with deionized water and anhydrous ethanol for three times. After extraction and filtration,nanocomposites with different ratios (Fe2O3: BiOCl mass ratio equating to 1:20, 1:40, 1:50, and 1:60, respectively)were obtained after being dried at 60 °C in air for 8 h, and were labeled as 1Fe/20Bi, 1Fe/30Bi, 1Fe/40Bi,1Fe/50Bi and 1Fe/60Bi, respectively. The mechanically mixed sample of M-1Fe/50Bi was also synthesized as a comparison. The process for synthesis of Fe2O3/BiOCl composites is shown in Figure 3.
2.3 Characterization
The crystallinity and structure of the as-prepared catalysts were characterized by powder X-ray diffraction(Panalytical Empyream Instrument) using CuKα(λ=1.5418 Å) radiation operating at a tube voltage of 40 kV and a tube current of 50 mA. Particle size and morphology of the samples were determined by using scanning electron micrographs (SEM) (HITACHI S-4800 with an acceleration voltage of 20.0 kV). The diffuse reflection spectrum of the photocatalyst was obtained by an UV-visible diffusive reflectance spectrophotometer(UVIKON XL/XS), and BaSO4was employed as the reference standard.
2.4 Photocatalytic degradation
The photocatalytic performance of the as-obtained catalysts were assessed by degrading RhB, MO and TC under irradiation with visible light using a UV-cutoff filter (λ> 420 nm) and a 500W Xe lamp. 0.025 g of the catalyst was added to 30 mL of aqueous solution(20 mg/L), and circulating water was employed to maintain a specified temperature in the process of reaction. Before illumination, the solution was continuously stirred for 2 h in darkness to establish an adsorption and desorption equilibrium of the dye solution on the samples. The concentration of the dye and TC aqueous solution was confirmed by the absorbance using a UV/Vis spectrophotometer (SPECORD 50 N).
3 Results and Discussion
3.1 Photocatalytic activities for RhB, MO and TC removal
The absorbance of the RhB, MO, and TC degradation was measured by an UV-visible spectrophotometer, the degradation rates were calculated by the Lambert-Beer
law (A=ε BC), and then the degradation curves were drawn to analyze the pollutant degradation performance of Fe2O3/BiOCl catalysts with different mole ratios.The calculation formula used to describe the photocatalytic reaction is as follows:

It is discovered that the degradation process well follows the first-order kinetic equation:


Figure 3 Schematic illustration of the synthetic processes of Fe2O3/BiOCl composites

Figure 4 (a) Normalized concentration of RhB versus visible light irradiation time in the presence of the samples, (b) UV-vis spectral changes of RhB in aqueous 1Fe/50Bi composite dispersion as a function of irradiation time, (c) Liner transform ln(C/C0) =kt of RhB degradation over the as-prepared catalysts, (d) Rate constants over Fe2O3/BiOCl with various ratio under visible light

Figure 5 (a) Normalized concentration of MO versus visible light irradiation time in the presence of the samples, (b) UV-vis spectral changes of MO in aqueous 1Fe/50Bi composite dispersion as a function of irradiation time, (c) Liner transform ln(C/C0) =kt of MO degradation over the as-prepared catalysts, (d) Rate constants over Fe2O3/BiOCl with various ratio under visible light
The degradation effect of RhB and MO by various samples under visible light is shown in Figure 4 (a) and Figure 5 (a). It was discovered that in the absence of the catalysts, the decomposition of RhB and MO was negligible. The performance of pure Fe2O3and BiOCl for degradation of RhB and MO was not ideal under visible light, which might be due to the wide bandgap of single Fe2O3and BiOCl that could not be excited by visible light to generate photoelectron-hole pairs.However, Fe2O3/BiOCl demonstrated good efficiency for degradation of RhB and MO under visible light, in which the photocatalytic efficiency of 1Fe/50Bi was particularly excellent. After irradiation for 15 min under visible light, the degradation rate of RhB reached 95%.The degradation rate of MO reached 39% after 125 min of irradiation under visible light, but the degradation rate achieved by pure BiOCl only reached 28%, indicating that the photocatalytic performance of BiOCl could be improved owing to the combined effect of Fe2O3and BiOCl. The degradation efficiency of RhB was better than that of MO. The photocatalytic performance of 1Fe/60Bi was worse than that of 1Fe/50Bi, which might be due to the decreased amount of Fe2O3. Judging from the pollutant degradation performance of 1Fe/40Bi, 1Fe/30Bi and 1Fe/20Bi, the photocatalytic efficiency decreased with an increasing amount of Fe2O3in the composite material and the photocatalytic efficiency of 1Fe/30Bi and 1Fe/20Bi is even lower than that of pure BiOCl. This may be due to the fact that the heterogeneous junction was formed in the composite material, but the excessive Fe2O3loading would reduce the specific surface area, resulting in the low photocatalytic efficiency. Therefore, the amount of Fe2O3was one of the important factors affecting the photocatalytic efficiency of composite materials.Although M-1Fe/50Bi had photocatalytic efficiency for degrading RhB and MO, its photocatalytic efficiency was not ideal and was far less than that of 1Fe/50Bi and pure BiOCl. Therefore, it was inferred that mechanical mixing could not make Fe2O3and BiOCl establish a close contact interface to form heterojunction for improving the photocatalytic efficiency of BiOCl, rather than obscuring the surface of BiOCl and reducing the photocatalytic efficiency of BiOCl. It also indicated that the formation of Fe2O3/BiOCl heterojunction could greatly improve photocatalytic efficiency.
The UV-visible absorption spectrum of RhB degraded by 1Fe/50Bi under visible light is shown in Figure 4 (b).The maximum absorption wavelength of RhB was 553 nm. Upon being subjected to illumination, the absorption peak intensity of RhB gradually weakened and then disappeared completely after illumination for 20 min.At the same time, the maximum absorption peak of RhB was generally blue shift during the degradation process,which might be caused by the gradual removal of ethyl radical in the degradation process of RhB[16]. The UVvisible absorption spectrum of MO degraded by 1Fe/50Bi under visible light is shown in Figure 5 (b). It can be seen that the maximum absorption wavelength of methyl orange was 464 nm, and the intensity of absorption peak was gradually weakened under illumination, indicating that MO was gradually degraded with the increase of illumination time.
Judging from Figure 4 (c) and Figure 5 (c), the kinetic fitting curves of degradation of RhB by different photocatalytic materials under visible light were calculated according to Formula 2, with their kinetic rate constants presented in Figure 4 (d) and Figure 5 (d). The kinetic rate constants of 1Fe/50Bi composites were higher than pure BiOCl and Fe2O3/BiOCl with other composite ratios, indicating that semiconductor composites significantly improved the photocatalytic efficiency and the degradation performance of 1Fe/50Bi was optimal.

Figure 6 Concentration of TC versus time over the samples
As shown in Figure 6, the photocatalytic performance of Fe2O3/BiOCl composites were estimated by degrading TC (20 mg/L) with visible light illumining (λ> 420 nm). The adsorption equilibrium between the catalysts and TC aqueous solution is reached within 120 min. In addition, the degradation rate of TC is only 15% after 140 min of visible light irradiation in the presence of pure BiOCl. Obviously, 1Fe/50Bi nanocomposite presents the excellent photocatalytic activity, corresponding to a TC degradation of 98%.
3.2 Structure and morphology of the as-prepared catalysts
As shown in Figure 7, the crystal structure of the samples was examined by XRD analysis. It can be seen that the diffraction peaks of synthetic pure BiOCl are consistent with those of BiOCl standard card (JCPDS 85-0861), so it is confirmed that the pure BiOCl has been successfully synthesized. Obviously, BiOCl was the predominant constituent in the composite samples of 1Fe/50Bi and the diffraction peak of Fe2O3was scarcely observed, which might be caused by the low content of Fe2O3in 1Fe/50Bi.It was also possible that the diffraction peak at 33.5° and 35.6° overlapped the diffraction peak of BiOCl[17]. In addition, the peak intensity of BiOCl in 1Fe/50Bi was significantly weaker than that of pure BiOCl, indicating that the presence of Fe2O3affected the growth of BiOCl.

Figure 7 XRD patterns of pure Fe2O3, BiOCl and Fe/50Bi composite

Figure 8 SEM images of: (a) BiOCl, and (b) Fe2O3/BiOCl
As shown in Figure 8, the morphology and particle size were determined by SEM. It can be seen from Figure 8(a) that pure BiOCl had a rose flower-like structure and an obvious layered structure. It can be seen from Figure 8 (b) that the obvious layered structure disappeared,indicating that Fe2O3was well loaded on BiOCl and evenly dispersed. At the same time, the disappearance of lamellar structure also indicated that the presence of Fe2O3inhibited the growth of BiOCl, which was consistent with the XRD results.
3.3 Optical properties
To investigate the optical absorption performance, UVvisible diffuse reflectance spectra of BiOCl and 1Fe/50Bi composite are shown in Figure 9, denoting that pure BiOCl had an absorption band edge at 370 nm, while 1Fe/50Bi had an absorption band edge at 580 nm.
According to the literature, both Fe2O3and BiOCl are semiconductors with an indirect band gap[18-21]. Thereby,the indirect band gap of these nanocrystals is estimated from the graph ofhνversus (αhν)1/2for the absorption coefficientα. The absorption coefficientαis related to the band gapEgas:

Figure 9 (a) UV-visible diffuse reflectance spectroscopy of pure BiOCl and 1Fe/50Bi, (b) the optical band gap of pure BiOCl and 1Fe/50Bi

wherehνis the incident photon energy andAis a constant. As shown in Figure 10 (b), the energy band gapEgis determined as 2.8 eV for BiOCl nanocrystal and 1.8 eV for 1Fe/50Bi nanocrystal. Apparently, the composite of Fe2O3led to a red shift of the absorption edge, which is mainly due to the interaction between Fe2O3and BiOCl.
3.4 Possible photocatalytic mechanism
To further understand the nature of the primary active species involved in degradation of RhB and MO in an aqueous phase over 1Fe/50Bi nanocrystal, we have carried out control experiments by adding some active species scavengers. In this experiment, sodium oxalate,silver nitrate,tert-butanol and p-benzoquinone were selected as sacrificial agents of hole (h+), electron (e-),hydroxyl radical (·OH) and superoxide radical (·O-2),respectively[22-23]. The concentration of sacrificial agents in the system was 1.0 mmol/L. The experimental results for the visible-light-driven catalytic degradation of RhB and MO are shown in Figure 10 and Figure 11. It can be seen from Figure 10 that the degradation of RhB demonstrated a significantly lower efficiency in the reaction system after adding sodium oxalate and a most significant inhibitory effect, which decreased in the following order: sodium oxalate > benzoquinone > silver nitrate >tert-butyl alcohol > no sacrificial agent. From Figure 11 (a), the concentration of methyl orange in the system withp-benzoquinone was even higher than that in the original solution when measuring the methyl orange content. According to the UV-visible absorption spectrum in Figure 11(b), the absorption peak at 200-250 nm changed after p-benzoquinone was added to the system,while the absorption peak at 464 nm remained almost unchanged. It is suggested that the presence of benzoquinone affected the determination of MO. This outcome might be due to the color of benzoquinone itself affecting the detection of MO, or it might be due to the reaction ofp-benzoquinone with the reducing photogenic electron, which made p-benzoquinone reducec to red hydroquinone, which interfered in the determination of MO. Therefore, in addition to the interference ofp-benzoquinone on MO, it was shown that sodium oxalate had the most obvious inhibition effect on MO, and the inhibition effect decreased in the following order: sodium oxalate >tert-butanol >1Fe/50Bi> silver nitrate. The strong inhibition of sodium oxalate indicated that photogenic holes (h+) were the main active species in the photocatalytic degradation of RhB and MO, while the electron (e-), hydroxyl radical (·OH) and superoxide radical (·O-2) were weak.As shown in Figure 12, to validate the predominant active species that play a significant role in the degradation process of TC, a free radical capture experiment was performed by adding different trapping agents into the solution with 1Fe/50Bi. As a result, it showed the same mechanism as the dyes degradation that photogenic holes (h+) play leading roles in the process of photocatalytic degradation of TC.

Figure 10 The experiment of capturing active species in the degradation process of RhB

Figure 11 (a) The experiment of capturing active species in the degradation process of MO; and (b) UV-visible diffuse reflectance spectroscopy with BQ

Figure 12 The experiment of capturing active species in the degradation process of TC
According to the above results, we proposed a possible mechanism about RhB and MO degradation over Fe2O3/BiOCl as shown in Figure 13. Being irradiated with visible light, both Fe2O3and BiOCl could be excited to generate electrons and holes. In the process of degradation, the heterojunction complex is responsible for suppressing recombination of electrons and hole pairs.Hence, the Fe2O3/BiOCl composite exhibits a significantly enhanced photocatalytic activity.

Figure 13 The possible photocatalytic mechanism over Fe2O3/BiOCl
4 Conclusions
Fe2O3was synthesized by a solvothermal method which was added in the process of hydrolysis synthesis of BiOCl to form Fe2O3/BiOCl composite. In summary, a string of Fe2O3/BiOCl photocatalysts were fabricated.The photocatalytic performance was evaluated by catalytic degradation of cationic RhB, anionic MO and TC under visible light irradiation. The structural and morphological analysis of Fe2O3/BiOCl composite samples was conducted by the XRD and SEM. The optical properties were studied by the UV-vis diffuse reflectance spectroscopy. It reveals that the 1Fe/50Bi composites show the best photocatalytic activity, and the photo-production holes are the main active species in photocatalytic reactions. The good photocatalytic performance for 1Fe/50Bi could be assigned to its good visible light absorption and efficient separate electronhole pairs based on the relative band gap position of these two independent components and the integrated heterostructure. The present work can be extended to other bismuth-containing heterostructured photocatalysts with high efficiency for elimination of the water pollutants from wastewater.
Acknowledgement: This work was financially supported by the National Natural Science Foundation of China (51901209).
- 中国炼油与石油化工的其它文章
- Study on Viscosity Reducing and Oil Displacement Agent for Water-Flooding Heavy Oil Reservoir
- Electrospinning Nanofiber Membrane Reinforced PVA Composite Hydrogel with Preferable Mechanical Performance for Oil-Water Separation
- Preparation of Solid Waste-Based Activated Carbon and Its Adsorption Mechanism for Toluene
- Antibacterial and Corrosion Inhibition Properties of SA-ZnO@ODA-GO@PU Super-Hydrophobic Coating in Circulating Cooling Water System
- Investigation of Nitrite Production Pathway in Integrated Partial Denitrification/Anammox Process via Isotope Labelling Technique and the Relevant Microbial Communities
- Heteroatom-Doped Carbon Spheres from FCC Slurry Oil as Anode Material for Lithium-Ion Battery

