Removal of Heteroaromatic Sulfur Compounds by a Non-noble Metal Fe Single-atom Adsorbent
Lü Yanjun; Wen Jie; Gong Qinmei; Zhang Lianhong; Li Airong;Arshid Mahmood Ali; Zhang Hui
(College of Chemistry and Chemical Engineering, Southwest Petroleum Uniνersity, Chengdu 610500)
Abstract: Sulfur-containing compounds (SCCs) must be removed from fuels before use. In this study, a novel non-noble metal Fe single-atom adsorbent (SA-Fe/CN) was synthesized using a core-shell strategy and applied for the adsorptive removal of benzothiophene (BT) and dibenzothiophene (DBT). The adsorption isotherms, thermodynamics, kinetics, and adsorption-regeneration cycles of DBT and BT on SA-Fe/CN were studied. SA-Fe/CN exhibited a significant capacity to adsorb DBT, and the isothermal equilibrium was well described by the Langmuir isotherm. The Gibbs free energy values were negative (ΔG0 <0), indicating that the adsorption of DBT and BT was favored and spontaneous. The adsorption process conformed to the pseudo-second-order kinetic model with high R2 values (0.9994, 0.9987). The adsorption capacity of SA-Fe/CN for DBT and BT reached 163.21 mg/g and 90.35 mg/g, respectively, due to the highly active sites of the single atom and electrostatic interaction with the sulfide. Therefore, SA-Fe/CN may be a promising adsorbent for SCC removal.
Key words: single-atom adsorbent; adsorptive desulfurization; thermodynamics and kinetics; heteroaromatic sulfur compounds
1 Introduction
Sulfur oxides (SOx) are produced from sulfur-containing compounds (SCCs) in fuels during the combustion process, which not only has a pernicious impact on the catalyst activity in catalytic converters, but also results in environmental problems such as acid rain and smog[1-2].To overcome these negative effects, deep desulfurization of fuels for the preparation of oil-based products has attracted much attention worldwide[3]. It is necessary to find various effective methods to realize simple and rapid desulfurization[4-5]. At present, various desulfurization technologies have been developed to remove the inactive sulfur, including extractive desulfurization(EDS)[2,6], bio-desulfurization (BDS)[7-8], oxidative desulfurization (ODS)[9-10], adsorptive desulfurization(ADS)[11-13], and hydrodesulfurization (HDS)[4,14].HDS, as a conventional desulfurization technology used industrially, is very effective in removing low molecular weight sulfur compounds such as thioether and thiols[4,15-17]. However, HDS cannot efficiently remove thiophene-containing compounds, is conducted at high temperature and pressure, and consumes expensive hydrogen[4,18]. To overcome the above limitations, ADS technology has received widespread attention owing to its simple operation, rapid reaction, and environmentally friendly nature[19-21]. The selective removal of SCCs has been explored in the presence of a wide variety of adsorbents, including activated carbon sorbents[22-24],zeolite[25-27], silica-based sorbents[28-30], metal-organic frameworks[12,31-32], and metal adsorbents (reduced metals and metal oxides)[10,33-34]. Dasgupta et al.[15]investigated the ADS process for refinery diesel based on NiMCM-41 and NiY adsorbents and found that the sulfur concentration was reduced from 450 μg/g to <50 μg/g in real refinery diesel under optimized conditions. Huo et al.[35]successfully prepared CuZn@C bimetallic adsorbents for ADS by a carbonization auto-reduction strategy. The results indicated that the bimetallic active sites were uniformly distributed on the surface of CuZn@C with a good porous structure, andan adsorption capacity for dibenzothiophene (DBT) of up to 60 mg/g. Subhan et al.[36]successfully synthesized highly dispersed La2O3over mesoporous silica, which made the desulfurization capacity higher for thiophene.Among these sorbents, LaAS-20 could capture 0.12 mmol thiophene per gram and retained good regeneration ability after five cycles.
Iron, a non-noble metal material with abundant reserves,is applied for adsorption desulfurization. Bandosz et al.[37]investigated the adsorption desulfurization of activated carbons with iron (C-Fe) deposited on their surfaces, and found that C-Fe exhibited a capacity of 25.13 mg/g for DBT removal. Danmaliki et al.[38]reported that activated carbon was loaded with cerium and iron (AC/Ce/Fe) to achieve adsorption desulfurization, and the adsorption capacities of AC/Ce/Fe for DBT and benzothiophene(BT) reached 26.63 mg/g and 7.23 mg/g, respectively.Louis et al.[39]developed Fe3O4nanoparticles supported on activated carbon (Fe@AC-2) and used this to remove thiophene, BT, and DBT. The results demonstrated that Fe@AC-2 could effectively remove DBT and the maximum capacity of 29 mg/g. Although the adsorptive capacity for different sulfides could be improved in the presence of Fe, the pores of activated carbon were blocked and the available volume of micropores was reduced. To solve the problem of blocked pores in Febased adsorbents and enhance their adsorption capacity,the development of highly dispersed iron single-atom materials has an important effect on SCC.
Metal single-atom materials can achieve ultimate atomic utilization efficiency and maximize catalytic activity by reducing existing metals to the single atom level to expose isolated single atomic sites and improve the surface area and active sites of adsorbents[40]. In addition,the active sites in single-atom materials are distributed in a positive valence state, which is more conducive to the adsorption of negatively charged sulfides[41]. In this study, a novel non-noble metal Fe single-atom adsorbent was synthesized according to the core-shell strategy,and was used to investigate the adsorption process of representative thiophene compounds, such as DBT and BT. This work provides a basis for the development of adsorption materials for the application of desulfurization of oil-based products.
2 Experimental
2.1 Reagents
All chemical reagents used in this study were of analytical grade and required no further purification.Iron (III) nitrate (Fe(NO3)3·9H2O), sodium hydroxide(NaOH), ethanol, n-octane, and hydrochloric acid (HCl,36 wt.%) were purchased from Chengdu Kelong Reagent Factory. Dopamine hydrochloride (PDA-HCl) and tris(hydroxymethyl)aminomethane were provided by Adamas Reagent Co., Ltd. and Aladdin Reagent Co., Ltd.,respectively. DBT and BT were purchased from Macklin Reagent Co., Ltd.
2.2 Preparation of adsorbent
2.2.1Synthesis ofα-FeOOH nanorads
α-FeOOH nanorods were synthesized using a hydrothermal method. In a typical procedure, 2.02 g Fe(NO3)3·9H2O (5 mmol) was dissolved in 30 mL deionized water to form a transparent solution. Then,1.2 g NaOH was dissolved in 30 mL deionized water and a yellow suspension was formed by the addition of the above Fe(NO3)3solution. After stirring for 30 min,the obtained suspension was transferred into a 50 mL autoclave and hydrothermally reacted at 180 °C for 6 h. When the autoclave was naturally cooled to room temperature, the formed yellow sample was collected by centrifugation, washed three times with deionized water and ethanol, and dried at 80 °C in a vacuum oven. Finally,the α-FeOOH nanorods were obtained.
2.2.2Synthesis of SA-Fe/CN
SA-Fe/CN was prepared by a metal (hydr)oxide@polymer core-shell strategy, which was adopted from Zhang et al.[42]with minor modifications. Typically, a fixed 2:1 ratio of pre-synthesized α-FeOOH (500 mg) and dopamine-HCl (250 mg) was homogeneously dispersed in 100 mL freshly prepared Tris buffer solution (10 mM) using ultrasound and then stirred for 72 h at room temperature. Next, the obtained products were collected by centrifugation, washed three times with deionized water and ethanol, and dried at 80 °C in vacuum oven.The attained α-FeOOH@PDA was annealed at 500 °C for 2 h in an Ar atmosphere (heating rate of 5 °C/min).The temperature was increased to 700 °C at a heating rate of 5 °C/min and the system was maintained at this temperature for 2 h. During this time, the PDA layer was decomposed and carbonized at high temperatures to form a hollow nitrogen-doped carbon (CN) shell, and α-FeOOH was reduced into Fe atoms. A strong interaction was formed between the CN shell and Fe atoms, which caused the isolated Fe atoms to be firmly anchored in the CN shell. To purify the samples, the unstable species were etched with HCl for 12 h. Finally, the above iron simple atoms (SA-Fe/CN) were filtered, washed with deionized water until the solution filtrate became neutral, and dried in a vacuum oven at 80 °C.
2.3 Structural characterization
To verify theSA-Fe/CN structure, the powder X-ray diffraction (XRD) patterns were obtained using a PANalytical X’Pert Pro MPD Phaser X-ray diffractometer with high-intensity Cu Kα radiation as the excitation source (2θ= 10°-80° andλ= 1.5406 Å). The surface morphology of the adsorbent was studied using a FEI Quanta 650FEG scanning electron microscope (SEM)and a JEM-2100F transmission electron microscope(TEM) operated at 100 kV. High-angle annular dark-field scanning transmission electron microscopy (HADDFSTEM) was performed using a JEOL-TEM operated at 200 kV for further characterization of the dispersion of single-atom materials. The Fe content was determined by inductively coupled plasma optical emission spectrometry(ICP-OES).
2.4 Determination of adsorption isotherms and kinetics
The ADS experiments of simulated fuel compounds (BT and DBT) were conducted in a 20 mL sealed reaction vessel with magnetic stirring in a thermostatic water bath.A stock solution containing 800 μg/g of each compound(prepared by using 0.59 g of BT and 0.82 g of DBT in 250 mL ofn-octane) was diluted further inn-octane to obtain concentrations in the range from 200 μg/g to 800 μg/g. In an adsorption isotherm experiment, 5 mL each of 200 μg/g, 400 μg/g, 600 μg/g, and 800 μg/g BT and DBT was added to 0.010 g of adsorbent contained in 20 mL sealed reaction vessel, and stirred for 2 h until adsorption equilibrium was reached. Then, a certain amount of the adsorbed sample was taken out and analyzed by using gas chromatography (GC, SP-6890) equipped with a flame ionization detector (FID). For the adsorption kinetics assay, 0.010 g SA-Fe/CN and 5 mL of 800 μg/g model fuel (BT or DBT) were mixed in a 20 mL sealed glass tube under constant stirring in a thermostatic water bath for 2 h at 313 K. At the same time, the concentration of adsorbate was also analyzed by GC. The equilibrium adsorption capacity (qe) of the adsorbent were calculated by Equation (1)[43]:

whereC0is the initial concentration of the adsorbate(μg/g),Ceis the equilibrium concentration of the adsorbate (μg/g),Vis the volume of the model fuel (L),ρis the density ofn-octane (kg/m3),mis the mass of the adsorbent (g), andqeis the equilibrium adsorption capacity of the adsorbent (mg/g).
2.5 Regeneration experiment
The performance of adsorption-regeneration and reusability is an important factor for evaluating adsorbents. Thermal regeneration method was employed in this paper. The regeneration of the SA-Fe/CN was evaluated after saturation of adsorbed DBT at 20 ℃and BT at 40 ℃. Spent SA-Fe/CN in DBT and BT was extracted in acetonitrile at room temperature.Subsequently, the extracted sample was dried at 20 ℃for 12 h. Then, the treated sample was pyrolyzed under an Ar flow at 550 °C for 2 h. The regenerated SA-Fe/CN was reused to adsorb DBT and BT under identical conditions.
3 Results and Discussion
3.1 Characterization of SA-Fe/CN
The XRD pattern of SA-Fe/CN exhibits a single broad peak in the 2θrange of 15°-30° assigned to the (002)plane of graphitized carbon, as shown in Figure 1. The stacks of rod-shaped well-dispersed crystals with lengths and diameters of 200 nm and 50 nm were observed in the TEM images, as shown in Figure 2(a). As shown in Figure 2(b), a uniform PDA coating layer was formed on the surface of α-FeOOH nanorods. In Figure 2(c), the coating layer (CN) was uniformly coated on the surface of the nanorods after annealing and Fe species weregenerated. In addition, SEM was used to confirm that the morphology of α-FeOOH@PDA was not significantly changed before and after the annealing process, as shown in Figure 3. It was shown from the HAADF-STEM images (Figure 4(a)) that a uniform CN layer was formed on the surface of the nanorods and Fe species were exposed on the inner wall of it. A hollow tube with a shell thickness of 5 nm and a length range of 200-250 nm was constructed, as illustrated in Figure 4(b). In Figure 4(c),the elemental distribution of SA-Fe/CN was analyzed by using EDX mapping, which revealed that C, N, and Fe were the major elements and were homogeneously distributed along with rod-like morphology. The Fe content, measured by ICP-OES, was approximately 0.3%.To identify and characterize the dispersion of Fe in SAFe/CN, AC HAADF-STEM analysis was employed. The presence of high-density single atoms Fe (bright dots marked with yellow circles) can be seen in Figure 4(d).According to the above characterization, the single-atom adsorbent has been successfully synthesized.

Figure 1 XRD patterns of SA-Fe/CN, α-FeOOH@PDA,and the standard PDF #81-0463, respectively

Figure 2 TEM images of (a) rod-shaped α-FeOOH, (b) α-FeOOH@PDA, and (b) annealed α-FeOOH@PDA

Figure 3 SEM images of (a) α-FeOOH@PDA and (b) annealed α-FeOOH@PDA
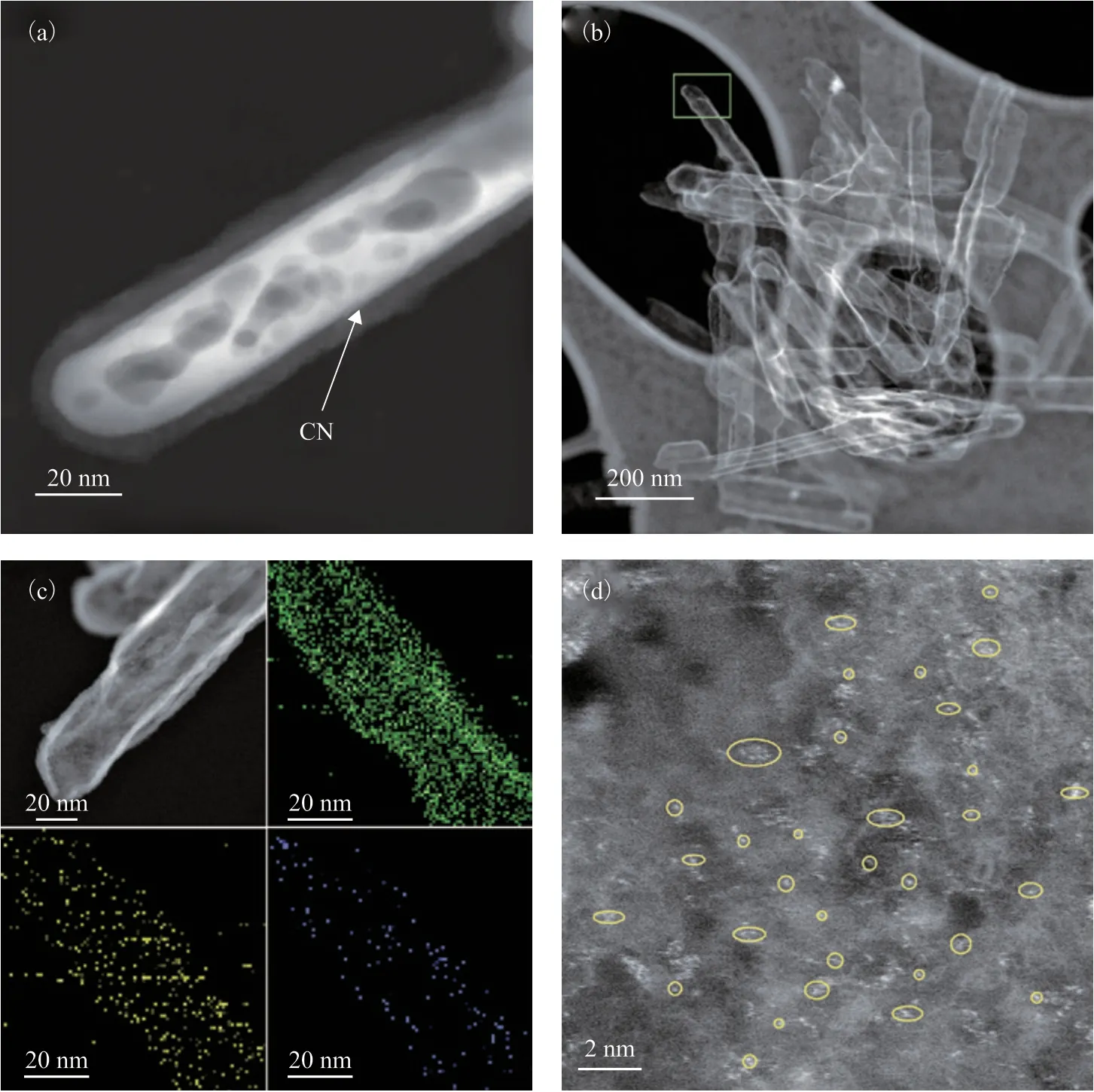
Figure 4 HAADF-STEM images of (a) annealed α-FeOOH@PDA and (b) SA-Fe/CN. (c) EDX mapping image of SA-Fe/CN.(d) AC HADDF-STEM image of SA-Fe/CN
3.2 Adsorption isotherms
To understand the adsorption mechanism, various isotherm models, such as the Langmuir isotherm and Freundlich isotherm, have been widely used to study adsorption equilibria[40,41,43]. The Langmuir adsorption isotherm is based on a monolayer adsorption model,which means that surface properties of the adsorbent are uniform[44]. Therefore, a maximum adsorption capacity of the adsorbent is attributed to the finite number of adsorption sites. The nonlinear and linear forms of the Langmuir isotherm equations are as follows[45-47]:


whereqeis the equilibrium adsorption capacity of the adsorbent (mg/g),Ceis the equilibrium concentration of adsorbate (mg/L),qmis the maximum adsorption capacity of the adsorbent (mg/g), andKLis the Langmuir adsorption constant (L/mg)[48-50].
In comparison with the Langmuir adsorption isotherm,Freundlich adsorption isotherm is an empirical model that is assumed to have heterogeneous adsorption owing to the diversity of adsorption sites[51]. The nonlinear and linear forms of the Freundlich isotherm equations are obtained from Equations (4) and (5):

whereKFis a constant related to the adsorption capacity(mg/g)·(mg/L)nandnis the adsorption intensity, which represents the degree of deviation from linear adsorption.In general, 1/n< 1, 1/n= 1, and 1/n> 1 show that the adsorption is a physical nonlinear, linear, and chemical nonlinear process, respectively[49,52-53]. The fitting parameters for both BT and DBT adsorbed on SA-Fe/CN at different adsorption temperatures (293 K, 303 K,and 313 K) are presented in Tables 1-3, respectively. The adsorption mechanism was physical for both DBT and BT onto SA-Fe/CN, as shown by the 1/n values.
The adsorption capacity of the SA-Fe/CN adsorbent for both DBT and BT in the Langmuir isotherm model was higher than that of the Freundlich isotherm model at all three different temperatures with higherR2values. Based on the Langmuir isotherm model, the adsorption process was monolayer adsorption. Once an active site is filled,it is not replaced by further adsorption. The maximum adsorption capacity was obtained when the adsorption reached a saturation point. Therefore, the maximum adsorption capacities of SA-Fe/CN for DBT and BT were 221.16 mg/g at 293 K and 137.46 mg/g at 313 K,respectively. Based on the Freundlich isotherm model,the Freundlich constantKFcan be used to represent the adsorption ability of the adsorbent for the adsorbate.The adsorption intensity becomes greater asKFvalue increases[54].
3.3 Adsorption thermodynamics
To reveal the inherent energetic changes in the adsorption process, the thermodynamic parameters (ΔG0, ΔH0,ΔS0) of adsorption were calculated from the following equations[55-58]:

where ΔG0is the Gibbs free energy change of adsorption(kJ/mol),Ris the universal gas constant (8.314 J/(mol/K)),T is the absolute temperature (K),Kis the thermodynamic equilibrium constant, ΔH0is the standard enthalpy change of adsorption (kJ/mol), and ΔS0is the standard entropy change of adsorption (J/mol/K). The value ofKdepends on ΔH0and ΔS0in the adsorption process, and its change over temperature was calculated according to Equation(7). The apparent equilibrium constant (Ke) is the ratio of the quantity of adsorbed DBT and BT to the concentration of DBT and BT in the solution at equilibrium[48,59].

The values of K at various temperatures were calculated by computing the Keat differentC0and extrapolating it to zero through a figure ofqe/Cevs.C0[48,60-61].The values of ΔG0in the adsorption process were determined by Equation (6). The ΔG0andKvalues for DBT and BT at the three studied temperatures are listed in Table 4. It should be noted that the values ofKdecreased as the adsorption temperature increased for DBT. Nevertheless, for BT, theKvalues increased as temperature increased. The overall negative values of ΔG0indicated the feasibility and spontaneity of the adsorption for both DBT and BT on SA-Fe/CN at various reaction temperatures. Moreover, ΔG0decreased with an increase in temperature from 293 K to 313 K, which revealed that the driving force for the adsorption of DBT and BT increased as the temperature increased. Similar results were found in the literature[58,62-63].
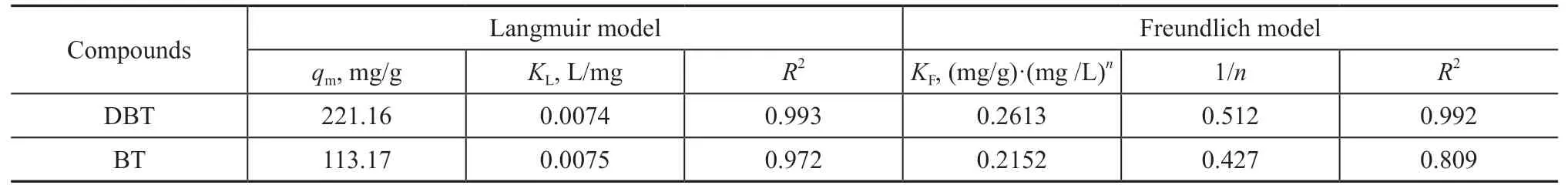
Table 1 Langmuir and Freundlich isotherms fitting parameters of SA-Fe/CN (T = 293 K)

Table 2 Langmuir and Freundlich isotherm fitting parameters of SA-Fe/CN (T = 303 K)
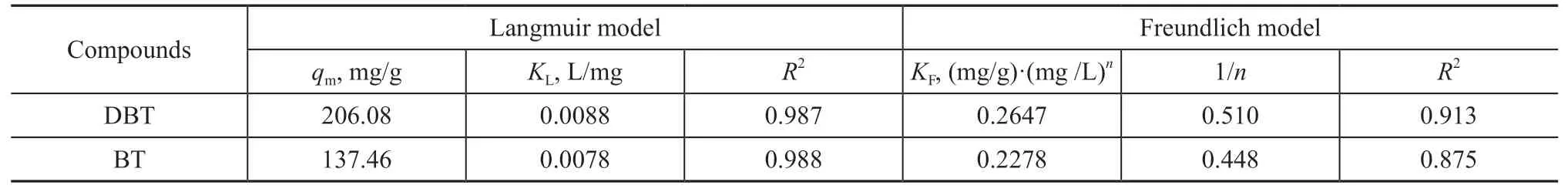
Table 3 Langmuir and Freundlich isotherm fitting parameters of SA-Fe/CN (T = 313 K)

Table 4 Summary of ΔG0 for DBT and BT adsorption onto SA-Fe/CN adsorbent at different temperatures
The linear relationship between ln K and 1/T for DBT and BT is shown in Figure 5. The values of ΔS0and ΔH0could be calculated from the slope and intercept of the linear relationship; the results are listed in Table 5. The adsorption of DBT on SA-Fe/CN was an exothermic process and a relatively low temperature contributed to the adsorption of DBT due to the negative value of ΔH0(ΔH0<0). As indicated by the negative ΔH0value, its adsorption on the surface of SA-Fe/CN was more ordered due to the larger molecules of DBT[48]. In contrast, the ΔH0value for BT was positive, confirming that the adsorption was an endothermic process and became more favorable as the adsorption temperature increased.

Figure 5 Plot of (ln K ) vs (1/T ) for DBT and BT (catalyst weight: 10 mg SA-Fe/CN, reaction time: 2 h, adsorbate concentration: 800 μg/g)
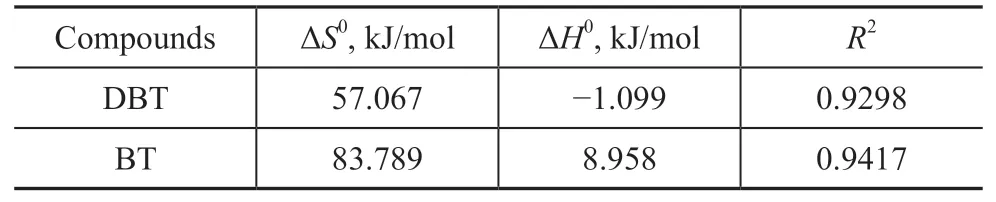
Table 5 Enthalpy and entropy for the adsorption of DBT and BT onto SA-Fe/CN
3.4 Adsorption Kinetics
The effect of contact time on the adsorption performance for both DBT and BT at 293 K is shown in Figure 6. The adsorption capacity gradually increased as the reaction time increased, with saturation was achieved at 120 min for both DBT and BT adsorption. The occupation of available active sites by sulfur compounds made it difficult to adsorb more SCCs; hence, the adsorption equilibrium was reached[48]. It also could be clearly seen that the maximum adsorption of DBT and BT were approximately 163.21 mg/g and 90.35 mg/g, respectively.The comparison of the adsorption capacity of Fe-based adsorbents for DBT and BT is presented in Table 6. The adsorption capacities of SA-Fe/CN for DBT and BT removal are higher than in the reported work. Therefore,SA-Fe/CN may be used as a potential adsorbent for ADS.To better elucidate behavior during adsorption, frequently used kinetic models, including the pseudo-first-order model and the pseudo-second-order model, have been applied to investigate and describe the adsorption of DBT and BT onto SA-Fe/CN[49,52,70]. The Lagergren pseudofirst-order kinetic model is described by the following equation[44,49,71]:
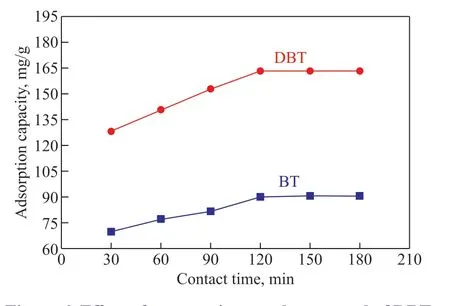
Figure 6 Effect of contact time on the removal of DBT and BT (catalyst weight = 10 mg SA-Fe/CN, reaction temperature = 293 K, reaction time = 3 h, adsorbate concentration = 800 μg/g)

Table 6 Comparison of the adsorption capacity of Fe-based adsorbent for sulfides

After integrating Equation (9) using the boundary conditionst= 0 tot=tandqt= 0 toqt=qton the integrated form, the expression becomes:

The pseudo-second-order model is given by the following expression:

By integrating equation (11), the expression simplifies to:

whereqeis the amount of adsorbate at equilibrium (mg/g),qtis the concentration of adsorbate at timet(mg/g),k1is the constant of the pseudo-first-order kinetic equation(min−1), andk2is the constant of the pseudo-second-order kinetic equation ((g/mg)/min).
The values of the parameters obtained from the fitting of the experimental data to the pseudo-first-order and pseudo-second-order kinetic models are presented in Table 7. The pseudo-second-order model was better and superior to the pseudo-first-order model, with higher values ofR2. Moreover, the adsorption rate constant (k2)of DBT was higher than that of BT, which might arise from the larger molecules of DBT[48]. Therefore, the adsorption of DBT and BT onto SA-Fe/CN conformed to the pseudo-second-order kinetic model, which is similar to the results in the literature[44,49,72].

Table 7 Modeled kinetic rate constants for the adsorption of DBT and BT
3.5 Regeneration of SA-Fe/CN
The regeneration of synthesized materials is one of the major factors influencing the real-world application of the adsorption process[73]. Figure 7 shows the adsorption of DBT and BT on virgin SA-Fe/CN and thermally regenerated SA-Fe/CN over three cycles. The adsorption capacity of SA-Fe/CN for DBT and BT decreased was lower than the initial adsorption capacity after the catalyst was regenerated three times. The decrease in adsorption capacity may be due to the strong interaction between adsorbed sulfides and SA-Fe/CN, which also may be because DBT and BT were not completely removed in the pyrolyzing process. These reasons lead to a reduction in the number of active sites on SA-Fe/CN, consequently reducing the saturated adsorption capacity of SA-Fe/CN.

Figure 7 Effect of regeneration times on DBT and BT adsorption on SA-Fe/CN
4 Conclusions
This work reports the successful application of a novel synthetic SA-Fe/CN for ADS. The adsorption isotherms,thermodynamics, kinetics, and adsorption-regeneration of SCCs, such as DBT and BT, on SA-Fe/CN were also investigated. SA-Fe/CN exhibited a significant adsorption performance to DBT and BT during the adsorption process, which adsorption capacity for DBT and BT reached 163.21 mg/g and 90.35 mg/g, respectively. In the study of the adsorption thermodynamics, the negative ΔG0(ΔG0<0) values at the different temperatures analyzed indicated that the adsorption of DBT and BT using SAFe/CN was spontaneous. The adsorption of DBT on SAFe/CN was an exothermic process and a relatively low temperature contributed to the adsorption of DBT owing to the negative value of ΔH0(−1.099). In contrast, ΔH0for BT was 0.9417, which showed that the adsorption of BT was endothermic. The isothermal equilibrium was well described by the Langmuir isotherm model, and monolayer adsorption onto SA-Fe/CN occurred. The adsorptionkinetics study results showed that the adsorption of DBT and BT onto SA-Fe/CN followed pseudo-second-order kinetics, with high values ofR2(0.9994, 0.9987). Thermal regeneration experiments performed on SA-Fe/CN showed that the good adsorption capacity for DBT and BT onto SA-Fe/CN was maintained after three cycles. Therefore,progress on such non-noble metal adsorbents for use in the desulfurization of hydrocarbon fuels may drastically reduce refinery operating costs and downsize vehicle catalytic converters in the future.
- 中国炼油与石油化工的其它文章
- Amorphous Catalysts for Electrochemical Water Splitting
- Tetralin Hydrocracking Reaction Network to Single-Ring Aromatics on Bifunctional Catalysts
- Upgradation of Heavy Crude Oil Via Hydrodynamic Cavitation Through Variations in Asphaltenes
- Wet Desulfurization of High-Sulfur Petroleum Coke Improved via Pre-calcination, H2O2 Treatment, and Ultrasound
- A High-Performance Composite Epoxy Coating Based on the Cooperative Enhancement of 2D Co2(OH)2BDC and Electrospun PAN Nanofiber Membrane
- Influence of Ethanol Addition on the Spray Auto-ignition Properties of Gasoline and Its Relationship with Octane Number

