基于脑代谢组学研究羚珠散联合对乙酰基氨基酚对发热幼龄大鼠的解热作用
孙皊,沈存思,廖颖钊,单进军
(1.南京中医药大学中医儿科研究所,江苏省儿童呼吸疾病重点实验室,江苏 南京 210023;2.南京中医药大学深圳中医医院儿科,广东 深圳 518033)
There are a wide range of diseases that can cause fever. Fever is a part of defensive response when pathogens and microorganisms invade the host. Meanwhile, fever is one of the leading reasons children and infants come for medical evaluation and often lead to administration of antipyretic drugs[1].
Acetaminophen, ibuprofen and aspirin are commonly used antipyretic medicine for reducing body temperature in a short time. Acetaminophen (APAP) is one of the most widely used west medicine for antipyretic, analgesic and anti-inflammation. However, in some cases, the combination of traditional Chinese medicine (TCM) and western medicine could achieve better effects[2-4].In China, a developing country, TCM usage is regular policy because of the long-time effect and low toxicity[5]. TCM has been used to treat paediatric fever by practitioners for many years[6-7]. The clinical Chinese patent medicine Ling-Zhu-San (LZS) is a kind of powder derived from nine traditional drugs (Saigae Tataricae Cornu, Margarita, Bovis Calculus Artifactus, Bombyx Batryticatus, Cinnabaris, Ambrum, Arisaema Cum Bile, Borneolum Syntheticum, Acori Tatarinowil Rhizoma). Combinations of LZS and APAP may combine the advantages of both, so as to achieve better antipyretic effect.
Metabolomics is the untargeted and targeted analysis of small molecular metabolites, which has been widely applied in revealing new biomarkers of disease and finding out the underlying mechanisms of clinical drugs 8. Several studies have analysed the potential biomarkers of easily accessible biofluid samples, such as blood[5-8], hypothalamus microdialysates[9]and urine[10-11].However, the extent that the biomarkers from biofluids samples could reflect the brain activity is not clear because of the effect of blood-brain-barrier (BBB). BBB is a kind of special structure that tightly controls the passage of molecules between the brain and the blood[12]. Brain metabolomics has been used to monitor the effect of drugs which directly affect brain disease[13].In this study, we collected brain tissue samples from young rats and performed untargeted metabolomics research on this basis.
Mass spectrometry (MS) coupled with chromatographic separation apparatus, such as gas chromatography (GC), has been used as a powerful tool to analyse complex biological samples[14-15].GC-MS has the ability to detect the chromatographic peaks sensitively, accurately and reliably[16].
In this study, acetaminophen and LZS were treated on young rats with combined modality therapy. GC-MS based metabolomic experiments were applied to investigate the underlying mechanisms of the antipyretic effect.
1 Materials and methods
1.1 Chemicals and reagents
LZS was provided by Lei Yun Shang Pharmaceutical Co., Ltd. (no. MA6014 019,China); Yeast was purchased from ANGEL YEAST Co., Ltd. (no. CY0175, China); Acetaminophen; Ultra high-purity water was prepared by Millipore-Q system (Millipore Corporation, Billerica, MA, USA).
Methoxyamine hydrochloride, pyridine,N,O-Bis (trimethylsilyl) trifluoroacetamide (BSTFA) with 1% trimethylchlorosilane (TMCS), and 1,2-13C2-myristic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Carboxymethyl cellulose (CMC) was obtained from Aladdin Industrial Corporation (Shanghai, China).
1.2 Animals and temperature measurement
Male Sprague-Dawley rats (80±20) g were obtained from Shanghai JiesiJie Experimental Animal Co., Ltd. (Shanghai, China). All rats were acclimated for 7 days in a controlled room with temperature (23±2) ℃, humidity (60%±5%), and a light/dark cycle of 12 h before the formal study. The rats received a standard diet and water ad libitum. During last three days, the rectal temperatures were measured twice per day using a digital thermometer for the regular rhythm of body temperatures. The rats, whose body temperature fluctuations were not higher than 0.3 ℃, were selected for the study. The animals were subcutaneously injected with 20% aqueous suspension of yeast (15 mL·kg-1) in the back of rats. The rectal temperatures were measured 4 hours after the yeast injection and rats with temperature increased greater than 1 ℃ were used in the study. A total of 50 rats were randomly divided into for five groups: normal group (NG, no treatment,n=10); model group (MG, subcutaneous injection of yeast,n=10); LZS group (LZS treatment,n=10); combination group (CMT, LZS+Acetaminophen treatment); acetaminophen group (APAP, Acetaminophen treatment,n=10). The animal experiments were performed under the guidelines of Animal Ethics Committee of Nanjing University of Chinese Medicine.
1.3 Sample collection and cytokines
After the last rectal temperature measurement, all rats were anesthetized by peritoneal injection of chloral hydrate. The abdominal aorta blood was immediately collected in heparinized tubes. The obtained plasma then was frozen at -80 ℃. The rat brains were also stored at -80 ℃. For measuring cytokine levels in plasma, IL-6 and PGE2 ELISA kits were used by following manufacturer's instruction.
1.4 Sample preparation and GC-MS analysis
1.4.1 Drug extraction and determination LZS powders were extracted by ultrasonic with methanol for 60min. The ratio of the weight of LZS and the volume of methanol were 1:20. The methanol extractions were centrifuged at 18000 rpm before injection.
GC-MS analysis was performed using a Thermo Trace 8000 gas chromatograph system coupled with mass spectrometer. Each 1 μL of derivatized sample was injected and separated with a TG-5MS GC column (Thermo,0.25 mm×30 m, 0.25 μm) in split mode with 20∶1 ratio. Helium was used as the carrier gas and was maintained at a constant flow of 1.2 mL·min-1. The GC temperature method is as follows: 60 ℃ for 1 min and increased to 320 ℃ at 20 ℃·min-1for 12 min. The electron energy was 70 eV and MS data were acquired in full-scan mode with a mass range of 50-500m/zand a time range of 3.64-26 min.
1.4.2 Tissue extraction and analysis Each 200 mg of frozen brain tissue (whole brain) were homogenized in 4 mL MeOH. A 600 μL of homogenate was added with 20 μL of MeOH containing IS (1,2-13C2-Myristic acid) which final concentration was 10 μg·mL-1. The mixture was then centrifugate and 300 μL of supernatant was dried to allow the evaporation of MeOH. Samples were reconstituted in 50 μL of 15 mg·mL-1methoxyamine pyridine and oscillated for 1.5 h. 50 μL of BSTFA were added before oscillated for another 0.5 h. After centrifuging for 10 min, the supernatants were analysed by GC-MS.
GC-MS analysis was performed using Thermo Trace 8000 gas chromatograph system coupled with mass spectrometer. Each 1 μL of derivatized sample was injected and separated with a TG-5MS GC column (Thermo,0.25 mm×30 m, 0.25 μm) in split mode with 20∶1 ratio. Helium was used as the carrier gas and was maintained at a constant flow of 1.2 mL·min-1. The GC temperature method is as follows: 60 ℃ for 1 min; increased to 320 ℃ at 20 ℃·min-1; held at this temperature for 5 min. The electron energy was 70 eV and MS data were acquired in full-scan mode with a mass range of 50-500m/zand a time range of 3.5-19 min.
1.5 Data alignment and analysis
2 Results
2.1 Identification of ingredients of LZS
The methanol extract of LZS was analyzed by GC-MS platform. The typical base peak intensity chromatogram was shown in Figure 1. The peaks were identified by consulting NIST database, and a total of 23 chemical materials were accurately matched (Table 1).
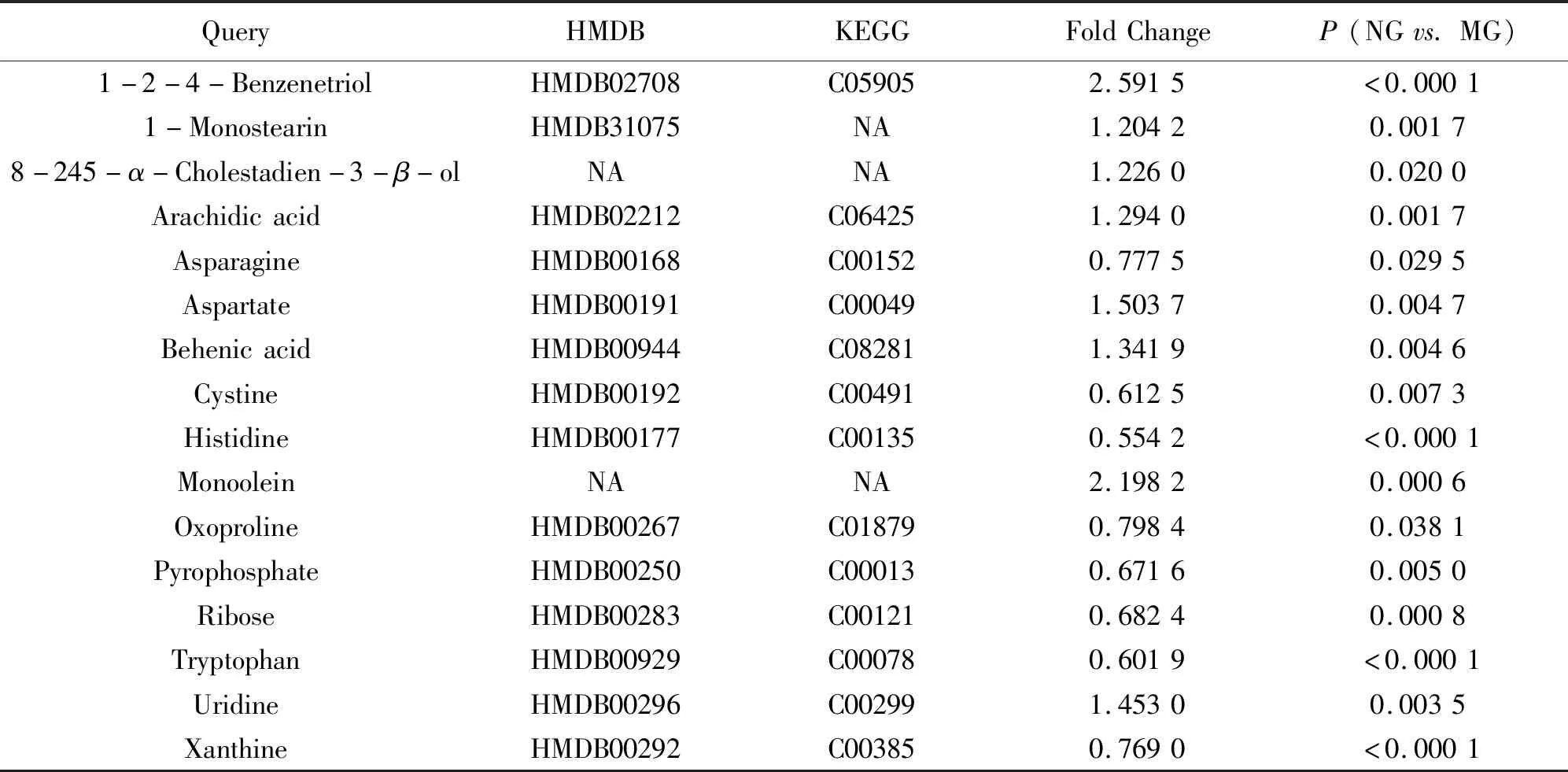
Table 2 Potential pyretic biomarkers
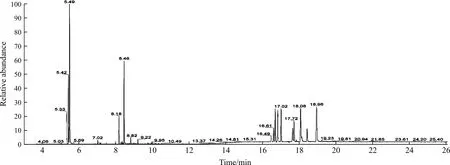
Fig.1 Typical base peak intensity chromatogram of methanol extract of LZS
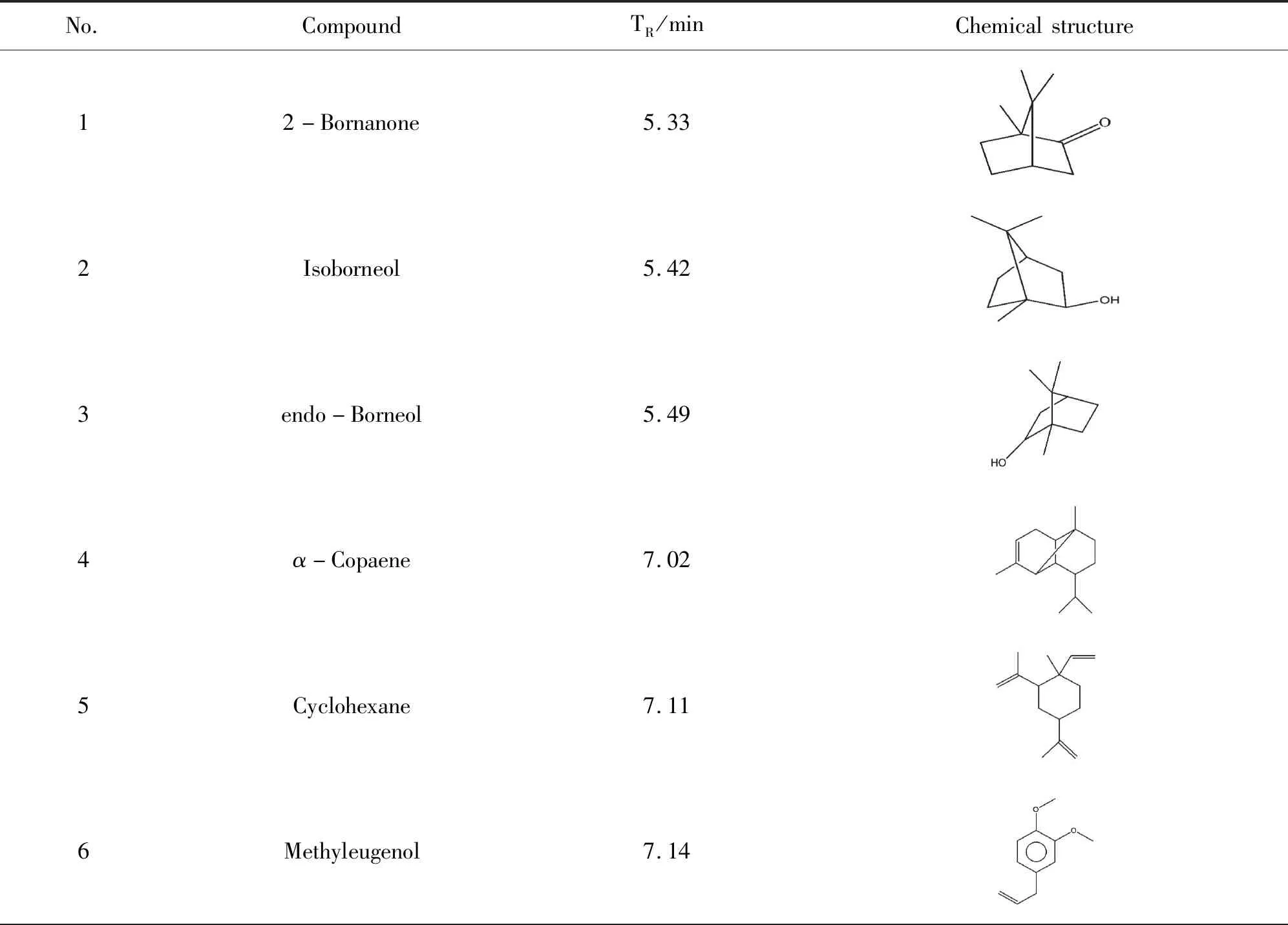
Table 1 Identified chemical materials of LZS
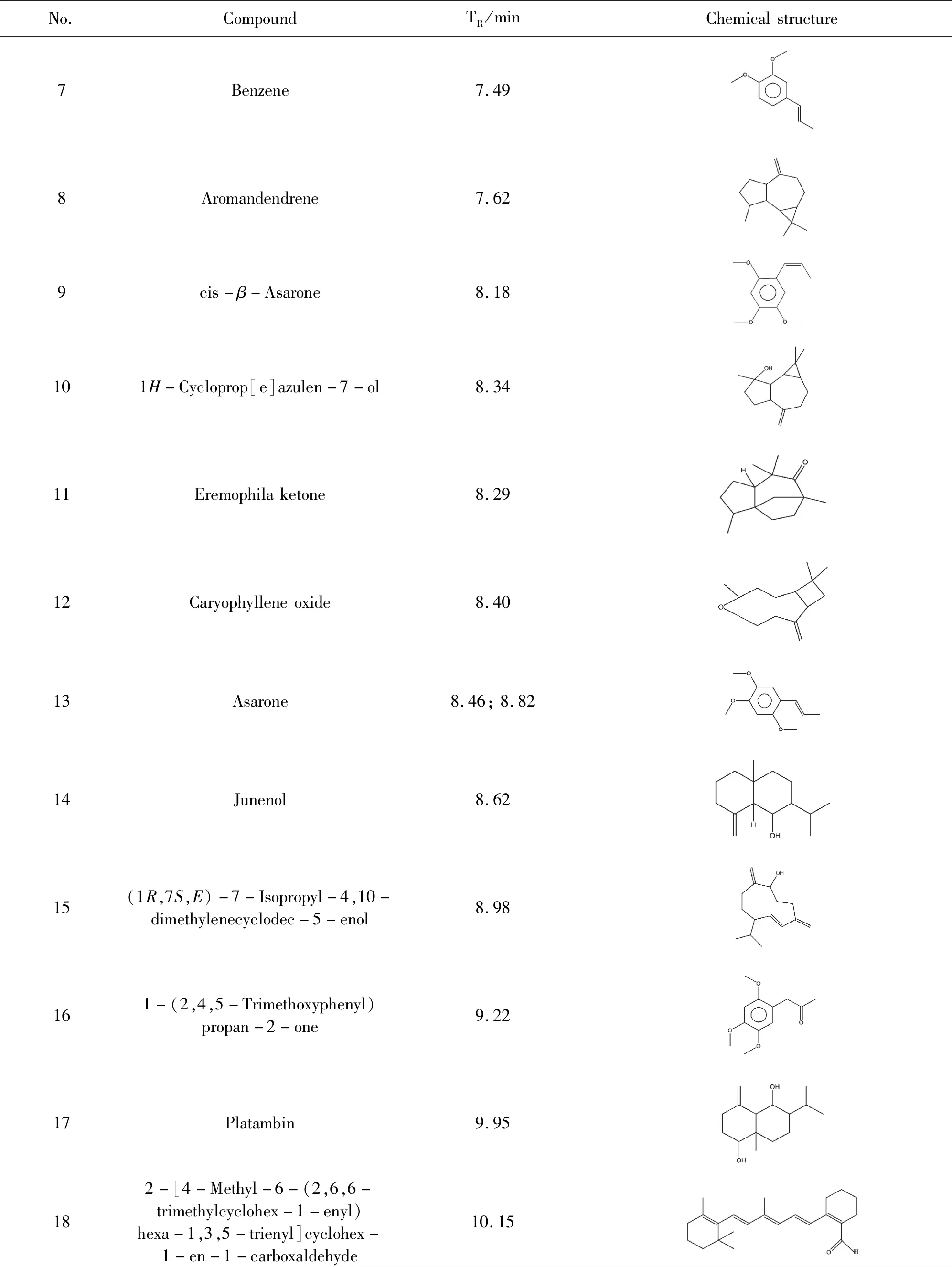
(Continued table Ⅰ)

(Continued table Ⅱ)
2.2 Antipyretic and anti-inflammation effects of LZS, APAP and GMT groups.
The rectal temperatures of rats in each group before and after drugs administration were recorded to monitor the body temperature changes. The rectal temperatures were measured at 0, 30, 60, 80, 90, 120, 180 min after drug administration. As shown in Figure 2A, the temperature fluctuations of NG group changed slightly. At the time-point of 0 min, the rectal temperatures of rats which were injected in yeast were significantly higher than the temperature of NG groups (P<0.01). After drug administration, the temperatures of CMT and APAP group cooled down quickly and last about 120 min. At the same time, the temperature fluctuation of LZS group was similar to MG group until 180 min when LZS begin to show its effect. It is noteworthy that the reduction of rectal temperature of GMT was quicker and the temperature bounce was slower than the APAP group.
The changing trends of two cytokines in rat plasma were shown in Figure 2B and Figure 2C. The levels of IL-1β and PGE2significantly increased in pyretic rats (P<0.01). The three administrations could obviously decrease the plasma inflammation levels. The combination of LZS and acetaminophen treatment showed the best anti-inflammation effect among these three administrations.
2.3 Multivariate data analysis of GC-MS data
Typical GC-MS base peak intensity chromatograms of brain samples from NG, MG, LZS, CMT, and APAP groups were shown in Figure 3. A total of 662 peaks were detected and 102 metabolites were accurately identified. Multivariate statistical analysis such as PCA and OPLS-DA analysis were applied for better visualizing the similarities and differences among the GC-MS data from these five groups (Figure 4). No obvious difference was found among five groups by PCA (Figure 4A). As shown in the OPLS-DA score plot, NG group was apparently separated from MG group which could reflect the fact that pyretic resulted in metabolomics disturbance in rat brain. (Figure 4B, C, D). The plot of OPLS-DA scores obtained from the GC-MS data of the brain showed a significant separation of NG, MG and drug treatment groups (Figure 4B, C, D). (Figure 4B:R2Y=0.928,Q2=0.538; Figure 4C:R2Y=0.895,Q2=0.477; Figure 4D:R2Y=0.919,Q2=0.503; Figure 4E:R2Y=0.889,Q2=0.576). The OPLS-DA model had anR2value of 0.034 8 andQ2value of -0.318 after 200 permutation (Figure 4F).
2.4 Identification of potential biomarkers and the changing trends in brain metabolomics
A total of 102 metabolites were identified by consulting the NIST database, HMDB website, KEGG database. The peak intensity of these metabolites was normalized by sum and log transferred. The data of normalized peak intensity finally were imported into SPASS 19.0 software forone-wayANOVAperformance and selected differential substances (P<0.05). At the same time, metabolites with fold change>1.5 were selected out as potential pyretic biomarkers. All 16 typical differential metabolites between NG and MG groups and the relative changing trend in five groups were shown in Figure 5. It did show that the relative concertation of most metabolites in drug treatment groups turned back to normal level and several of them showed statistical significance.

Note: A. The rectal temperatures were measured at 0, 30, 60, 90, 120, 180 min after drug administration; B, C. The concentration of IL-1β and PGE2 in rat plasma from NG, MG, LZS, CMT and APAP groups. ##P<0.01, compared with NG; *P<0.05, **P<0.01, compared with MG.Fig. 2 The changing trends of rectal temperature and relative cytokine concentrations in rat plasma
Fig.3 Typical base peak intensity chromatogram of brain samples from NG, MG,LZS, APAP and CMT group

Note: A. PCA score plot of five groups; B. OPLS-DA score plot of NG, MG, LZS, CMT and APAP group; C. OPLS-DA score plot of NG, MG and LZS group; D. OPLS-DA score plot of NG, MG and CMT group; E. OPLS-DA score plot of NG, MG and APAP group; F. Validation of OPLS-DA model by permutation test (n=200). R2=0.034 8, Q2=-0.318.Fig.4 PCA and OPLS-DA score plots of the NG, MG, LZS, APAP and CMT group
2.5 Pathways analysis
Pathways analysis then was performed based on these 16 potential pyretic biomarkers. The pathways that were closely associated with the antipyretic and anti-inflammation effects were shown in Figure 6. The results indicated that these metabolites could regulate pathways associated with carbohydrate metabolism (TCA cycle), lipid metabolism (Arachidic acid metabolism) and energy metabolism (Glutathione metabolism).
3 Discussion
In this study, we first monitored the rectal temperature of pyretic rats in 180 min and the result suggested that co-administration of LZS and acetaminophen could rapidly and continuously cool down the temperature of fever rats. After this, we detected the relative cytokine concentrations in rat plasma and this experiment verified the efficacy of co-administration.
The metabolic profiles of brains from young rats have rarely been studied by GC-MS. In fact, other works on pyretic have been mainly focused on samples such as plasma[5-7, 10]and urine sample[11]. In the present study, the biological relationships between the potential pyretic biomarkers and inflammation, as well as the mechanism underlying the antipyretic effect of combination of LZS and APAP, are discussed according to the pathways showed in Figure 6.

Note: The bottom and the top of the box are the 25th and the 75th percentile, a horizontal line near the middle of the box represents the 50th percentile, or the median. #P<0.05, ##P<0.01, compared with NG; *P<0.05, **P<0.01, compared with MG.Fig.5 Box and whisker plots of typically altered metabolites in brain samples as measured by GC-MS
Tryptophan metabolic pathways are usually associated with immunosuppression[17]. Degradation of tryptophan to kynurenine depletes the microenvironment of this amino acid and starves immune cells. The kynurenine produced by it binds to kynurenine to induce the modulation of AchR cells by aromatase receptors and active immunosuppression. It reported that 5-hydroxytryptamine (5-HT) could participate in the regulation of body temperature and the increased level of 5-HT induced pyretic[18]. As shown in Figure 5, co-administration of LZS and APAP could decrease the concentration of tryptophan and result in a lower level of kynurenine and 5-HT and finally reduced the body temperature.
Oxoproline (5-oxoproline) could transfer to glutamate which is one of the precursors to glutathione synthesis. Glutathione metabolism could increase intracellular NADPH and reduce oxidative stress[19].Glutathione (GSH) plays an important role as a neuroprotective structure against oxidative stress in its reduced form by removing peroxides and avoiding the origin of more damaging oxygen radicals. Glutamate generated into gamma-Aminobutyric acid (GABA) through glutamic acid decarboxylase[20].GABA is a major inhibitory neurotransmitter of the nervous system involved in mammalian thermoregulation and associated with febrile seizures. One homeostatic mechanism that prevents brain hyperthermia from ambient heat is elevation of GABA in interstitial fluid[21].It is reported that GABA could contribute as endogenous cryogens and the level of GABA was significantly increased during HS-induced hyperthermia[22].

Note: Metabolites in black bold fonts were potential biomarkers that were affected by CMT.Fig.6 The pathways closely associated with the antipyretic and anti-inflammation effects
The release of GABA from the presynaptic terminals was inhibited by Prostaglandin E2(PGE2). The metabolites arachidic acid and behenic acid were related to the biosynthesis pathway of arachidic acid. It is reported that arachidic acid generated PGE2which could get through the BBB and permeate into hypothalamus regions. Then fever occurs when PGE2binds to the EP3 receptor on hypothalamic neurons 5. Metabolites in CMT group related to these pathways returned to normal level compared to the disordered condition in MG group. Additionally, the changing trend of cytokine concentrations of IL-1β and PGE2in rat plasma in Figure 2 could verify the hypothesis above indirectly. Simultaneously, the mechanism of combination therapy on pyretic could be further explained.
Interestingly, we found that APAP did not regulate the metabolism of biomarkers for fever, but LZS could significantly improve the metabolism of aspartate and cystine. After CMT, the metabolites such as ribose and uridine can be significantly improved. And all three drug therapies can reverse arachidonic acid and xanthine levels. These results indicated that LZS contributed more to glutathione pathway and aspartic acid pathway. It can better regulate the neurotransmitter and TCA circulation. This may explain that although LZS has poor antipyretic effect, the therapeutic effect of combined with APAP is better than APAP alone.
4 Conclusion
In this study, we verified the excellent antipyretic effect of combination of LZS and APAP from the experimental results of body temperature test and cytokines test. At the meantime, brain metabolomics of pyretic mice was performed by GC-MS platform combined with multivariate data analysis (PCA and OPLS-DA) and pathways analysis to preliminary investigate the therapeutic mechanism. A total of 16 metabolites in brain significantly altered between NG and MG mice which could be considered as potential pyretic biomarkers. The properties of these potential biomarkers suggested that the pyretic effect could result in dysfunction of carbohydrate metabolism, lipid metabolism and energy metabolism. The mechanism of sustained and rapid antipyretic activity of combination of LZS and APAP supposed to be related to these metabolic pathways. In summary, combination of LZS and APAP could achieve better therapeutic effect and this may provide experimental basis for their clinical combined usage.

