Tetralin Hydrocracking Reaction Network to Single-Ring Aromatics on Bifunctional Catalysts
Ju Xueyan; Huang Zhen; Zhang Rui; Wang Lixin; Hu zhihai; Li Dadong
(SINOPEC Research Institute of Petroleum Processing, Beijing 100083)
Abstract: Conversion of LCO (light cycle oil) to BTX (benzene, toluene, and xylene) is an economically valuable method for refineries. However, this approach still faces difficulties as the main reactions are not clearly understood. Here we study the detailed hydrocracking pathway of typical reactants, 1-methylnaphthalene and tetralin, through molecular simulations and experiments to improve our understanding of the conversion process of LCO to BTX. Molecular simulations demonstrate that the rate-determining step is the isomerization pathway of six-membered ring to five-membered ring in tetralin as its activation energy (ΔEa) is the highest among all the reactions and the order of ΔEa of reactions is isomerization> ring-opening ≈ side-chain cleavage. The results of experiments show that with the increase in reaction depth, i.e., through a high temperature (350 - 370 °C) and low LHSV (4.5 - 6.0 h−1), isomerization, ring-opening, and side-chain cleavage reactions occurred, thus improving the selectivity and yield of alkyl aromatics.
Key words: tetralin, hydrocracking network, single-ring aromatics
1 Introduction
Because of the stringent requirement of environment protection, new vehicle diesel standards have proposed more strict requirements on the sulfur, nitrogen, and aromatics content as well as a high cetane value. At present, the proportion of light cycle oil (LCO) delivered from fluid catalytic cracking (FCC) units can amount to over 30% in the Chinese diesel pool. However, when the mass fraction of aromatics is about 70% - 90%, LCO becomes one of major concerns for Chinese refiners as a poor blending component. Especially, most hydrocarbons in LCO are bicyclic aromatics with a low cetane number,which limits its application as a vehicle fuel. On the other hand, the aromatics can be converted into highvalue products with a specific aromatic structure. The hydrocracking process has been widely used to convert bicyclic aromatics to tetralin by partial hydrogenation,followed by ring-opening, cracking, and side-chain cleavage reactions in the hydrocracking reaction zone[1].Then, the monocyclic aromatics generated in the hydrocracking process, such as benzene, toluene, and xylene (BTX), are high-value chemicals with extensive applications in the petrochemical, polymer, and fine chemicals industries[2-3].
1-Methylnaphthalene (1-MN), a typical bicyclic aromatic model compound, exhibits a strong adsorption tendency on the surface of bifunctional catalysts, and the hydrogenation reaction rate of converting naphthalene(NA) to tetralin is much higher than that of converting tetralin to decalin (DN). Hydrogenation reaction mainly occurs on the metal active sites, whereas the cracking reaction, a secondary reaction, occurs on the Brønsted acid sites and metal active sites of catalysts[4].On the metal-zeolite bifunctional catalysts, reactions first occur on the hydrogenation active sites and then on the acidic sites for isomerization, ring-opening, and side-chain cleavage[5].
As discussed in a previous study, on zeolite-supported catalysts, more than 300 types of hydrocracking products can be obtained due to cracking (benzene),hydrogenation (DN), isomerization (isomer of DN),ring-opening (alkylbenzene, monocyclic naphthene),and dehydrogenation (NA)[6]. Nickel and other metal components on the catalysts can facilitate the hydrogenation reaction of unsaturated hydrocarbons such as aromatics and olefins and provide overflow hydrogen for acid centers on the catalyst. When the amount of active hydrogen provided by the active phase is insufficient, tetralin can form heavier products via the carbene reaction path[7]. The properties of catalysts, such as acidity, porosity, and metal properties, have prominent influence on the bicyclic aromatics reaction[8].
At present, studies on LCO model compounds can be mainly divided into two types[9-11]: One is to study the direct hydrocracking reaction of 1-MN on bifunctional catalysts to determine the reaction process and the effect of catalyst. The disadvantage of this method is that it cannot simulate the two-stage reactor widely used to remove the sulfur and nitrogen impurities in LCO. The other is to use tetralin to simulate the reaction in the second reactor of hydrocracking[12]. In typical LCO,the mass fraction of NA with a substituent group is more than 30%, whereas that of NA is only 1% - 3%.Therefore, it is difficult to accurately simulate the actual reaction of LCO due to the improper carbon number.To the best of our knowledge, hydrotreated 1-MN has been rarely studied. The aim of this study is to achieve isomerization, ring-opening, cracking, and side-chain cleavage reaction network of hydrotreated 1-MN and simulate the LCO reaction process in the secondstage reactor of hydrocracking. This paper reports the simulation results and processing conditions that could support network identification and catalyst design reasonably.
2 Experimental
2.1 Preparation of bifunctional catalysts
Commercial HY zeolite and pseudoboehmite powder provided by the SINOPEC Research Institute of Petroleum Processing (RIPP) were used as the support.The bifunctional catalyst was prepared as follows: A certain proportion of HY zeolite, pseudoboehmite powder,extrusion agent, and adhesive were evenly mixed,extruded, and then dried in an oven at 120 °C for 3 h.The product was calcined at 590 °C for 3 h in a tubular furnace under a flowing air atmosphere. The incipientwetness impregnation method was used with basic nickel carbonate and molybdenum trioxide in a phosphoric acid solution. After drying in the oven at 120 °C for 3 h, the mixture was heated in flowing air and calcined at 450 °C for 3 h in a tubular furnace.
2.2 Preparation of raw materials
First, 1-MN purchased from Aladdin Reagent Company was dissolved in cyclohexane as the refining feedstock that can simulate the reaction of polycyclic aromatic hydrocarbons (PAHs) in LCO. The solvent was used as a hydrotreating reaction zone feedstock to generate methyltetralin. Table 1 shows the property of feedstock.Under mild hydrogenation conditions, the conversion ratio of 1-MN was 93.04%, and the selectivity of 1-MN hydrogenation to tetralin was 84.98%. The hydrocracking feedstock was rich in tetralin (-1 and -5 substituents), the main probe compounds discussed next.
2.3 Experimental instrument
As shown in Figure 1, experiments were carried out in a fixed-bed stainless steel microreactor with a catalyst loading of 0.1 - 1.0 g, a hydrogen flow rate of 300 mL/min, temperature of 330 - 370 °C, and a hydrogen partial pressure of 3.0 - 4.5 MPa. Before the hydrogenation reaction, the oil which was composed of 3.0% of CS2, and 97.0% of cyclohexane was introduced to fully presulfurize the catalyst. After a certain reaction time (2 h, 4 h, 6 h) under the set hydrogenation reaction conditions, the liquid products were collected and quantitatively analyzed.

Figure 1 Process flow diagram of experimental device
2.4 Product analysis
The hydrocracking products on the bifunctional catalyst are complex. Taking 1-MN as an example,the hydrocracking products showed more than 100 characteristic peaks just by a gas chromatography (GC)analysis. To improve the accuracy of product analysis, the products were analyzed using a GC-mass spectrometry(GC-MS) method and an Agilent GC 7890A equipped with a HP-PONA (50 m × 0.2 mm × 0.5 μm) column.
3 Products Analysis and Molecular Simulation of Reaction Pathways
3.1 Detailed product analysis
In this section, the product was analyzed as monomer hydrocarbons, and the main reaction product was classified. The hydrocarbon composition of the product is shown in Table 2. To facilitate the analysis of results,the hydrocracking products of hydrotreated 1-MN were divided into the following four types:
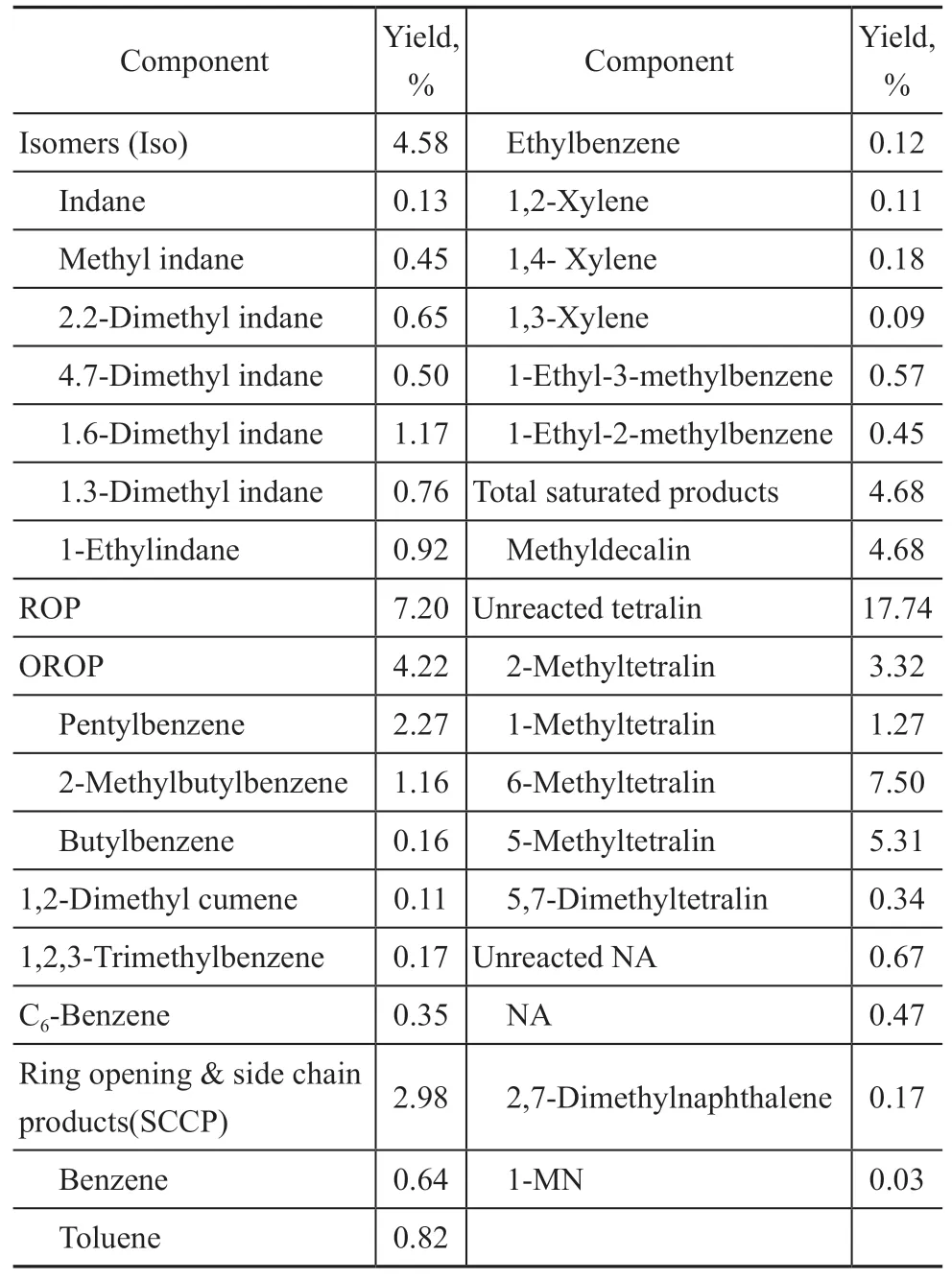
Table 2 Composition of hydrocracking products
1) Only ring-opening products (OROP) mainly include alkylbenzenes with a long side chain, such as butylbenzene, amylbenzene, and their isomers, among which the content of amylbenzene is the highest.
2) Side-chain cracking products (SCCP) mainly include trimethylbenzene, methylethyl benzene, xylene, and benzene. Ring-opening products (ROP) are composed of OROP and SCCP products.
3) Isomeric products (ISO) include ROP and indane[13].
4) Bicyclic saturated products such as DN and the related alkyl substituents.
3.2 Molecular simulation of pathway results
Molecular simulation was applied to calculate the activation energy (ΔEa) of possible reaction paths by which the optimal reaction pathway can be further obtained. According to the transition state theory, reaction energy barrier refers to the maximum energy needed to cross in the conversion process of reactants. The lower the reaction energy barrier, the easier the occurrence of reaction in kinetics.
The specific process was to construct and optimize a structural model of reactants and products, and to search the transition state by LST/QST (linear synchronous transit/quadratic synchronous transit) method. Based on the process mentioned above, the transition state was confirmed by vibration frequency analysis. Finally, the reaction energy barrier and reaction heat were determined.In Dmol3 calculation, GGA/BLYP (generalized gradient approximation/Becke-Lee-Yang-Parr) was selected as the exchange correlation function; DND (double numerical plus d-functions) was selected as the basis set; and the convergence accuracy was 0.05 kJ/mol with a force of 1×1014N and displacement of 5×10−13m.
For step 1, the carbon atoms on 5-methyltetralin (5-MT) were labeled as shown in Figure 2 to calculate the activation energy in different positions to generate carbocations. The cracking of 5-MT showedβ-scission on the catalyst with abundant Brønsted acid sites, while the naphthenic ring of tetralin can be first dehydrogenated to alkenes on the bifunctional catalyst, and then combine with protons to form carbocations in the center of Brønsted acid site. Finally, β-scission occurred, and the ring-opening reaction completed. The activation energy of the reaction of carbon atoms in different positions calculated using molecular simulation is shown in Table 3. It was found that tetralin can be combined with H+with a lower activation energy at 5 - 10 sites in the acid center and lose H−to form carbocations at 1 - 4 sites as shown in Figure 2. Therefore, in addition to the classical dehydrogenation olefin mechanism, H+will be generated on the aromatic ring to form carbocations at the same time. The H−generated with carbocations can be removed from the naphthenic ring, and cracking reaction will occur further.
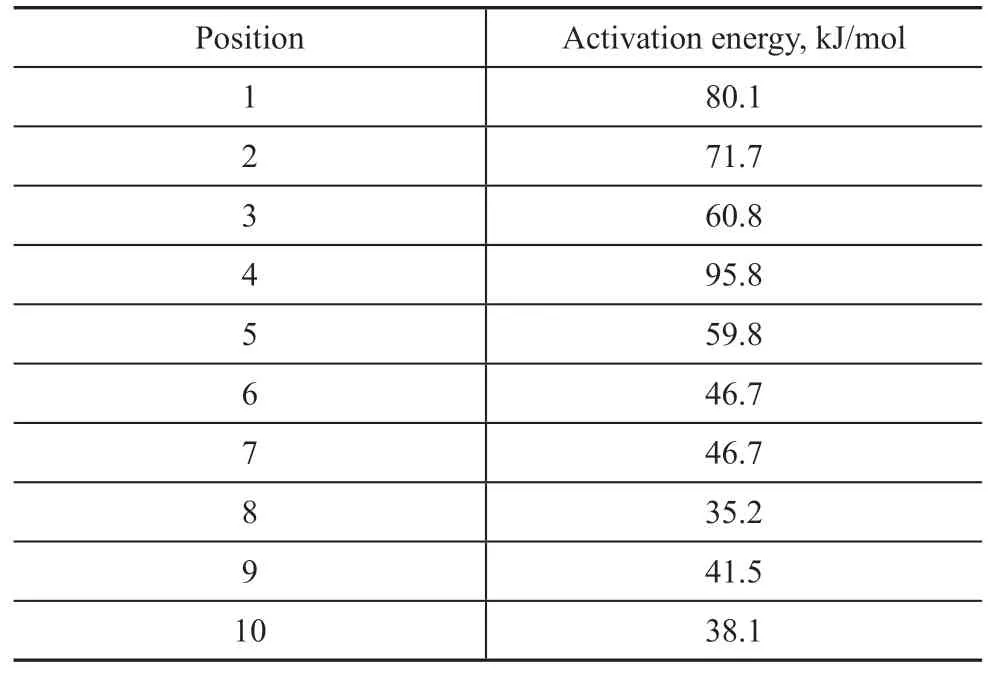
Table 3 Activation energy data of carbocation formation at different positions on tetralin
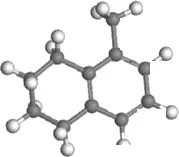
Figure 2 Label of carbon atoms at different positions on 5-MT
Under typical reaction and catalyst conditions, the choice of reaction path is mainly related to the activation energy.According to Figure 3(a), the ΔEaof alkylbenzene generated by 5-MT containing a carbocation direct ring-opening is up to 276.6 kJ/mol, whereas the ΔEaof isomerization process is obviously lower.Therefore, tetralin preferentially isomerizes to indane in hydrocracking process. The isomerization processconsists of two steps: 1) The first step is to generate the transition state of ternary cyclocarbocation, which needs an activation energy of 15.1 kJ/mol. 2) The second step involves the C-C bond fracturing to form indane, which needs a higher activation energy of 169.0 kJ/mol, that is still far lower than that of direct ring-opening.

Figure 3 Activation energy data of tetralin hydrocracking reaction path
Figure 3(b) shows that the ΔEaof further ringopening after the isomerization of 1-MT and 5- MT is substantially lower than those of direct ring-opening of tetralin shown in Figure 3(a). It is considered that the isomerization of tetralin to indane is a necessary process for ring-opening as reported in literature[14]. Table 2 shows that the content of alkylbenzene in the product is as high as 7.2% including benzene, toluene, ethyltoluene,butylbenzene, and pentylbenzene. Figure 4 shows the ΔEadata for further side-chain cleavage of alkylbenzenes with multiple carbon substituents. It can be concluded that alkylbenzene is prone to isomerization following the carbocation formation, so many types of alkylbenzenes are generated in the side-chain cleavage process. The simulated ΔEaof alkylbenzenes’ side-chain cleavage reaction pathway ranges from 65.4 kJ/mol to 209 kJ/mol.
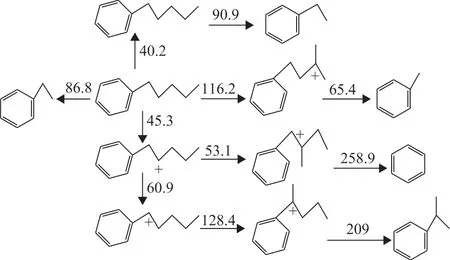
Figure 4 Activation energy data of side-chain breaking pathway alkylbenzenes
Based on the molecular simulation results of feasible hydrocracking network mentioned above, the isomerization of tetralin requires the highest ΔEa, and the ΔEaof reactions follows the order: isomerization > ringopening ≈ side-chain cleavage. There are various reaction paths in the side-chain cleavage process.
4 Effects of Reaction Parameters
As concluded in the simulation results mentioned above,isomerization, ring-opening, and side-chain cleavage show positive effects on the conversion of hydrotreated 1-MN to short-chain alkylbenzenes (SABs). Therefore,according to the previous hydrocarbon classification, in addition to defining tetralin conversion, the reaction path selectivity is described by S-iso, S-ring opening, and S-side cracking. The specific calculation formulas are shown in Equations (1)-(4).

The parameters can be defined as follows:
S-iso—Isomerization selectivity of tetralin;
S-ring opening—Ring-opening pathway selectivity of tetralin to alkylbenzene;
S-side cracking—Side-chain cleavage selectivity of tetralin to <C10alkylbenzene;
AMN= Mass fraction of 1-MN in raw materials;
BMN= Mass fraction of 1-MN in product;
ATN= Mass fraction of tetralin in raw materials;
BTN= Mass fraction of tetralin in product;
BIN= Mass fraction of indane in raw materials;
BAB= Mass fraction of indane in product;
BSAB= Mass fraction of <C10alkylbenzenes in product.
4.1 Effect of LHSV on product distribution
Space velocity (LHSV) refers to the raw material volume of per unit catalyst volume in unit time that can reflect the pathway. The hydrocracking reaction LHSV was studied in the range of 4.5 - 8.2 h−1, while the temperature, hydrogen partial pressure, and hydrogen flow rate were 370 °C, 4.0 MPa, and 300 mL/min,respectively. The hydrocracking products were divided into benzene (Benzene), alkylbenzene (AB), longchain alkylbenzenes (LABs), SABs, indane (Indane),1-methyltetralin (1-MT), 5-MT, DN, NA, and polyalkylsubstituted tetralin (DMT). From the conversion rules of 1-MT and 5-MT shown in Figure 5, it can be concluded that the conversion of 1-MT increased with the decrease in space velocity, and the conversion of 1-MT was slightly higher than that of 5-MT, which is due to the presence of substituents with electron-donating effect on the 1-MT saturated naphthenic ring.
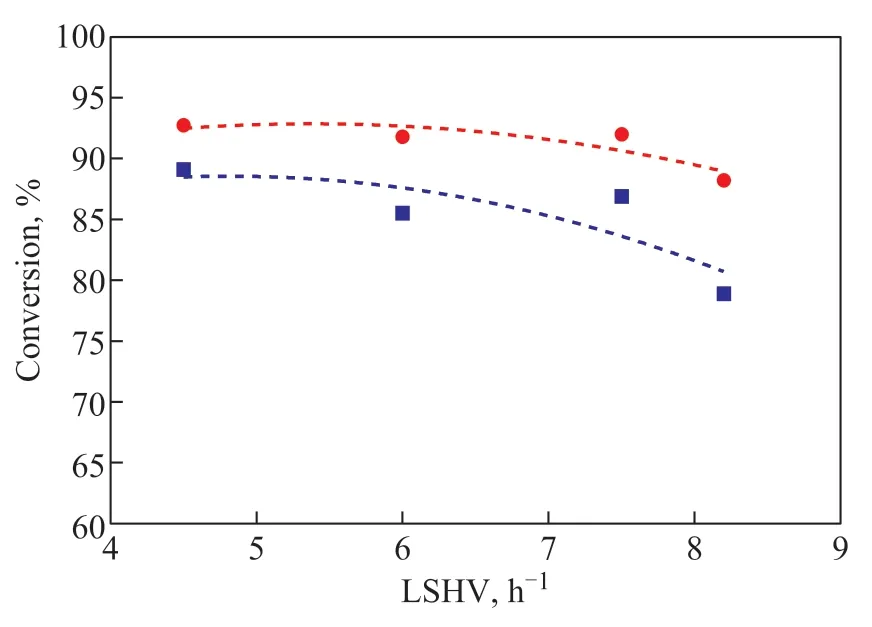
Figure 5 Conversion of 1-MT and 5-MT under different LHSV conditions

Figure 6 Product distribution under different LHSV conditions
As shown in Figure 6, the content of indane first increased and then decreased with the decrease in LHSV. As an intermediate product in the hydrocracking process[15-16],indane generated from tetralin at the initial stage of reaction further converted into alkylbenzene as the reaction progressed. The content of alkylbenzene products monotonously increased, especially the difference in content between SABs and LABs became narrow when the LHSV reached 4.5 h−1.
From the perspective of alkylbenzene content in the products, tetralin reaction is dominated by monomolecular path. Combining the simulation result with the product distribution under different LSHVs, the monomolecular mechanism reaction path can be speculated and confirmed as shown in Figure 7.
By ignoring the bimolecular reaction path and dehydrogenation reaction of tetralin, isomerization,ring-opening, and side-chain cleavage reactions can substantially promote the formation of SABs. According to the classification mentioned above, the effect of LSHV on reaction selectivity is proposed, as shown in Figure 8.

Figure 7 Monomolecular reaction pathway of 1-MT hydrocracking
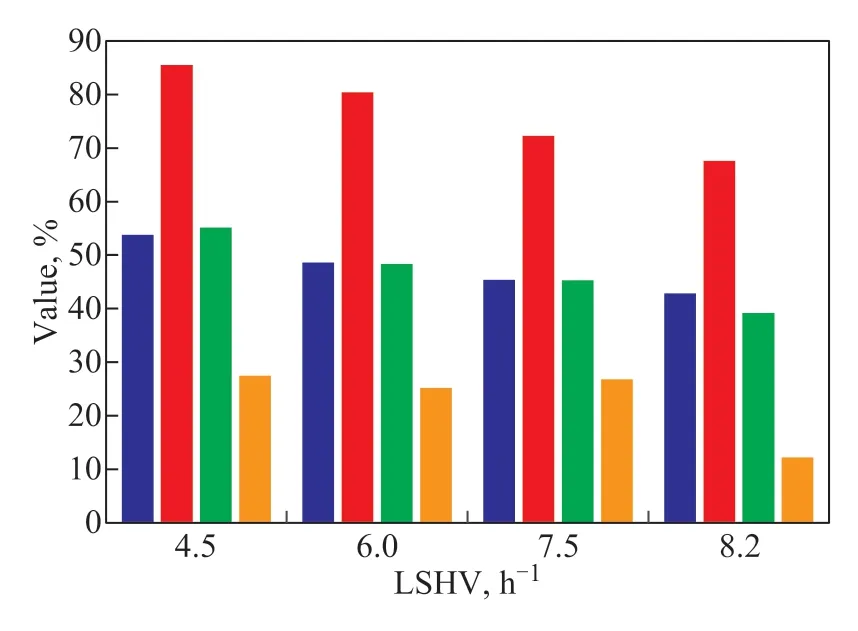
Figure 8 Effect of LHSV on the reaction of hydrotreated 1-MN
Figure 8 shows that the conversion of tetralin substantially increased with the decrease of LHSV. TheS-iso increased from 67.6% to 85.5%, whereas the S-ring opening and S-chain breaking increased from 39.2% to 55.1% and from 12.2% to 27.5%, respectively. The results show that hydrogenation and other rapid reactions preferentially occurred with a high LSHV. A low LHSV is obviously beneficial to isomerization, cracking, and other reaction paths that require a rather longer reaction time. Therefore,a relatively low LSHV not only improves the conversion of tetralin, but also promotes the reaction to isomerization path that is conducive to the formation of SABs from indene.
4.2 Effect of temperature on product distribution
According to the activation energy data mentioned above,a suitable temperature is crucial to 1-MN hydrocracking.Therefore, the effect of temperature was studied in the range of 340 - 370 °C when the hydrogen partial pressure, hydrogen flow rate, and LHSV were 4.0 MPa,300 mL/min, and 6.0 h−1, respectively. The distribution of products at different reaction temperatures is shown in Figure 9.
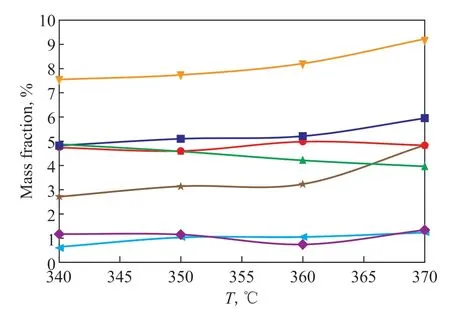
Figure 9 Effect of temperature on hydrocracking product distribution
The effect of temperature on hydrocracking reaction can be determined from the product distribution shown in Figure 9. When the temperature increased from 340 °C to 370 °C,the DN content decreased from 4.88% to 3.96%. However,the content of indane and AB increased from 4.82% to 5.95% and 7.55% to 9.22%, respectively. At present,studies[17]proposed that the reaction rate of tetralin is controlled by isomerization process, and the isomerization,cracking, and side-chain cleavage process of tetralin is a thermodynamically favorable reaction. According to the simulation calculation shown in Section 3, the ΔEaof isomerization process is higher than that of ring-opening and side-chain cleavage process. A higher temperature is beneficial to the isomerization path with a higher ΔEawhich is conducive to the formation of SABs[18].
As shown in Figure 10, the conversion of tetralin increased from 38.4% to 48.6% with increasing temperature, and the S-iso increased substantially,reaching 76.3% at 370 °C. Moreover, the S-side breaking also reached 24.0%. When the reaction temperature was higher than 360 °C, not only the strong acid center but also the weak acid center in the catalyst started to work,substantially improving the S-iso reaction.
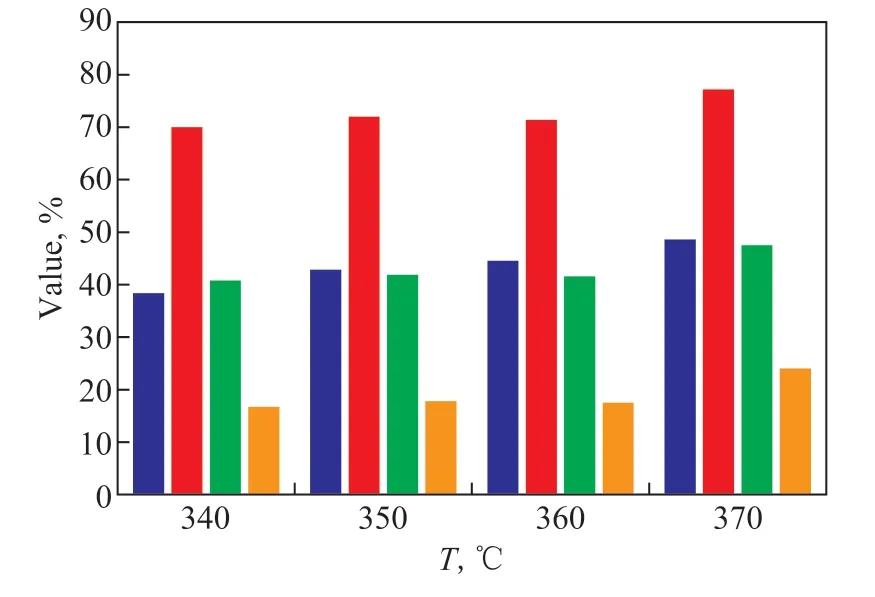
Figure 10 Effect of temperature on reaction of hydrotreated 1-MN
4.3 Effect of hydrogen pressure on product distribution
The effect ofPH2was evaluated in the range of 3.0 -4.5 MPa, whereas the temperature hydrogen flow rate and LHSV were 370 °C, 300 mL/min, and 6.0 h−1,respectively. The main product distribution is shown in Figure 11.

Figure 11 Effect of PH2 on hydrocracking product distribution
From the perspective of kinetics and thermodynamics,a high hydrogen partial pressure is beneficial to the saturation conversion of tetralin to DN. As shown in Figure 11, the content of DN substantially increased with the increase of hydrogen partial pressure.
The effect of hydrogen pressure on hydrocracking process is rather complex, and the macroscopic performance can be described as follows: 1) Zeolite and γ-Al2O3constitute the catalyst support, which favors the carbocation mechanism and β-scission reaction. With the increase in hydrogen pressure, the coke deposit on catalyst decreased,and a high cracking activity can be maintained. 2) Within a certain range, the solubility of H2in the liquid phase changes linearly with hydrogen pressure, which can reduce the effect of external diffusion and provide active hydrogen for catalyst. As shown in Figure 11, the content of AB, LAB, and SABs in cracking product obviously follows the same trend as hydrogen pressure, while the indane content shows an opposite trend.

Figure 12 Effect of PH2 on the reaction of hydrotreated 1-MN
As shown in Figure 12, the trend of tetralin conversion is opposite to that of S-iso in the range of experiments.Moreover, theS-ring opening andS-side breaking changed slightly. On one hand, a high hydrogen pressure was unfavorable to the classical hydrocracking path.On the other hand, it was obviously beneficial to the saturation of isomerization and ROPs. The change trend of reaction selectivity was affected by two aspects, so there is no obvious change in S-ring opening and S-side breaking. The enhanced amount of ROP and SCCP products can be considered to be directly related to the improvement of tetralin conversion[19].
Compared with the isomerization path, hydrogenation route is more sensitive to hydrogen pressure. As 3 mol H2participates in the reaction during the hydrogenation of tetralin to DN, the increase in hydrogen pressure is beneficial to the formation of DN. When PAHs are present in the system, it can be predicted that the effect of hydrogen pressure will be more complex[20].
5 Conclusion
(1) Based on the study of bicyclic aromatics in hydrocracking reaction, a detailed pathway is proposed by combining a novel product analysis and molecular simulation. The results of molecular simulation confirmed that the isomerization of tetralin to indane occurs prior to the ring-opening process, and the order of activation energy is isomerization > ring-opening ≈ side-chain cleavage, indicating that the isomerization path is the reaction-controlling step.
(2) The LHSV, hydrogen pressure, and temperature parameters were systematically studied. Tetralin tends to isomerize and open as the reaction progresses.The selectivity of alkylbenzene can be improved by monomolecular reaction. A high temperature is beneficial to the isomerization, ring-opening, and sidechain cleavage of tetralin. An increase in hydrogen pressure in the hydrocracking section slightly affects the isomerization and ring-opening selectivity of tetralin,but it is obviously conducive to further hydrogenation of tetralin to DN.
Acknowledgement:This work was financially supported by the SINOPEC Science and technology Development Funds (No.12005-1) and the Hydrogenation Process and Hydrogenation Catalyst Laboratory (RIPP, SINOPEC). All the staff in our laboratory provided support in the process study and simulation calculation.
- 中国炼油与石油化工的其它文章
- Amorphous Catalysts for Electrochemical Water Splitting
- Upgradation of Heavy Crude Oil Via Hydrodynamic Cavitation Through Variations in Asphaltenes
- Wet Desulfurization of High-Sulfur Petroleum Coke Improved via Pre-calcination, H2O2 Treatment, and Ultrasound
- Removal of Heteroaromatic Sulfur Compounds by a Non-noble Metal Fe Single-atom Adsorbent
- A High-Performance Composite Epoxy Coating Based on the Cooperative Enhancement of 2D Co2(OH)2BDC and Electrospun PAN Nanofiber Membrane
- Influence of Ethanol Addition on the Spray Auto-ignition Properties of Gasoline and Its Relationship with Octane Number

