高低肌内脂肪猪背最长肌关键基因表达差异分析
谭娅 李亮 雷宇航 黄志洋 赵春萍 张静 张正群 齐婧 朱砺 史开志

摘要:【目的】篩选出高肌内脂肪(Intramuscular fat,IMF)沉积的关键基因并进行表达验证,为揭示猪肌内脂肪沉积的分子调控机制提供理论依据。【方法】根据NCBI-GEO数据库中高、低肌内脂肪巴克夏群体的转录组数据,利用DESeq2进行差异表达基因筛选(|log2 Fold Change|≥1,FDR<0.05),采用Metascape对差异表达基因进行功能富集分析,通过MCODE筛选及鉴定出高肌内脂沉积的关键基因,并采用实时荧光定量PCR在地方猪(柯乐猪)和外种猪(杜长大猪)上进行关键基因表达量验证。【结果】从高、低肌内脂肪巴克夏群体6个文库中共鉴定出7661个基因,高肌内脂肪群体样本的脂质代谢相关基因总表达量显著高于低肌内脂肪群体样本(P<0.05,下同),其中差异表达基因有133个[110个上调(在高肌内脂肪群体高表达),23个下调(在低肌内脂肪群体高表达)]。上调差异表达基因主要富集于胶原蛋白绑定、细胞外基质组装、脂肪酰辅酶A 生物合成过程、固醇代谢过程和脂质代谢调控过程等GO功能条目,以及脂肪乙酰辅酶A生物合成、ChREBP激活代谢基因、脂肪酸代谢等信号通路上;下调基因主要富集于线粒体组装、多细胞生物学过程、辅酶绑定和缺氧反应等GO功能条目,以及基于NFKB的TNFA信号、白细胞介素4和白细胞介素13信号和干扰素信号等信号通路上。通过MCODE分析共筛选获得5个关键基因,分别是SLC25A5、FN1、FASN、ACACA和PRKDC基因;挑选SCD、FASN和ACACA基因进行表达量验证,结果发现这3个基因的相对表达量均表现为柯乐猪高于杜长大猪,其差异达显著或极显著(P<0.01)水平,进一步佐证SCD、FASN和ACACA基因在高肌内脂肪猪群体中高表达。【结论】在巴克夏猪高肌内脂肪群体中高表达的SCD、FASN和ACACA基因,在高肌内脂肪的柯乐猪中也呈显著或极显著高表达,因此这3个关键基因有望作为筛选和培育高肌内脂肪猪品种的分子标记,同时为研究肌内脂肪沉积的分子调控机制提供技术支撑。
关键词: 猪;肌内脂肪;背最长肌;差异表达基因;脂肪沉积
中图分类号: S828.1 文献标志码: A 文章编号:2095-1191(2022)04-0899-09
Expression difference analysis of hub genes between high and low intramuscular fat content in longissimus dorsi of pigs
TAN Ya1,2,3, LI Liang1,2, LEI Yu-hang2, HUANG Zhi-yang2, ZHAO Chun-ping1,3, ZHANG Jing1,3, ZHANG Zheng-qun1, QI Jing1,3, ZHU Li2, SHI Kai-zhi1,3*
(1Institute of Animal Husbandry and Veterinary, Guizhou Academy of Agricultural Sciences, Guiyang, Guizhou 550005, China; 2College of Animal Science and Technology, Sichuan Agricultural University, Chengdu, Sichuan 611130, China;
3Guizhou Engineering Research Center of Local Pig Protection and Breeding, Guiyang, Guizhou 550005, China)
Abstract:【Objective】To screen the hub genes of high intramuscular fat (IMF) deposition and to verify their expression, so as to provide a theoretical basis for revealing the molecular regulation mechanism of IMF deposition in pigs.【Method】Based on the transcriptome data of Berkshire pigs with high and low IMF in NCBI-GEO database, the differentially expressed genes (DEGs) were screened by DESeq2 (|log2 Fold Change|≥1, FDR< 0.05), the functional enrichment of DEGs were analyzed by Metascape. Meanwhile, MCODE were used to identify the key genes of high IMF deposition, real-time fluorescence quantitative PCR (qRT-PCR) was used to verify the expression of hub genes in indigenous pigs (Kole) and foreign pigs (DLY pigs). 【Result】7661 genes were identified from six libraries of Berkshire pigs with high and low IMF. The total expression of genes related to lipid metabolism in high IMF group was significantly higher than that in low IMF group (P<0.05, the same below). Additionally, the total number of DEGs in two groups was 133 [110 were up-regulated (high expression in high IMF) and 23 were down-regulated (high expression in low IMF)]. The up-regulated DEGs were mainly enriched in GO items such as collagen binding, extracellular matrix assembly, fatty acetyl-coA biosynthesis, sterol metabolism and lipid metabolism regulation, and in signaling pathways such as fatty acetyl-coA biosynthesis, CHREBP activated metabolic genes and fatty acid metabolism. Meanwhile, the down-regulated genes were mainly enriched in GO items such as mitochondrial assembly, multi-multicellular biological processes, coenzyme bin-ding and hypoxia response, and in signaling pathways such as TNFA signaling via NFKB, interleukin-4 and interleukin-13 signaling and signaling by interleukins. Five hub genes, SLC25A5, FN1, FASN, ACACA and PRKDC, were identified by MCODE analysis, and then SCD, FASN and ACACA were selected for expression verification. The results showed that the relative expression of these three genes were significantly higher in Kole pigs than that in DLY pigs (P<0.01), and it was further proved that SCD, FASN and ACACA were highly expressed in high IMF pigs. 【Conclusion】SCD, FASN and ACACA genes that highly expressed in the high IMF group of Berkshire pigs are also significantly or extremely highly expressed in Kole pigs with the high IMF. Therefore, these three hub genes may be used as molecular markers for selecting and breeding pigs with high IMF, and provide technical support for studying the molecular regulation mechanism of IMF deposition.
Key words: pig; intramuscular fat; longissimus dorsi; differentially expressed genes (DEGs); fat deposition
Foundation items: Guizhou Department of Science and Technology Plan Project (QKHZC〔2019〕2278, QKHFQ〔2018〕4007, QKHCG〔2020〕1Y027); Guizhou Academy of Agricultural Sciences and Technology Achievements Transformation Guidance Fund Project (QNKYKJCX〔2022〕01)
0 引言
【研究意义】脂肪是动物体内存储能量的重要组织,脂质合成及沉积与动物体内营养物质的消化、吸收、代谢和能量周转存在密切联系(Yun et al.,2018)。脂肪沉积是猪生长的重要生物学过程,其中肌内脂肪(Intramuscular fat,IMF)是指位于肌纤维与肌束间的脂肪,其含量差异可促使肌肉呈现出不同程度的大理石花纹,同时影响肌肉的嫩度、多汁性、风味及脂肪酸含量等品质性状(Damian et al.,2016;陈静等,2021)。因此,肌内脂肪是评价肉品质的重要指标,解析其遗传机制对科学开展肉质调控具有重要意义。【前人研究进展】随着对西方猪种(杜洛克、大白猪及长白猪等)瘦肉率的高强度持续选育,外种猪的肌内脂肪已低于2%,但我国地方猪的肌内脂肪一般都高于3%(熊远著,2000;李鹏和斯日吉楞,2010;张雄等,2019)。长期以来,通过提高肌内脂肪含量以改善猪肉品质是生猪产业面临的着要问题之一,其分子调控机制也受到广泛关注。肌内脂肪沉积同时受到动物遗传背景、饲料、年龄等因素的影响(Katsumata,2011;Bosch et al.,2012;Pena et al.,2016;Malgwi et al.,2022),如通过蛋氨酸和赖氨酸限饲可显著提高育肥猪的肌内脂肪含量(Palma-Granados et al.,2019;Wu et al.,2019),在饲粮中添加共轭亚油酸可有效提高肌内脂肪含量(Wang et al.,2021),但这些调节因子功能的发挥均是通过影响脂肪生成及脂肪分解过程中的关键基因来实现。目前,有关肌内脂肪沉积相关基因的研究报道较少。Cho等(2019)对西方猪种和韩国本地猪种背最长肌的肌内脂肪进行比较,鉴定出猪12号染色体上的MYH3基因是影响肌内脂肪沉积的因果基因,可通过启动子上6-bp缺失的结构变异而抑制肌源性调节因子结合,进而促进肌内脂肪沉积。Huang等(2021)在我国地方猪、西方猪种及其杂交猪种上进行验证,得出的研究结果均不支持 MYH3基因6-bp缺失与肌内脂肪间的因果关系,提示12号染色体上QTL的因果突变有待进一步探究。由于肌内脂肪属于中等偏低的遗传力性状(h2=0.2),因此可通过合理的品种选育得到明显改善与提高。【本研究切入点】分子标记辅助选择(Marker-assisted selection,MAS)的诞生可实现从分子水平上快速准确地分析个体遗传组成,从而实现对基因型的直接選择,极大提高育种效率,最终加快对目的性状的遗传选择进展。虽然目前关于猪肌内脂肪沉积的机制研究较多,但对于外种猪和地方猪的肌内脂肪沉积机制是否具有一致性尚无定论。【拟解决的关键问题】利用现有的NCBI-GEO数据库资源,挖掘高肌内脂肪猪与低肌内脂肪猪的转录组差异,明确高、低肌内脂肪沉积的功能基因差异,筛选出高肌内脂肪沉积的关键基因并进行表达验证,以期为揭示猪肌内脂肪沉积的分子调控机制提供理论依据。
1 材料与方法
1. 1 试验动物及样本采集
供试的地方猪(柯乐猪)和外种猪(杜长大)样本分别来自贵州优农谷生态产业有限公司柯乐猪原种场和四川省某规模化猪场,各6头,所有供试猪均按NY/T 65—2004《猪饲养标准》进行饲喂,饲喂至360日龄左右进行屠宰,采集背最长肌(最后肋骨处),液氮保存备用。
1. 2 转录组数据来源
转录组数据来源于GEO数据库(登录号GSE86086),猪品种为巴克夏,共6个样本,高肌内脂肪群体(n=3)的肌内脂肪含量为(3.80±0.03)%,低肌内脂肪群体(n=3)的含量为(0.47±0.04)%(Lim et al.,2017)。
1. 3 数据分析
采用CPM(Counts per million)法校正基因表达水平,转录本表达量统计采用edgeR(Robinson et al.,2010),并根据 CPM 值分析样本间的相关性。使用DESeq2筛选差异表达基因(Differentially expressed genes,DEGs)(Love et al.,2014;孙瑞萍等,2020),筛选标准为FDR<0.05 且|log2 Fold Change|>1。然后采用Metascape(https://metascape.org/gp/index.html#/main/step1)对差异表达基因进行功能注释分析,并以超几何分布(P<0.05)在分子特征数据库(MSigDB)的KEGG、Reactome、GO和Hallmark等4个数据库中进行信号通路富集分析。
1. 4 关键基因筛选
通过STRING构建差异表达基因编码蛋白的相互作用网络(Protein-protein interaction network,PPI),导入Cytoscape 3.9.0进行网络可视化处理和子模块筛选,再利用MCODE筛选及鉴定出关键基因(张斌等,2021)。
1. 5 基因表达验证
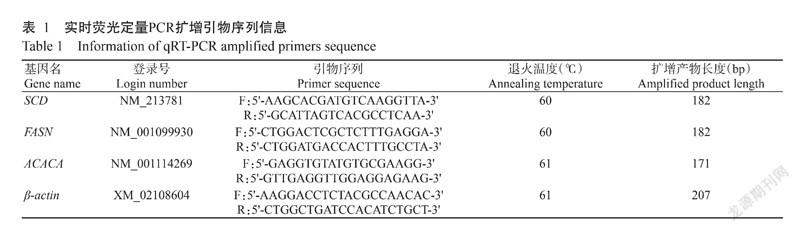
采用实时荧光定量PCR验证关键基因在地方猪和外种猪背最长肌中的表达情况。在NCBI中搜索关键基因的mRNA序列,通过Primer Premier 6.0设计实时荧光定量PCR扩增引物(表1),并以β-actin为内参基因。采用TRIzol法提取样本总RNA,通过PrimeScriptTM RT reagent Kit with gDNA Eraser试剂盒(TaKaRa)反转录合成cDNA后,参照TB Green® PrimeScript™ RT-PCR(TaKaRa)说明在CFX-96荧光定量 PCR 仪上进行实时荧光定量PCR检测,扩增程序:95 ℃预变性 1 min;95 ℃ 5 s,退火30 s,72 ℃ 1 min,进行40个循环。每个样本设3个重复,通过2-△△Ct法换算目的基因相对表达量。
1. 6 统计分析
采用Excel 2017和GraphPad Prism 7进行统计分析,并以双尾非配对t检验进行差异显著性分析。
2 结果与分析
2. 1 转录组数据特征分析结果
利用edgeR对基因的原始表达量进行CPM值计算,筛选出至少在1个文库中CPM值大于0.5的基因进行分析,6个文库共鉴定出7661个基因。对6个样本[高肌内脂肪样本(H1、H2和H3),低肌内脂肪样本(L1、L2和L3)]的基因表达量进行相关分析,结果发现各生物学重复样本间的相关性均高于不同组间的样本(图1-A)。同时,对表达量排名前1000的基因进行聚类分析,结果发现每个组内的3个生物学重复样本均聚为一支(图1-B)。
2. 2 脂质代谢相关基因表达分析结果
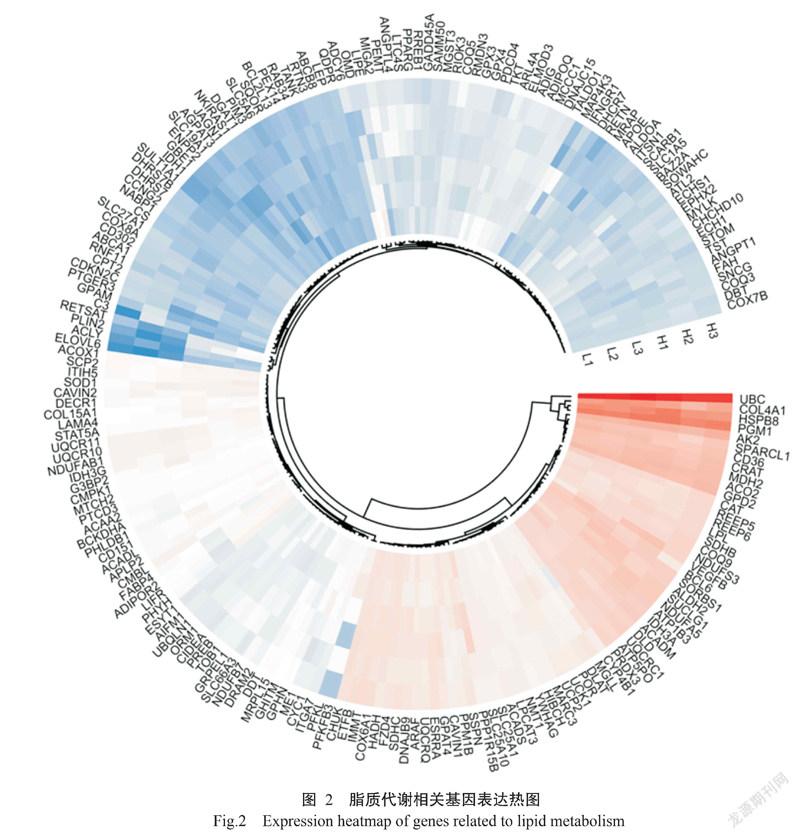
脂质代谢相关基因集来自MSigDB(https://www. gsea-msigdb.org/gsea/msigdb/index.jsp)的REACTOME_ metabolism of lipids(739个脂质代谢基因)和KEGG_fatty acid metabolism(39个脂肪酸酸代谢基因),共发现有199个脂质代谢相关基因在6个样本中表达(图2),其表达模式表现为高肌内脂肪群体样本的脂质代谢相关基因总表达量显著高于低肌内脂肪群体样本(P<0.05,下同)。
2. 3 差异表达基因分析结果
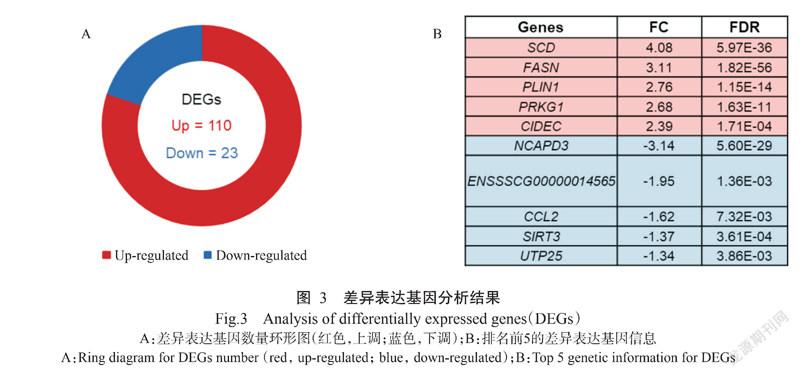
利用DESeq2進行差异表达基因筛选(|log2 Fold Change|≥1,FDR<0.05),共筛选得到133个差异表达基因,其中110个上调、23个下调(图3-A)。排名前5的上调差异表达基因分别是硬脂酸乙酰辅酶A去饱和酶(Stearoyl-coA desaturase)编码基因(SCD)、脂肪酸合酶(Fatty acid synthase)编码基因(FASN)、围脂滴蛋白1(Perilipin 1)编码基因(PLIN1)、CGMP依赖性蛋白激酶1(Protein kinase CGMP-dependent 1)编码基因(PRKG1)和细胞死亡诱导DFFA样效应蛋白C(Cell death inducing DFFA like effector C)编码基因(CIDEC);排名前5的下调差异表达基因分别是NCAPD3(Non-SMC condensin II complex subunit D3)基因、ENSSSCG00000014565、C-C基序趋化因子配体2(C-C motif chemokine ligand 2)编码基因(CCL2)、去乙酰化酶3(Sirtuin 3)编码基因(SIRT3)和U3小核仁RNA相关蛋白25(UTP25 small subunit processor component)编码基因(UTP25)。
2. 4 基因功能富集分析结果
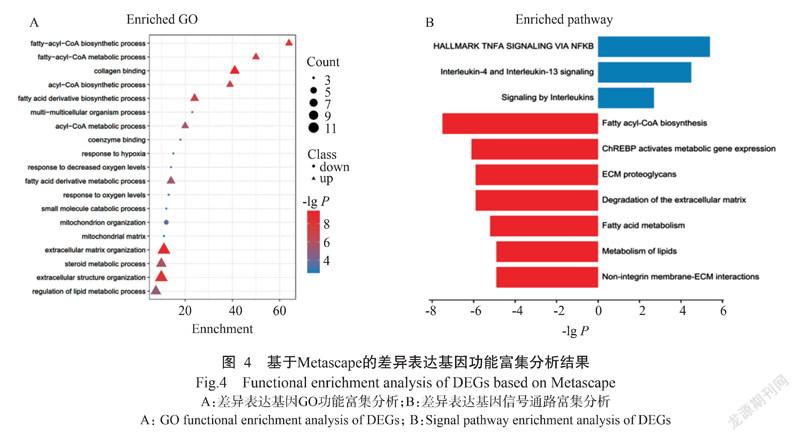
利用Metascape对差异表达基因进行功能富集分析,结果显示133个差异基因共富集到406条功能条目上。其中,上调差异表达基因(在高肌内脂肪群体高表达)主要富集于胶原蛋白绑定(Collagen bin-ding,P=3.98E-10)、细胞外基质组装(Extracellular matrix organization,P=3.16E-09)、脂肪酰辅酶A生物合成过程(Fatty-acyl-CoA biosynthetic process,P=1.26E-08)、固醇代谢过程(Steroid metabolic process,P=1.58E-06)和脂质代谢调控过程(Regulation of lipid metabolic process,P=1.00E-05)等GO功能条目(图4-A),以及脂肪乙酰辅酶A生物合成(Fatty acyl-CoA biosynthesis,P=3.16E-08)、ChREBP激活代谢基因(ChREBP activates metabolic gene expression,P=7.94E-07)、脂肪酸代谢(Fatty acid metabolism,P=6.31E-06)等信号通路(图4-B)上;下调差异表达基因(在低肌内脂肪群体高表达)主要富集于线粒体组装(Mitochondrion organization,P=2.51E-04)、多细胞生物学过程(Multi-multicellular organism process,P=2.51E-04)、辅酶绑定(Coenzyme binding,P=6.31E-04)和缺氧反应(Response to hypoxia,P=1.00E-03)等GO功能条目(图4-A),以及基于NFKB的TNFA信号(HALLMARK TNFA SIGNALING VIA NFKB,P=3.98E-06)、白细胞介素4和白细胞介素13信号(Interleukin-4 and interleukin-13 signaling,P=3.16E-05)和干扰素信号(Signaling by interleukins,P=1.99E-03)等信号通路(图4-B)上。
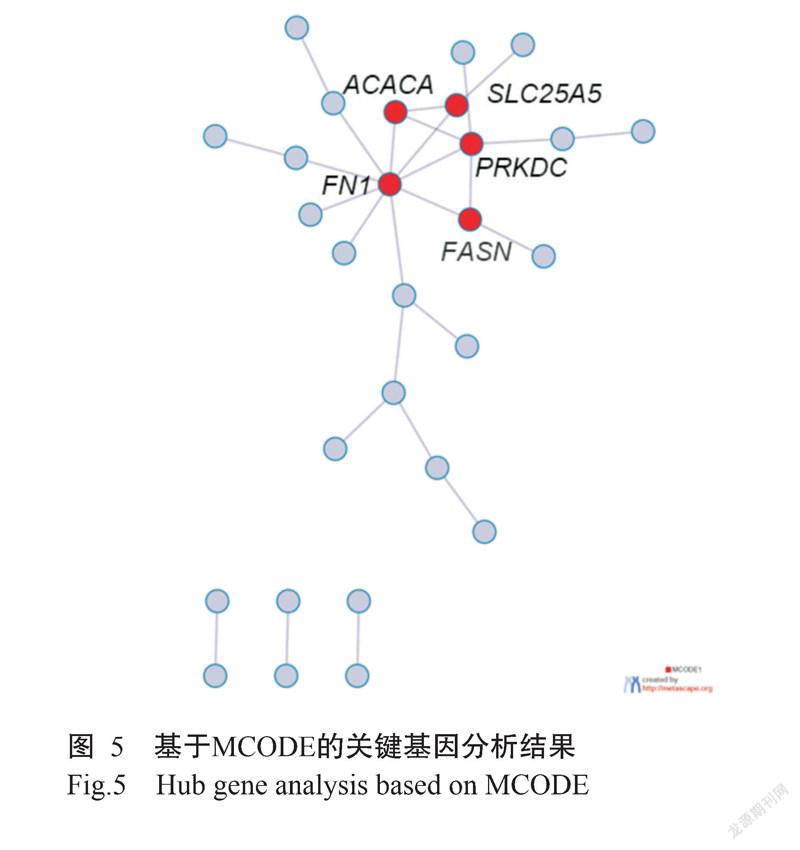
利用MCODE进行关键基因分析,共筛选获得5个关键基因(图5),分别是溶质载体家族25成员5(Solute carrier family 25 member 5,SLC25A5)、纤连蛋白1(Fibronectin 1)编码基因(FN1)、脂肪酸合成酶(Fatty acid synthase)编码基因(FASN)、乙酰辅酶A羧化酶α(Acetyl-CoA carboxylase alpha)编码基因(ACACA)和DNA依赖性蛋白激酶催化亚基(Protein kinase,DNA-activated,catalytic subunit,PRKDC)。除SLC25A5基因呈下调表达外,其余4个关键基因均呈上调表达。
2. 5 关键基因表达验证结果
综合Gallardo等(2009)、Yang等(2013)、Crespo-Piazuelo等(2020)的研究结果,且收录在猪QTLdb与肌内脂肪含量相关的基因数据库中,挑选出FASN和ACACA基因进行实时荧光定量PCR验证。此外,由于SCD基因的上调表达倍数变化最大,且其参与脂质代谢调控已在畜禽的相关研究中得到证实(Uemoto et al.,2012;Yokota et al.,2012;Henriquez-Rodriguez et al.,2016),故本研究选取SCD、FASN和ACACA基因进行表达验证。背最长肌的肌内脂肪表型观察结果(图6-A)表明,柯乐猪的肌内脂肪含量显著高于杜长大猪(8.46% vs 1.19%);实时荧光定量PCR验证结果(图6-C)显示,SCD、FASN和ACACA基因的相对表达量均表现为柯乐猪高于杜长大猪,其差异达显著或极显著(P<0.01,下同)水平,与RNA-Seq测序结果(图6-B)基本一致,即在背最长肌肌内脂肪表型差异明显的柯乐猪与杜长大猪上SCD、FASN和ACACA基因表达差异极显著。
3 讨论
肌内脂肪是衡量猪肉质性状的重要指标之一,其含量直接影响肉色、嫩度、大理石纹及滴水损失等,对猪肉的食用价值和营养价值有直接贡献(Hocquette et al.,2010;Liu et al.,2021)。本研究对高、低肌内脂肪含量巴克夏猪的差异表达基因分析发现,上调基因Top5(在高肌内脂肪组中高表达排名前5)分别为SCD、FASN、PLIN1、PRKG1和CIDEC,其中,SCD和FASN基因在脂肪酸组成中的重要作用已被多项研究证实(Yokota et al.,2012;Maharani et al.,2013)。本研究通過MCODE其挖掘出5个关键基因,分别是SLC25A5、FN1、FASN、ACACA和PRKDC基因,综合前人的相关研究结果(Gallardo et al.,2009;Yang et al.,2013;Crespo-Piazuelo et al.,2020),以及差异表达基因的RNA-Seq测序结果,重点讨论SCD、FASN和ACACA基因与猪肉品质的调控关系。
SCD 基因编码的硬脂酰辅酶A去饱和酶是一种内质网酶,在将饱和脂肪酸(Saturated fatty acids,SFA)转化为单不饱和脂肪酸(Monounsaturated fatty acids,MUFA)的过程中发挥关键作用(Maharani et al.,2013)。MUFA被认为是对人类健康有益的一类脂肪酸,有助于降低低密度脂蛋白胆固醇含量。此外,SCD基因在脂肪组织和骨骼肌中高表达(Voss et al.,2005),是位于猪14号染色体上的脂肪沉积候选基因,其多态性研究已有较多报道。Uemoto等(2012)在SCD基因启动子区鉴定出2个SNPs(g.-353C>T和g.-233T>C),并证实其单倍型与杜洛克猪群体的脂肪酸组成及脂肪熔点间存在显著相关性。Maharani等(2013)为了评价SCD基因与950个韩国本地猪×长白猪F2杂交群体脂肪酸组成的关联性,对SCD基因的6个SNPs(启动子区的g.-353T>C和g.-233T>C;外显子区的g.817C>T;3'-UTR区的g.13311C>G、g.14384g>A和g.14424C>T)进行基因型分析,结果发现F2杂交群体SCD基因与脂肪酸组成存在很强的关联性。Lim等(2015)对巴克夏猪的研究表明,SCD基因3'-UTR区的c*2041T>C多态性会影响脂肪酸组成、脂肪沉积和大理石纹。Henriquez-Rodriguez等(2016)研究表明,对SCD基因T基因型(g.2228T>C)和LEPR基因C基因型(g.1987C>T)的联合选择可有效提高杜洛克猪MUFA/SFA比例。
FASN基因编码脂肪酸合酶,其主要功能是在NADPH存在下催化乙酰辅酶A和丙二酰辅酶A合成棕榈酸酯(C16:0)和硬脂酸酯(C18:0),进而生成长链饱和脂肪酸(Jensen-Urstad and Semenkovich,2012)。Grzes等(2016)研究发现,FASN基因有4个SNPs(c.-2908G>A、c.-2335C>T、c.*42_43insCCCCA和c.*264A>G)与背膘厚相关,其中c.-2335C>T多态性还影响杜洛克胸最长肌胆固醇水平和皮特兰皮下脂肪组织多不饱和脂肪酸(PUFA)含量。Renaville等(2015)研究证实,FASN基因多态性显著影响意大利系大猪的肉品质。Zappaterra等(2019)研究报道,FASN基因的c.265T>C多态性能显著改变意大利系大白猪胸最长肌中硬脂酸、花生四烯酸、γ-亚麻酸和花生四烯酸的含量。PLIN1基因也与脂质功能相关,其编码蛋白覆盖在脂肪细胞中的脂滴上,从而保护脂肪细胞,直至被激素敏感的脂肪酶分解,因而在抑制脂肪分解过程中发挥重要作用(Beller et al.,2008;Li et al.,2020)。本研究结果表明,脂质代谢相关基因表达总量表现为高肌内脂肪群体样本显著高于低肌内脂肪群体样本,且上调差异表达基因(在高肌内脂肪群体高表达)主要富集在脂质代谢相关的GO功能条目及信号通路上;利用MCODE进行关键基因分析,共筛选获得5个关键基因(SLC25A5、FN1、FASN、ACACA和PRKDC),但至今尚无SLC25A5、FN1和PRKDC基因参与脂质代谢的研究报道,因此有待进一步探究其是否在脂质代谢过程发挥重要作用。
ACACA基因编码的乙酰辅酶A羧化酶α是一种含有生物素的活性酶,能催化乙酰辅酶A羧化成丙二酰辅酶A,是脂肪酸合成的限速酶。Gallardo等(2009)研究表明,猪ACACA基因编码区(CDS)存在2个SNPs(c.4899G>A和c.5196T>C),且这2个SNPs与胴体瘦肉含量、肌内脂肪含量及血清高密度脂蛋白胆固醇浓度密切相关。Stachowiak等(2013)对波兰猪群体ACACA基因的分析结果表明,3'-UTR区2个SNPs(c.*99T>A 和 c.*195C>A)的突变会影响背膘厚和瘦肉率。本研究基于巴克夏猪的高、低肌内脂肪群体进行肌内脂肪数据分析,筛选出高肌内脂肪的关键基因有SCD、FASN和ACACA;在高肌内脂肪的柯乐猪和低肌内脂肪的杜长大猪群体内也发现这3个基因的表达趋势与在外种猪巴克夏体内的一致。因此,SCD、FASN和ACACA基因可作为筛选和培育高肌内脂肪猪品种的分子标记,同时为研究肌内脂肪沉积的分子调控机制提供技术支撑。
4 结论
在巴克夏猪高肌内脂肪群体中高表达的SCD、FASN和ACACA基因,在高肌内脂肪的柯乐猪中也呈显著或极显著高表达,因此这3个关键基因可作为筛选和培育高肌内脂肪猪品种的分子标记,同时为研究肌内脂肪沉积的分子调控机制提供技术支撑。
参考文献:
陈静,尤瑞国,刘慧敏,杨国庆. 2021. 柠檬醛对小鼠生长性能、肌内脂肪含量及脂肪酸代谢酶的影响[J]. 河南农业大学学报,55(4):721-726. [Chen J,You R G,Liu H M,Yang G Q. 2021. Effects of citral on growth performance,intramuscular fat content and fatty acid metabolizing enzymes in mice[J]. Journal of Henan Agricultural University,55(4):721-726.] doi:10.16445/j.cnki.1000-2340.2021 0531.001.
李鹏,斯日古楞. 2010. 营养调控影响猪肌内脂肪沉积的研究进展[J]. 饲料广角,(22):34-35. [Li P,Siriguleng. 2010. Research advances on influence of nutrition regulation in porcine intramuscular fat deposition[J]. Feed China,(22):34-35.] doi:10.3969/j.issn.1002-8358.2010.22.012.
孙瑞萍,王峰,晁哲,刘海隆,郑心力,刘圈炜,黄丽丽,邢漫萍,魏立民. 2020. 1月龄五指山猪与长白猪骨骼肌mi-RNA转录组比较[J]. 江苏农业学报,36(3):620-625. [Sun R P,Wang F,Chao Z,Liu H L,Zheng X L,Liu Q W,Huang L L,Xing M P,Wei L M. 2020. Comparative analysis on miRNA transcriptomes of skeletal muscle between one-month-old Wuzhishan pig and Landrace[J]. Jiangsu Journal of Agricultural Sciences,36(3):620-625.] doi:10.3969/j.issn.1000-4440.2020.03.013.
熊远著. 2000. 瘦肉猪育种的发展及展望[J]. 中国工程科学,2(9):42-46. [Xiong Y Z. 2000. Development and prospects of lean-type swine breeding[J]. Engineering Science,2(9):42-46.] doi:10.3969/j.issn.1009-1742.2000.09.007
张斌,杨昕霞,袁志辉. 2021. 水稻响应热胁迫核心基因的筛选与鉴定[J]. 江苏农业学报,37(4):817-822. [Zhang B,Yang X X,Yuan Z H. 2021. Screening and identification of core genes responding to heat stress in rice[J]. Jiangsu Journal of Agricultural Sciences,37(4):817-822.] doi:10. 3969/j.issn.1000-4440.2021.04.001.
張雄,尚以顺,史开志,张勇,黄波,韩雪,王婧. 2019. 从江香猪胴体及肉品质性状研究[J]. 家畜生态学报,40(1):36-40. [Zhang X,Shang Y S,Shi K Z,Zhang Y,Huang B,Han X,Wang J. 2019. Study on carcass performance and meat quality of Congjiang pigs[J]. Acta Ecologae Animalis Domastici,40(1):36-40.] doi:10.3969/j.issn.1673-1182.2019.01.007.
Beller M,Sztalryd C,Southall N,Bell M,Jäckle H,Auld D S,Oliver B. 2008. COPI complex is a regulator of lipid homeostasis[J]. PLoS Biology,6(11):e292. doi:10.1371/journal.pbio.0060292.
Bosch L,Tor M,Reixach J,Estany J. 2012. Age-related changes in intramuscular and subcutaneous fat content and fatty acid composition in growing pigs using longitudinal data[J]. Meat Science,91(3):358-363. doi:10.1016/j.meatsci.2012.02.019.
Cho I C,Park H B,Ahn J S,Han S H,Lee J B,Lim H T,Yoo C K,Jung E J,Kim D H,Sun W S,Ramayo-Caldas Y,Kim S G,Kang Y J,Kim Y K,Shin H S,Seong P N,Hwang I S,Park B Y,Hwang S,Lee S S,Ryu Y C,Lee J H,Ko M S,Lee K,Andersson G,Pérez-Enciso M,Lee J W. 2019. A functional regulatory variant of MYH3 influences muscle fiber-type composition and intramuscular fat content in pigs[J]. PLoS Genetics,15(10):e1008279. doi:10.1371/journal.pgen.1008279.
Crespo-Piazuelo D,Criado-Mesas L,Revilla M,Castelló A,Noguera J L,Fernández A I,Ballester M,Folch J M. 2020. Identification of strong candidate genes for backfat and intramuscular fatty acid composition in three crosses based on the Iberian pig[J]. Scientific Reports,10(1):13962. doi:10.1038/s41598-020-70894-2.
Damian F,Seon-Tea J,Robyn W. 2016. Consumer acceptability of intramuscular fat[J]. Korean Journal for Food Scien-ce of Animal Resources,36(6):699-708. doi:10.5851/kosfa.2016.36.6.699.
Gallardo D,Quintanilla R,Varona L,Díaz I,Ramírez O,Pena R N,Amills M. 2009. Polymorphism of the pig acetyl-coenzyme A carboxylase alpha gene is associated with fatty acid composition in a Duroc commercial line[J]. Animal Genetics,40(4):410-417. doi:10.1111/j.1365-2052.2009. 01854.x.
Grzes M,Sadkowski S,Rzewuska K,Szydlowski M,Switonski M. 2016. Pig fatness in relation to FASN and INSIG2 genes polymorphism and their transcript level[J]. Mole-cular Biology Reports,43(5):381-389. doi:10.1007/s11033- 016-3969-z.
Henriquez-Rodriguez E,Bosch L,Tor M,Pena R N,Estany J. 2016. The effect of SCD and LEPR genetic polymorphisms on fat content and composition is maintained throughout fattening in Duroc pigs[J]. Meat Science,121:33-39. doi: 10.1016/j.meatsci.2016.05.012.
Hocquette J F,Gondret F,Baéza E,Médale F,Jurie C,Pe-thick D W. 2010. Intramuscular fat content in meat-produ-cing animals:Development,genetic and nutritional control,and identification of putative markers[J]. Animal:An International Journal of Animal Bioscience,4(2):303-319. doi:10.1017/S1751731109991091.
Huang C,Zhong L P,Zou X X,Huang Y Z,Cai L P,Ma J W. 2021. Evidence against the causal relationship between a putative cis-regulatory variant of MYH3 and intramuscular fat content in pigs[J]. Frontiers in Veterinary Science,8:672852. doi:10.3389/fvets.2021.672852.
Jensen-Urstad A P L,Semenkovich C F. 2012. Fatty acid synthase and liver triglyceride metabolism:Housekeeper or messenger[J]. Biochimica et Biophysica Acta,1821(5):747-753. doi:10.1016/j.bbalip.2011.09.017.
Katsumata M. 2011. Promotion of intramuscular fat accumulation in porcine muscle by nutritional regulation[J]. Animal Science Journal,82(1):17-25. doi:10.1111/j.1740-0929.2010.00844.x.
Li S J,Raza S H A,Zhao C P,Cheng G,Zan L S. 2020. Overexpression of PLIN1 promotes lipid metabolism in bovine adipocytes[J]. Animals:An Open Access Journal from MDPI,10(11):E1944. doi:10.3390/ani10111944.
Lim K S,Kim J M,Lee E A,Choe J H,Hong K C. 2015. A candidate single nucleotide polymorphism in the 3' untranslated region of stearoyl-CoA desaturase gene for fatness quality and the gene expression in Berkshire pigs[J]. Asian-Australasian Journal of Animal Sciences,28(2):151-157. doi:10.5713/ajas.14.0529.
Lim K S,Lee K T,Park J E,Chung W H,Jang G W,Choi B H,Hong K C,Kim T H. 2017. Identification of differentially expressed genes in longissimus muscle of pigs with high and low intramuscular fat content using RNA sequen-cing[J]. Animal Genetics,48(2):166-174. doi:10.1111/age.12518.
Liu J Q,Li J,Chen W T,Xie X T,Chu X G,Valencak T G,Wang Y Z,Shan T Z. 2021. Comprehensive evaluation of the metabolic effects of porcine CRTC3 overexpression on subcutaneous adipocytes with metabolomic and transcriptomic analyses[J]. Journal of Animal Science and Biotechnology,12(1):19. doi:10.1186/s40104-021-00546-6.
Love M I,Huber W,Anders S. 2014. Moderated estimation of fold change and dispersion for RNA-Seq data with DESeq2[J]. Genome Biology,15(12):550. doi:10.1186/s13059-014-0550-8.
Maharani D,Park H B,Lee J B,Yoo C K,Lim H T,Han S H,Lee S S,Ko M S,Cho I C,Lee J H. 2013. Association of the gene encoding stearoyl-CoA desaturase (SCD) with fatty acid composition in an intercross population between Landrace and Korean native pigs[J]. Molecular Biology Reports,40(1):73-80. doi:10.1007/s11033-012-2014-0.
Malgwi I H,Halas V,Grünvald P,Schiavon S,Jócsák I. 2022. Genes related to fat metabolism in pigs and intramuscular fat content of Pork:A focus on nutrigenetics and nutrigenomics[J]. Animals:An Open Access Journal from MDPI,12(2):150. doi:10.3390/ani12020150.
Palma-Granados P,Seiquer I,Benítez R,Óvilo C,Nieto R. 2019. Effects of lysine deficiency on carcass composition and activity and gene expression of lipogenic enzymes in muscles and backfat adipose tissue of fatty and lean piglets[J]. Animal:An International Journal of Animal Bioscience,13(10):2406-2418. doi:10.1017/S17517311190 00673.
Pena R N,Ros-Freixedes R,Tor M,Estany J. 2016. Genetic marker discovery in complex traits:A field example on fat content and composition in pigs[J]. International Journal of Molecular Sciences,17(12):E2100. doi:10.3390/ijms17122100.
Renaville B,Bacciu N,Lanzoni M,Corazzin M,Piasentier E. 2015. Polymorphism of fat metabolism genes as candidate markers for meat quality and production traits in heavy pigs[J]. Meat Science,110:220-223. doi:10.1016/j.meatsci.2015.07.014.
Robinson M D,McCarthy D J,Smyth G K. 2010. edgeR:A Bioconductor package for differential expression analysis of digital gene expression data[J]. Bioinformatics (Oxford,England),26(1):139-140. doi:10.1093/bioinforma-tics/btp616.
Stachowiak M,Nowacka-Woszuk J,Szydlowski M,Switonski M. 2013. The ACACA and SREBF1 genes are promising markers for pig carcass and performance traits, but not for fatty acid content in the longissimus dorsi muscle and adipose tissue[J]. Meat Science,95(1):64-71. doi:10.1016/ j.meatsci.2013.04.021.
Uemoto Y,Nakano H,Kikuchi T,Sato S,Ishida M,Shibata T,Kadowaki H,Kobayashi E,Suzuki K. 2012. Fine mapping of porcine SSC14 QTL and SCD gene effects on fatty acid composition and melting point of fat in a Duroc purebred population[J]. Animal Genetics,43(2):225-228. doi:10.1111/j.1365-2052.2011.02236.x.
Voss M D,Beha A,Tennagels N,Tschank G,Herling A W,Quint M,Gerl M,Metz-Weidmann C,Haun G,Korn M. 2005. Gene expression profiling in skeletal muscle of Zucker diabetic fatty rats:Implications for a role of stea-royl-CoA desaturase 1 in insulin resistance[J]. Diabetologia,48(12):2622-2630. doi:10.1007/s00125-005-0025-2.
Wang L Y,Huang Y Q,Wang Y Z,Shan T Z. 2021. Effects of polyunsaturated fatty acids supplementation on the meat quality of pigs:A meta-analysis[J]. Frontiers in Nutrition,8:746765. doi:10.3389/fnut.2021.746765.
Wu L,Zhang H W,Na L,Zhou X H,Li X,Zhao Y R,Wen Z,He Q H. 2019. Methionine restriction at the post-weanling period promotes muscle fiber transition in piglets and improves intramuscular fat content in growing-fini-shing pigs[J]. Amino Acids,51(10-12):1657-1666. doi:10.1007/s00726-019-02802-6.
Yang B,Zhang W C,Zhang Z Y,Fan Y,Xie X H,Ai H S,Ma J W,Xiao S J,Huang L S,Ren J. 2013. Genome-wide association analyses for fatty acid composition in porcine muscle and abdominal fat tissues[J]. PLoS One,8(6):e65554. doi:10.1371/journal.pone.0065554.
Yokota S,Sugita H,Ardiyanti A,Shoji N,Nakajima H,Hosono M,Otomo Y,Suda Y,Katoh K,Suzuki K. 2012. Contributions of FASN and SCD gene polymorphisms on fatty acid composition in muscle from Japanese black cattle[J]. Animal Genetics,43(6):790-792. doi:10.1111/j.1365- 2052.2012.02331.x.
Yun J Y,Jin H G,Cao Y,Zhang L C,Zhao Y M,Jin X,Yu Y S. 2018. RNA-Seq analysis reveals a positive role of HTR2A in adipogenesis in Yan yellow cattle[J]. International Journal of Molecular Sciences,19(6):1760. doi:10.3390/ijms19061760.
Zappaterra M,Luise D,Zambonelli P,Mele M,Serra A,Costa L N,Davoli R. 2019. Association study between backfat fatty acid composition and SNPs in candidate genes highlights the effect of FASN polymorphism in large white pigs[J]. Meat Science 156:75-84. doi:10.1016/j.meatsci.2019.05.013.
收稿日期:2022-02-19
基金项目:贵州省科技计划项目(黔科合支撑〔2019〕2278号,黔科合服企〔2018〕4007号,黔科合成果〔2020〕1Y027号);贵州省农业科学院科技成果转化引导资金项目(黔农科院科技创新〔2022〕01号)
通讯作者:史开志(1981-),http://orcid.org/0000-0001-7255-7180,研究員,主要从事地方猪培育及产业化发展研究工作,E-mail:shkzjjp@163.com
第一作者:谭娅(1989-),http://orcid.org/0000-0003-2128-2396,主要从事猪遗传与分子育种研究工作,E-mail:Tanya_Lee@126.com

