A Novel MIL-101(Cr) Acidified by Silicotungstic Acid and Its Catalytic Performance for Isomerization of n-Heptane
Zhang Wei; Liu Rongjiang;MaShoutao,2; Kuvshinov Dimitriy;Suo Yanhua; Wang Yingjun
(1. Proνincial Key Laboratory of College of Polyolefin New Materials, Chemistry and Chemical Engineering,Northeast Petroleum University, Daqing 163318;2. Daqing Chemical Research Center of PetroChina, Daqing 163714;3. School of Engineering, University of Hull, UK)
Abstract: The 0.4%Pt/xSTA-MIL-101(Cr) metal-acid bifunctional catalysts were prepared by impregnation using STAMIL-101(Cr) as the support. The synthesized samples were verified to exhibit a typical octahedral structure of MIL-101(Cr) and the pore structure was arranged orderly. The specific surface area of the samples was extremely high and the samples were made of micro-mesoporous composite materials. Silicotungstic acid could retain its Keggin structure in the 0.4%Pt/xSTA-MIL-101(Cr) samples and the catalyst possessed moderately strong Brønsted acid sites. Besides, the dispersion of Pt particles in MIL-101(Cr) was relatively high. n-Heptane isomerization was first used as a probe to test the novel 0.4%Pt/xSTA-MIL-10(Cr) catalyst. Compared with the conventional silicate catalysts, the catalytic performance of 0.4%Pt/30%STA-MIL-101(Cr) was significantly improved with a n-heptane conversion of 58.93% and an iso-heptane selectivity of 95.68%, respectively, under conditions covering a reaction time of 2 h and a reaction temperature of 260 °C.The catalyst could still maintain a relatively high catalytic performance after a reaction time of 5 h. Compared with the nonnoble metal catalyst, the catalytic efficiency of 0.4%Pt/30%STA-MIL-101(Cr) is relatively high. The mechanism model of n-heptane isomerization over 0.4%Pt/xSTA-MIL-101(Cr) catalyst was established.
Key words: MIL-101; silicotungstic acid; n-heptane; isomerization; platinum
1 Introduction
As environmental problems become more and more serious,the people’s demand for fuel quality becomes higher and higher. How to increase the octane number of gasoline without using methyltert-butyl ether (MTBE) has become an urgent problem to be solved by researchers. Since isomerization ofn-alkanes is an important way to increase the octane number of gasoline, among whichn-heptane isomerization is still in the laboratory research stage, hencen-heptane isomerization has been a research hotspot in recent years. Therefore, a series of molecular sieve catalysts that can catalyze isomerization ofn-heptane have appeared one after another. However, the conversion ofn-heptane and the selectivity ofiso-heptane achieved by such catalysts during the isomerization ofn-heptane are relatively low[1], so it is necessary to further explore efficient catalysts that not only can promote the conversion ofn-heptane but also can inhibit the side reactions of cracking.
Metal-organic frameworks (MOFs) are a kind of porous material with an orderly pore structure formed by selfassembly of metal ions and organic ligands. Since its discovery, it has been widely used in catalysis, gas separation and storage, slow-release drug, electronic devices, chemical sensing and fluorescent materials due to its unique advantages. MIL-101(Cr), which is a kind of MOFs with special structure, was first synthesized by Ferey,et al. in 2005[2], with its pore structure shown in Figure 1(a).Pure MIL-101(Cr) exhibits a zeolite-like cubic structure with three-dimensional pore channels, and it has special properties such as high crystallinity, high specific surface area and high porosity, making itself a promising carrier.Besides, its unique double-pore structure can have a unique shape-selective catalysis effect on the isomerization reaction.However, the few acidic active sites of MIL-101(Cr) would seriously limit its application in the field of catalysis. In order to solve the problem of its low acid strength, many researchers have doped heteropoly acid into MIL-101(Cr) to make up for the weak acidity of MIL-101(Cr)[3].
The anion structure diagram of silicotungstic acid (STA)with the Keggin structure ([XM12O40]n-) is shown in Figure 1(b). The anion of silicotungstic acid exhibits a Td symmetry, in which the central silicon atom coordinates with the four surrounding oxygen atoms in tetrahedral type,and the coordinating tungsten atom coordinates with the six surrounding oxygen atoms in octahedral type. Silicotungstic acid is a multifunctional solid catalyst with a strong Brφnsted acid center, which could provide a favorable acidic environment for catalytic reactions. However, the specific surface area of silicotungstic acid is too small to be in contact with the reactants fully, which would greatly limit its application in the catalytic field. Therefore, it is necessary to dope silicotungstic acid onto the material with a high specific surface area in order to play a good catalytic role[4-5].
A large number of studies have reported the application of catalysts containing the precious metal Pt in the isomerization reaction ofn-alkane[6-7]. Compared with the isomerization performance of non-precious metal catalysts,the catalysts containing precious metal Pt can exhibit better isomerization performance. Therefore, it is an ideal choice to apply Pt as the active component in then-heptane isomerization reaction. Moreover, H2PtCl6can be reduced in H2atmosphere at 200 °C, and the isomerization reaction can be carried out directly without cooling the catalyst after the reduction reaction, which can greatly reduce the reaction time to significantly improve the catalytic efficiency.

Figure 1 Pore structure of MIL-101(Cr) (a) and structure of STA (b)
In summary, the introduction of precious metal Pt and silicotungstic acid into MIL-101(Cr) material is expected to be an excellent isomerization catalyst. At present,many studies have been reported on the hydrocatalytic reaction using MIL-101(Cr) material as the support of the catalyst[8-10], but till now no literature has reported the application of MIL-101(Cr) material modified by STA inn-heptane isomerization reaction. Therefore, the STAMIL-101(Cr) micro-mesoporous composite materials were successfully synthesized by the hydrothermal method in this paper, and the precious metal Pt was introduced into STA-MIL-101(Cr) catalyst by the impregnation method. A series of 0.4%Pt/xSTA-MIL-101(Cr) bifunctional catalysts were prepared by changing the doping amount of silicotungstic acid on MIL-101(Cr)material, andn-heptane isomerization reaction was selected as the probe to study the influence of doping amount of silicotungstic acid, reaction temperature, and reaction time on the catalytic performance of the catalyst.
2 Experimental
2.1 Preparation of catalysts
MIL-101(Cr) (MIL refers to Matériaux de l′Institut Lavoisier, and the number n represents the serial number of preparation with a molecular formula of Cr3O(OH)(H2O)2(C8H4O4)3) was synthesized by the hydrothermal method[11]. H2BDC (terephthalic acid) (2.08 g),Cr(NO3)3·9H2O (5 g) and a given amount of silicotungstic acid were dissolved in 25 mL of distilled water. After stirring for 20 min, the resulting mixture was transferred into a 100-mL Teflon-lined stainless-steel autoclave and heated at 220 °C for 18 h. The product was filtered with a sand core funnel, and then was washed by centrifugation until the mother liquor was colorless and transparent.Finally, STA-MIL-101(Cr) material was obtained by dissolving the resulting sample into ethanol followed by drying in vacuum at 80 °C for 24 h. According to the difference in the doping amount of silicotungstic acid, the obtained sample was named asxSTA-MIL-101(Cr) (x= 0,20%, 30%, 40%), wherexrepresents the mass fraction of silicotungstic acid. Pt/xSTA-MIL-101(Cr) was prepared by the impregnation method. Firstly, a certain amount of H2PtCl6·6H2O and 0.5 g ofxSTA-MIL101 (Cr) was dissolved in 20 mL of deionized water. After stirring at room temperature for 24 hours, the solvent was removed by vacuum drying at 80 °C, and the Pt/xSTA-MIL-101(Cr) catalyst was finally obtained.
2.2 Catalyst characterization
The powder X-ray diffraction (XRD) patterns were recorded on a Rigaku D/Max-2200 diffractometer operating at a tube voltage of 40 kV and a tube current of 20 mA, using CuKα radiation in the scanning angle(2θ)range of 5o-20oat a scanning speed of 2(o)/min.The Fourier transform infrared (FT-IR) spectra of KBr pellets were recorded on a Thermo Nicolet iS10 FTIR spectrometer with a scanning range of 2 000 cm-1-500 cm-1. The specific surface area and total pore volume were analyzed by the BET method using a Micromeritics adsorption equipment ASAP 2020. The scanning electron microscopic (SEM) images were collected using a SIGMA field emission scanning electron microscope with an accelerating voltage of 20 kV. The transmission electron microscopy (TEM) analysis was performed using a FEI Tecnai F30 microscope. The elemental analysis was recorded on a XRF-1800 X-ray fluorescence spectrometer.The amount of acidic protons in 0.4%Pt/xSTA-MIL-101(Cr) (x= 0, 20%, 30%, 40%) was determined by the acid-base titration[12]. 0.5 g of catalyst was added to the excess sodium hydroxide solution calibrated with a potassium hydrogen phthalate solution, sealed and stirred at room temperature for 24 h. After the reaction mixture was filtered to remove solids, the resulting filtrate was back-titrated with the calibrated hydrochloric acid and the amount of acidic protons in the catalyst could be calculated from the volume of the consumed sodium hydroxide solution. Thermal gravimetric analysis (TGA)result was recorded on a SDT Q600T instrument using a constant heating rate of 10 °C/min under N2atmosphere.
2.3 Catalytic activity test
n-Heptane isomerization was carried out in a self-assembled continuous flow fixed-bed stainless steel reactor. A total of 0.2 g of the catalyst mixed with the quartz sand was placed in the reactor. The mixture was at first reduced at 473 K for 5 h,and then aSZB-1 double plunger micro metering pump was used to injectn-heptane into the reactor for conducting the catalytic reaction (operating under atmospheric pressure at an-heptane flow rate of 2 mL/h). After the reaction was stable for 30 min, the products were analyzed by a GC-7980A type gas chromatograph. Finally, the performance of the catalyst was evaluated by calculating the conversion ofn-heptane and the selectivity ofiso-heptane, which were defined as follows:
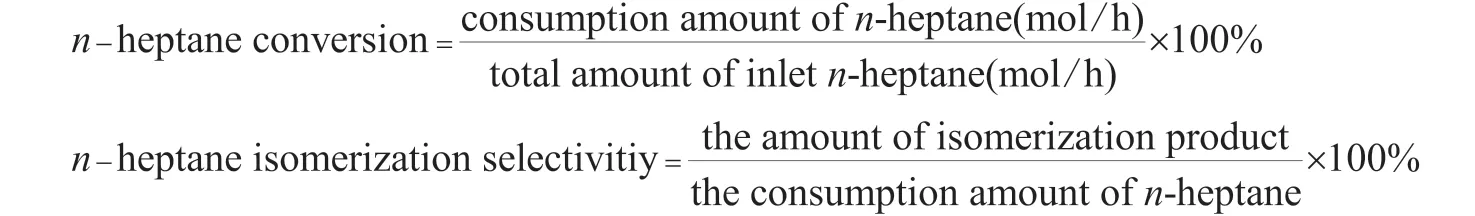
3 Results and Discussion
3.1 Catalyst characterization
The powder X-ray diffraction patterns of Pt/xSTAMIL-101(Cr) samples with different doping amounts of silicotungstic acid are shown in Figure 2. The characteristic diffraction peaks of the XRD patterns of the samples are basically the same as those of the simulated patterns. It can be seen from the XRD patterns that all the samples exhibited five obvious characteristic diffraction peaks at 2.8°, 3.3°, 5.2°, 5.8o, and 9.1°,respectively, corresponding to the crystallite phases of(200), (311), (222), (400), and (331) of MIL-101(Cr)[11]. It indicates that the MIL-101(Cr) material was successfully synthesized in this experiment, and the MIL-101(Cr)support could retain the typical zeolite-like cubic structure after doping with silicotungstic acid[13-15], denoting that the doping of STA to MIL-101(Cr) did not damage the skeleton structure of MIL-101(Cr). It can also be seen from Figure 2 that, with the increase of doping amount of STA, the intensity of most characteristic diffraction peaks of MIL-101(Cr) gradually decreases, while the intensity of characteristic diffraction peaks of STA gradually increases despite the weak diffraction peaks of STA. The difference in diffraction peak intensity of each sample is probably attributed to the orientation of the crystal in the powder sample[16]. In addition, the characteristic diffraction peaks of STA cannot be observed obviously from all of the Pt/xSTA-MIL-101(Cr) samples, from which it can be inferred that the dispersion of STA in MIL-101(Cr) is relatively high, and this could guarantee the STA performing its acidity function sufficiently. When the doping amount of STA is equal to 40%, the diffraction peaks change obviously compared with other 0.4%Pt/xSTA-MIL-101(Cr) samples, and the 0.4%Pt/xSTA-MIL-101(Cr) sample shows three characteristic diffraction peaks at 6.55o, 7.13oand 7.47o. This fact indicated that the high doping amount of STA will lead to the formation of heterocrystalline phase.peak of STA gradually increases, which is also consistent with the XRD characterization results.
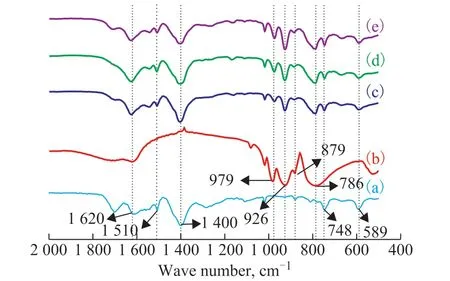
Figure 3 FT-IR spectra of pure STA (a) and 0.4%Pt/xSTAMIL-101(Cr) [x = 0 (b), 20% (c), 30% (d), 40% (e)]

Figure 2 Powder XRD patterns of simulated MIL-101(Cr)(a), 0.4%Pt/xSTA-MIL-101(Cr) [x = 0 (b), 20% (c), 30% (d),40% (e)] and pure STA (f)
Figure 3 shows the Fourier infrared spectra of 0.4%Pt/xSTA-MIL-101(Cr) samples with different doping amount of silicotungstic acid. The characteristic absorption peaks at 1620 cm-1and 1400 cm-1can be observed from Figure 3, which correspond to the asymmetric stretching vibration and symmetric stretching vibration of carboxyl group in the framework structure of MIL-101(Cr), respectively. The characteristic absorption peak at around 1510 cm-1is caused by the stretching vibration of the C=C bonds. The characteristic absorption peaks at around 589 cm-1and 748 cm-1are caused by the vibration of the Cr-O ions, which can verify the formation of the metal-organic framework structure[17-18]. There are four types of stretching vibrations of the oxygen atoms in the silicotungstic acid, including Si-Oa, W=Od, W-Ob-W, and W-Oc-W. These four stretching vibrations correspond to the four characteristic absorption peaks in the infrared spectrum, which are 926 cm-1, 979 cm-1, 879 cm-1and 786 cm-1, respectively[19-20]. This outcome indicates that the Keggin structure could be maintained after silicotungstic acid was doped into MIL-101(Cr). It can also be seen from Figure 3 that, with the increase of doping amount of STA, the characteristic absorption peak of MIL-101(Cr)gradually decreases, while the characteristic absorption
The N2adsorption-desorption isotherms and the pore size distribution of 0.4%Pt/xSTA-MIL-101(Cr) samples with different doping amount of STA are shown in Figure 4(a). It can be seen from Fig. 4 that all of the 0.4%Pt/xSTA-MIL-101(Cr) samples exhibit the characteristics of Langmuir type I and IV adsorption isotherms, and there is an obvious hysteresis loop of H4 in the high pressure region. It shows that the synthesized sample is a kind of micro-mesoporous composite materials. Whenp/p0is approximately equal to 0.1 and 0.2, there are secondary uptakes on the N2adsorption-desorption isotherm, which might be caused by the two kinds of pore size in MIL-101(Cr)[21]. It can also indicate that MIL-101(Cr) material has been successfully synthesized, and the doping of silicotungstic acid does not damage the framework structure of MIL-101(Cr), which is consistent with the characterization results of XRD. With the increase of the doping amount of silicotungstic acid, the nitrogen adsorption amount of the sample decreases significantly,which might be caused by the fact that a part of the pores of MIL-101(Cr) was occupied by silicotungstic acid.According to the pore size distribution of the samples,there are two kinds of pore size (1.54 nm and 2.51 nm,respectively) in all the 0.4%Pt/xSTA-MIL-101(Cr)catalysts, which further prove that the synthesized sample is a kind of micro-mesoporous composite material.
Furthermore, as the doping amount of silicotungstic acid increases, the peak intensity of pore size distribution in Figure 4(b) becomes smaller and smaller, indicating that the pore size distribution of the sample becomes more and more uneven and the silicotungstic acid is successfully encapsulated into the micro-mesoporous quasi spherical cages of MIL-101(Cr).
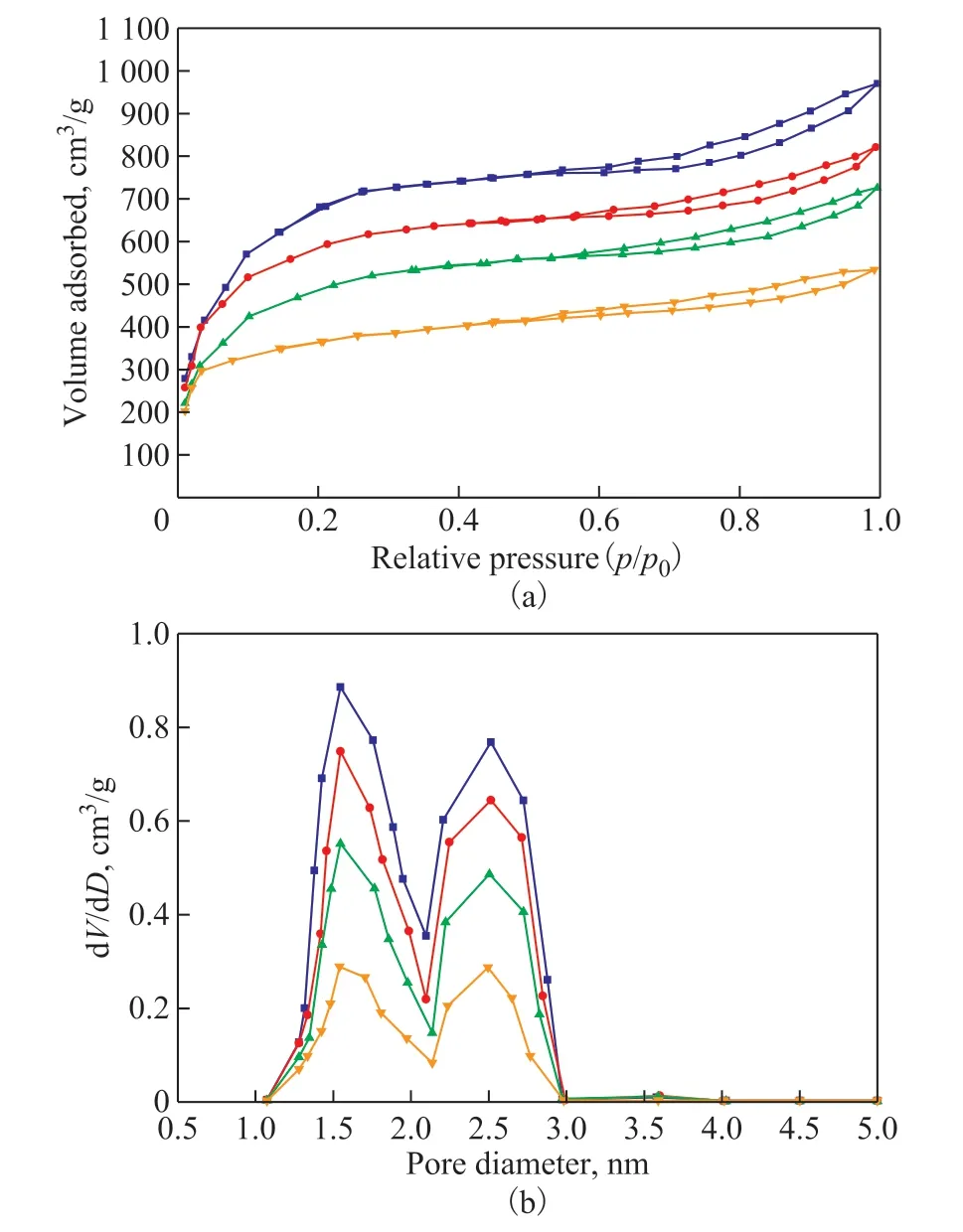
Figure 4 N2 adsorption-desorption isotherms (a) and pore size distribution curves (b) of 0.4%Pt/xSTA-MIL-101(Cr) (x=0, 20%, 30%, 40%)
The specific surface area and pore volume of 0.4%Pt/xSTA-MIL-101(Cr) samples doped with different amount of STA are shown in Table 1. As the doping amount of STA increases, both the specific surface area and pore volume of the sample decrease, indicating that STA is successfully introduced into the pores of MIL-101(Cr).

Table 1 Specific surface area and pore volume of 0.4%/xSTA-MIL-101(Cr) (x = 0, 20%, 30%, 40%)
Figure 5 shows the SEM and TEM images of 0.4%Pt/xSTA-MIL-101(Cr) samples with different doping amount of STA. It can be seen from Figure 5 that the synthesized sample exhibits a typical regular octahedral structure,and clear lattice fringes can be seen in Figure 5(a), denoting that the synthesized 0.4%Pt/MIL-101(Cr) sample exhibits a high degree of order. As the doping amount of silicotungstic acid increases, the lattice fringes of the samples in TEM images become more blurred [Figure 5(b), 5(c) and 5(d)],but the sample lattice fringes are still visible, indicating that the doping of silicotungstic acid does not destroy the framework structure of MIL-101(Cr), which is consistent with the characterization results of XRD and N2adsorptiondesorption analysis. In addition, it can be seen from Figure 5(c) that some substances with a rod-like structure could also be observed besides the regular octahedral structure of MIL-101(Cr), indicating that 0.4%Pt/30%STA-MIL-101(Cr)sample was not a single substance, since it contained two different products, which were consistent with the results reported in the literature[11,22]. It was reported by Zhao, et al. that acetic acid could take the place of hydrofluoric acid in the synthesis of MIL-101(Cr), but when the acetic acid concentration was too high, some rod grains with large size of MIL-88B(Cr) would be mixed in the grains of MIL-101(Cr). In this experiment, MIL-101(Cr) was modified by silicotungstic acid, which had the same effect as acetic acid, and then a small amount of MIL-88B(Cr) was formed,as shown in Figure 5(c), which might be caused by the relatively higher concentration of silicotungstic acid. Figure 5(c) shows the distribution of Pt particles on the surface of MIL-101(Cr). The average particle size of Pt was 4.96 nm,which was approximately determined by Nano Measurer software. It can be seen from Figure 5(a) that the lattice fringe is about 2.6 nm and it corresponds to the (222) plane of MIL-101(Cr)[8], which can also help to verify that the synthesized material is MIL-101(Cr). All the 0.4%Pt/xSTA-MIL-101(Cr)samples are aggregated to a certain extent after doping with STA, as compared with 0.4%Pt/MIL-101(Cr) samples presented in Figure 5(e), 5(f), 5(g) and 5(h). However, no aggregated silicotungstic acid can be observed on the surface of MIL-101(Cr) (x= 0, 20%, 30%, 40%) in Figure 5(a),5(b), 5(c) and 5(d), indicating that silicotungstic acid entered the pore cages of MIL-101(Cr). As shown in Figure 5(b),5(c) and 5(d), no obvious agglomeration of metal particles is found, indicating that Pt particles exhibit a high dispersion in MIL-101(Cr). The reason why some of the TEM images of 0.4%Pt/40%STA-MIL-101(Cr) sample are not clear is that the metal organic framework material is very sensitive to electron beam, and the framework will shake or drift during the shooting. Thus, it is difficult to obtain high-quality TEM images.
The content of elements and protonic acid in 0.4%Pt/xSTA-MIL-101 (Cr) catalyst is shown in Table 2. The content of elements in 0.4%Pt/xSTA-MIL-101(Cr)catalyst is measured by XRF. The content of Cr decreases with an increasing doping amount of STA as shown in Table 2. It is found that the molar ratio of silicon atoms and tungsten atoms in the sample is about 1:12,indicating that the Keggin structure was retained after the silicotungstic acid was doped into MIL-101(Cr).
Conventional techniques such as pyridine adsorption/desorption and temperature-programmed ammonia desorption (NH3-TPD) are not suitable for the determination of acid strength of MOF based catalysts due to the overlap of IR peaks of MOFs and probe molecules as well as the poor thermal stability of MOFs[11,23].

Figure 5 TEM images of 0.4%Pt/xSTA-MIL-101(Cr) [x = 0 (a), 20% (b), 30% (c), 40% (d)]; SEM images of 0.4%Pt/xSTAMIL-101(Cr) [x = 0 (e), 20% (f), 30% (g), 40% (h)]
The amount of acidic protons in the sample was determined by acid-base titration. It can be seen from Table 2 that with the increase of STA doping amount, the content of protonic acid in the sample increases gradually, which is consistent with the theoretical calculation value based on the fact that each Keggin anion contains four acidic protons. This indicates that silicotungstic acid could still retain its Keggin structure after being doped into MIL-101(Cr) and it could be assured that the acidity of silicotungstic acid would not be affected. Therefore, silicotungstic acid in the cage of MIL-101(Cr) can provide a good acidic environment for catalytic reactions. This outcome is also consistent with the characterization results of XRD, FT-IR, and XRF.In order to determine the thermal stability of the synthesized samples, the thermogravimetric analysis of the synthesized samples was carried out. During the test, all samples were heated in N2atmosphere from room temperature to 700 °C at a constant heating rate of 10 °C/min. Figure 6 shows the TGA curves of 0.4%Pt/xSTA-MIL-101(Cr) samples with different doping amount of STA. It can be seen from Figure 6 that the TGA curve trend of each sample is basically the same, but with the increase of STA doping amount, the weight loss rate of the sample gradually decreases. The first weightlessness stage of the sample proceeds from room temperature to 300 °C, and the weightlessness rate is about 10%. This result is mainly caused by the removal of guest water molecules from MIL-101(Cr) skeleton, indicating that MIL-101(Cr) can be stabilized at 300°C. The second weightlessness stage of the sample is between 300°C and 500°C, which is caused by the framework collapse of MIL-101(Cr) due to the thermal decomposition of the organic ligand of MIL-101(Cr)after heating. At about 500°C, the MIL-101(Cr) sample collapses completely, and the mass of the sample no longer changes when the temperature further increases[2].

Table 2 The elemental composition and amount of acidic protons of 0.4%Pt/xSTA-MIL-101(Cr) (x = 0, 20%, 30%, 40%).

Figure 6 TGA spectra of 0.4%Pt/xSTA-MIL-101(Cr) [x = 0(a), 20% (b), 30% (c), 40% (d)]
3.2 Catalytic performance
3.2.1 Effect of STA doping amount on the catalytic performance of 0.4%Pt/xSTA-MIL-101(Cr)
Figure 7 shows the influence of the doping amount of silicotungstic acid on the catalytic performance of 0.4%Pt/xSTA-MIL-101(Cr) catalysts. When 0.4%Pt/MIL-101(Cr)was used to catalyze the isomerization ofn-heptane, both the conversion ofn-heptane and the selectivity ofisoheptane were very low. It can also be observed obviously from Figure 7 that both the conversion ofn-heptane and the selectivity ofiso-heptane were greatly improved after doping silicotungstic acid into the catalyst. This result is due to the fact that the catalytic reaction of isomerization requires an acidic environment to produce the intermediate of carbocation, which would finally initiate the isomerization reaction[26]. The introduction of silicotungstic acid into pure MIL-101(Cr), in which there are initially few acid sites, would provide more acid sites for the catalyst, so that the dehydrogenated alkane(olefin) molecules via protonation and rearrangement could form the isomers more rapidly, which would finally promote the isomerization reaction ofn-heptane in the presence of hydrogen. Besides, according to the FT-IR characterization results, the structure of silicotungstic acid would not be damaged after it was doped to MIL-101(Cr),which guaranteed that it could implement its acid catalytic function effectively. When the doping amount of silicotungstic acid did not exceed 30%, with the increase of silicotungstic acid amount, the conversion ofn-heptane andiso-heptane selectivity gradually increased. It is most probable because there were relatively few acid sites in the catalyst compared with the metal sites when the doping amount of silicotungstic acid did not exceed 30%. The increase of silicotungstic acid doping amount could facilitate the balance between the metal sites and the acid sites in the catalyst, which was conducive to the isomerization reaction. When the doping amount of silicotungstic acid was 30%, both the conversion ofn-heptane and the selectivity ofiso-heptane could reach the highest values, which were 58.9% and 95.7%, respectively. When the doping amount of silicotungstic acid was 40%, the conversion ofn-heptane and the selectivity ofiso-heptane decreased to only 19.1%and 52.3%, respectively. It could be ascribed to the fact that when the doping amount of silicotungstic acid was 40%,the high amount of silicotungstic acid would clog the pores of MIL-101(Cr) seriously, reduce the specific surface area of the catalyst, break the balance between metal sites and acid sites, and intensify the cracking reaction. Therefore, the conversion ofn-heptane and the selectivity ofiso-heptane reduced significantly. Table 3 shows the activity comparison forn-heptane isomerization between the catalyst used in this experiment and the reported catalyst, from which it can be seen that the selectivity of the catalyst has been improved to some extent in comparison with the reported traditional silicate catalyst[24-27].

Figure 7 Effect of STA doping amount on catalytic performance of catalysts for n-heptane isomerization (under conditions covering a reaction temperature of 260 °C, a WHSV of 6.8 h-1, and a reaction time of 2 h)
In addition, the STA catalyst and the STA/MIL-101(Cr)catalyst were also used inn-heptane isomerization. The experimental results show that both STA and STA/MIL-101(Cr) catalysts do not exhibit any catalytic activity.This is true, because there are no metal active sites in both the STA and STA/MIL-101(Cr) catalysts, and the hydrogenation and dehydrogenation reaction cannot be conducted, which would lead to the result that both STA and STA/MIL-101(Cr) catalysts cannot carry out the isomerization reaction.

Table 3 Activity comparison between 0.4%Pt/30%STAMIL-101(Cr) and other reported catalysts.
3.2.2 Effect of reaction temperature on the catalytic performance of 0.4%Pt/30%STA-MIL-101(Cr)
Figure 8 shows the influence of the reaction temperature on the catalytic performance of the 0.4%Pt/30%STA-MIL-101(Cr) catalyst. It can be seen from Figure 8 that when the reaction temperature was 220 °C, the conversion ofn-heptane and the selectivity ofiso-heptane were relatively low, which were only 21.3% and 59.9%, respectively.When the reaction temperature was lower than 260 °C,the isomerization activity gradually increased with the increase of the reaction temperature. It could be inferred from the fact that the increase of temperature from 220 °C to 260 °C is conducive to the activation of the catalyst, which would make the catalyst play its catalytic role better, since the catalyst could not be activated until the reaction temperature reached a certain value. Thus,with the increase of reaction temperature, the number of activated molecules would increase, and finally the effective collisions of the reactants, which were conducive to the isomerization reaction, would also increase.When the temperature rose to 260 °C, the conversion ofn-heptane and the selectivity ofiso-heptane reached the maximum values, which were 58.9% and 95.7%,respectively. However, as the reaction temperature continues rising, the conversion ofn-heptane and the selectivity ofiso-heptane gradually would decrease.This result can ascertain the fact that isomerization reaction is exothermic, and when the reaction temperature rises, the exothermic reaction of isomerization will be inhibited and the endothermic reaction of cracking will be promoted according to the thermodynamic equilibrium or the Arrhenius equation. However, there was more carbon depositing on the catalyst surface when the reaction temperature was higher than 260 °C, and the deposited carbon would cover a part of the active sites of the catalyst[25]. In addition, higher reaction temperature would also cause the collapse of the framework structure of the catalyst[2]. Therefore, when the temperature was higher than 260 °C, the conversion ofn-heptane and the selectivity ofiso-heptane would reduce significantly.Table 4 shows the distribution ofn-heptane isomerization products over the 0.4%Pt/30%STA-MIL-101(Cr) catalyst at different reaction temperatures. It can be seen from Table 4 that the isomerization products mainly consisted of 2-methylhexane (2-MH), 3-methylhexane (3-MH),and multi-branched isomers (DMP). The mass percentage of 2-methylhexane and 3-methylhexane is very close,and it is mainly related to the dehydrogenation process ofn-heptane. Due to the similar thermodynamic properties of different secondary carbon atoms ofn-heptane, the probability of 2-heptyl radicals, 3-heptyl radicals, and 4-heptyl radicals generated in the isomerization process is approximately equal[28]. The C-H bond of primary carbon atom is more difficult to give rise to homolysis than that of secondary carbon atom, and thus the dehydrogenation of 2-heptyl radical mainly produces 2-heptene. While the probability of the formation of 2-heptene and 3-heptene after the dehydrogenation of 3-heptyl radical is approximately equal, the dehydrogenation of 4-heptyl radical can produce only 3-heptene[28]. The content of multi-branched isomers is the highest among the reaction products. With the increase of reaction temperature, the content of multi-branched isomers increases at first and then decreases. When the reaction temperature is 260 °C, the mass percent of multi-branched isomers is 24.6%, and then-heptane conversion and isoheptane selectivity are 58.9% and 95.7%, respectively.Therefore, the 0.4%Pt/30%STA-MIL-101(Cr) catalyst can be used forn-heptane isomerization to obtain gasoline components with higher octane number, which has a very promising application prospect.
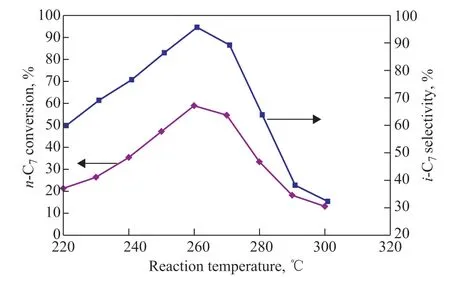
Figure 8 Effect of reaction temperature on the n-heptane isomerization performance of 0.4%Pt/30%STA-MIL-101(Cr) (under conditions covering a WHSV of 6.8 h-1, and a reaction time of 2 h)
The cracking products consist of mainly C3and C4alkanes, and there are no hydrocarbon products with a carbon number of more than seven, indicating that the isomerization reaction ofn-heptane is carried out according to the mechanism of monomolecular reaction.
3.2.3 Effect of reaction time on the catalytic performance of 0.4%Pt/30%STA-MIL-101(Cr)
Figure 9 shows the variation ofn-heptane isomerization performance over 0.4%Pt/30%STA-MIL-101(Cr) catalyst at a reaction temperature of 260 °C. It can be seen from Figure 9 that the conversion ofn-heptane and the selectivity ofiso-heptane gradually increased with an increasing reaction time at the initial stage of the reaction. It is due to the fact that the activation of the catalyst needed a certain time, and the first two hours of the reaction belonged to thematurity stage of the catalyst. As the reaction went on, the catalyst was gradually activated, so the catalytic activity of the catalyst increased gradually. The conversion ofn-heptane and the selectivity of iso-heptane reached the maximum values after around 2 h, which were 58.9% and 95.7%,respectively. When the reaction time was about 2 hours,the catalyst could attain the optimum activation state, and just at this moment both the metal sites and acid sites could be activated to the full extent, so the conversion ofn-heptane and the selectivity ofiso-heptane reached the maximum values. With the reaction proceeding further, the conversion ofn-heptane and the selectivity ofiso-heptane decreased slightly.This is probably true as the reaction had proceeded more than 2 h, and a certain amount of carbon would be deposited on the active centers of the catalyst, which could lead to the blocking of some pores in the catalyst, and the framework structure of the catalyst may also change after a period of reaction.

Table 4 Product distribution of n-heptane isomerization over 0.4%Pt/30%STA-MIL-101(Cr) at different reaction temperature

Figure 9 Effect of reaction time on the n-heptane isomerization performance of 0.4%Pt/30%STA-MIL-101(Cr) (under conditions covering a reaction temperature of 260 °C, and a WHSV of 6.8 h-1)
3.2.4 Mechanism ofn-heptane isomerization over 0.4%Pt/xSTA-MIL-101(Cr)
According to the types of catalysts, the isomerization mechanism ofn-heptane can be divided into the acid catalysis mechanism and the metal-acid bifunctional catalysis mechanism. The mechanism of isomerization ofn-heptane over 0.4%Pt/xSTA-MIL-101(Cr) belongs to the metal-acid bifunctional catalysis mechanism. The equilibrium between the metal sites and the acid sites plays a key role in the catalytic performance of catalysts for isomerization ofn-heptane[29-32]. When the catalyst did not contain silicotungstic acid, the isomerization performance of the catalyst was very low, but it still exhibited a certain catalytic activity forn-heptane isomerization. This is due to the fact that MIL-101(Cr)is prone to losing guest water molecules after heating to form coordinatively unsaturated metal sites[11], and these unsaturated metal sites are the Lewis acid sites that could provide a certain amount of active sites forn-heptane isomerization. However, more hydrogenolysis reactions would also occur in tandem with the isomerization reaction, which is consistent with the results reported in the literature[33]. The Al-MCF-17/Pt and MCF-17/Pt catalysts were applied in hexane isomerization by Nathan Musselwhite, et al.[33], and it was found that the unmodified MCF-17/Pt catalyst exhibited a low hexane isomerization performance by 2 orders of magnitude lower than that of the modified catalyst, and the isomerization selectivity was only 50%. The experimental results were consistent with the results obtained in this paper. After doping silicotungstic acid, the acid sites of the catalyst increased obviously, and the isomerization performance of the catalyst was improved prominently. When the doping amount of silicotungstic acid was 30%, the catalytic activity was the highest, which could be attributed to the following three aspects: (1) According to the size of the cage of MIL-101(Cr) and the Keggin anions, the maximum number of STA molecules encapsulated in the large cages and small cages of MIL-101(Cr) is 5 and 3, respectively, and each cage could encapsulate 3.7 STA molecules at most theoretically. When the doping amount of silicotungstic acid in MIL-101 was 20%, 30%and 40%, each cage of MIL-101(Cr) could encapsulate 1.1, 2, and 2.9 STA molecules on average, respectively,calculated according to the topological structure of MIL-101(Cr). The results of IR, XRF, and acid-base titration studies showed that the Keggin structure of silicotungstic acid was not destroyed, so silicotungstic acid was combined with MIL-101(Cr) by the van der Waals force to provide a good acidic environment for catalytic reaction in the cages of MIL-101(Cr). Therefore,MIL-101(Cr) could provide more acid sites as well as larger reaction space for the isomerization reaction after doping with silicotungstic acid; (2) When the doping amount of silicotungstic acid was 30%, the number of the metal sites and acid sites for the isomerization reaction could reach a best matching state, which was conducive to the isomerization reaction; (3) The cages of MIL-101(Cr) with two different sizes could provide a shapeselective catalytic space for isomerization, which would effectively reduce the conversion of iso-heptane, and the isomerization selectivity of the catalyst could be improved. Therefore, the 0.4%Pt/30%STA-MIL-101(Cr)catalyst would exhibit the best isomerization performance.According to this experiment, when the doping amount of silicotungstic acid was 30%, the metal sites and acid sites were just in an equilibrium state, so the catalytic activity of 0.4%Pt/30%STA-MIL-101(Cr) catalyst was the highest. As the doping amount of silicotungstic acid increased to 40%, not only some pores of MIL-101(Cr)could be blocked, resulting in reduction of the specific surface area of the catalyst, but also the equilibrium state between metal sites and acid sites would be broken. Since the excessive acid sites could lead to the intensification of cracking reaction, the isomerization performance of the catalyst would reduce significantly. The isomerization reaction ofn-heptane catalyzed by bifunctional catalyst is carried out through the high active carbocations, so the formation of carbocations is the key step of the isomerization reaction. Due to the lack of electrons, the carbocations are able to conduct a series of reactions that form the basis of the isomerization mechanism, and this could also explain the fact that many of the side reactions would occur in tandem with the isomerization reaction.According to the metal-acid bifunctional catalysis mechanism, the metal sites can provide the active centers for the hydrogenation and dehydrogenation necessary for the isomerization ofn-alkane, and the main role of acid sites is to make the intermediates of the alkene products generate carbocations, which then undergo the rearrangement reaction. Thus, the reaction mechanism ofn-heptane isomerization could be deduced as follows:
(1) Dehydrogenation reaction on the metal sites

(2) Protonation reactions and rearrangement reactions on the acid sites

(3) Hydrogenation reaction on the metal sites

According to the catalytic mechanism of metal-acid bifunctional catalyst, the reaction process ofn-alkane isomerization on the 0.4%Pt/xSTA-MIL-101(Cr)bifunctional catalyst is shown in Figure 10. Firstly,n-heptane is dehydrogenated on the active component of Pt to form an alkene with the same number of carbon atoms. Then the alkene is transferred to the acid site formed mainly by the silicotungstic acid followed by protonation into primary carbocation. Based on the stability of the carbocation, the primary carbocation will undergo a rearrangement reaction on the acid site to form the stable tertiary carbocation, which in turn can remove a proton from the carbocation to form the isomeric alkene. Finally, the isomeric alkene can react with hydrogen on the metal site of Pt to formiso-heptane.
4 Conclusions
In this study, silicotungstic acid was used to modify MIL-101(Cr) with different doping amount to synthesize novelxSTA-MIL-101(Cr) samples. The 0.4%Pt/xSTA-MIL-101(Cr) metal-acid bifunctional catalysts were prepared by the impregnation method, which were characterized by XRD, N2adsorption-desorption, SEM, TEM, FT-IR,XRF, acid-base titration and TGA characterizations. The characterizations illustrated that the skeleton structure of MIL-101(Cr) was not destroyed by doping with silicotungstic acid, and the synthesized samples exhibited a high thermal stability, a typical octahedral structure, and a large specific surface area (1437.1 m2/g). Besides, it was also demonstrated that the pore structure was arranged orderly and silicotungstic acid could retain its Keggin structure in the 0.4%Pt/xSTA-MIL-101(Cr) samples, which could provide a good acidic environment for the catalytic reaction in the micro-mesoporous quasi spherical cages of MIL-101(Cr).Furthermore, the Pt particles could be proved to exhibit a good dispersion in MIL-101(Cr) by TEM characterization.The effect of doping amount of silicotungstic acid, reaction temperature, and reaction time on the catalytic performance of 0.4%Pt/xSTA-MIL-101(Cr) catalysts was investigated usingn-heptane isomerization as the probe for the first time.The results showed that the bestn-heptane isomerization performance of 0.4%Pt/30%STA-MIL-101(Cr) catalyst was obtained when the reaction time was 2 h at a reaction temperature of 260 °C. Under the specified reaction conditions, the match of acid sites and metal sites could reach an optimal state for then-heptane isomerization reaction,and the conversion ofn-heptane and the selectivity ofisoheptane reached 58.93% and 95.68%, respectively. Besides,compared with the traditional silicate catalyst, the selectivity of the said catalyst had been significantly improved, and the stability of 0.4%Pt/30%STA-MIL-101(Cr) was high enough for achieving a constant high catalytic performance within a reaction time of 5 h. In addition, the catalytic mechanism ofn-heptane isomerization over 0.4%Pt/xSTA-MIL-101(Cr)catalyst was studied and the model of the mechanism was established. This study broadened the application of MOF and could provide the theoretical basis and technical support for the application of MOF materials inn-heptane isomerization.
- 中国炼油与石油化工的其它文章
- Study on Viscosity Reducing and Oil Displacement Agent for Water-Flooding Heavy Oil Reservoir
- Electrospinning Nanofiber Membrane Reinforced PVA Composite Hydrogel with Preferable Mechanical Performance for Oil-Water Separation
- Preparation of Solid Waste-Based Activated Carbon and Its Adsorption Mechanism for Toluene
- Antibacterial and Corrosion Inhibition Properties of SA-ZnO@ODA-GO@PU Super-Hydrophobic Coating in Circulating Cooling Water System
- Investigation of Nitrite Production Pathway in Integrated Partial Denitrification/Anammox Process via Isotope Labelling Technique and the Relevant Microbial Communities
- Heteroatom-Doped Carbon Spheres from FCC Slurry Oil as Anode Material for Lithium-Ion Battery

