Production of functional sperm from in vitro-cultured premeiotic spermatogonia in a marine fish
Hong Zhang ,Wan-Wan Zhang ,Cheng-Yu Mo ,Meng-Dan Dong ,Kun-Tong Jia ,Wei Liu,* ,Mei-Sheng Yi,*
1 School of Marine Sciences, Sun Yat-Sen University, Guangzhou, Guangdong 510275, China
2 Southern Marine Science and Engineering Guangdong Laboratory (Zhuhai), Zhuhai, Guangdong 519000, China
3 Guangdong Provincial Key Laboratory of Marine Resources and Coastal Engineering, Guangzhou, Guangdong 510000, China
ABSTRACT In vitro production of functional gametes can revolutionize reproduction by reducing generation intervals and accelerating genetic breeding in aquaculture,especially in fish with relatively long generations.Nevertheless,functional sperm production from in vitro-cultured spermatogonia remains a challenge in most aquaculture fish.In this study,we isolated and characterized premeiotic spermatogonia from marine four-eyed sleepers(Bostrychus sinensis),which are prone to ovotesticular or sterile testicular development,and induced the differentiation of the spermatogonia into flagellated sperm in a three-dimensional (3D) culture system.Artificial insemination indicated that the in vitro-derived sperm were capable of fertilizing mature oocytes to develop into normal larvae.Furthermore,melatonin significantly promoted spermatogonia proliferation and differentiation through the ERK1/2 signaling pathway,and thus increased the efficiency in functional sperm production.The 3D culture system and resulting functional sperm hold great promise for improving the genetic breeding of aquaculture fish.
Keywords:In vitro spermatogenesis;3D culture model; Spermatogonia; Four-eyed sleeper;Melatonin;Genetic breeding
INTRODUCTION
Commercial aquaculture has made enormous contributions to global food production,especially high-quality fish protein (Gui et al.,2022;Mei &Gui,2015;Naylor et al.,2021).Over the past decade,innovations in biotechnology have driven major advances in the molecular and genetic improvement of economically important traits in fish,leading to a flourishing aquaculture seed industry (Gui et al.,2022;Houston et al.,2020).Despite rapid development,the insufficient supply of good-quality seeds,especially for species with relatively long generations,remains a considerable obstacle in the sustainable development of fish aquaculture (Zhang et al.,2019,2021a).Advanced germline manipulation biotechnologies,such as surrogate broodstock andin vitroproduction of functional gametes,may serve as potential strategies to accelerate genetic breeding by reducing generation intervals of target species,such as salmon,black carp,grouper,sturgeon,and grass carp (Goszczynski et al.,2019;Gui et al.,2022;Zhang et al.,2021a).Functional donorderived gametes have been generated by surrogate broodstock in salmon,rainbow trout,flounder,and turbot(Okutsu et al.,2006,2007;Takeuchi et al.,2004;Zhou et al.,2021).Nevertheless,anin vitroculture system to produce functional sperm from germ stem cells in aquaculture fish has not yet been reported.
Spermatogenesis is a long,complex,and sequential process during which spermatogonial stem cells (SSCs)proliferate and differentiate into functional sperm (Schulz et al.,2010;Subash &Kumar,2021).Spermatogenesis includes self-renewal of SSCs,transformation of SSCs into differentiated spermatogonia,and meiosis events to produce functional sperm (Kawasaki et al.,2016;Xie et al.,2020).The fate of SSC self-renewal or differentiation is fine-tuned by intrinsic signals within cells and extracellular signals from the germ cell niche,such as cell-cell communication,endocrinal factors,and extracellular matrix (ECM) (Mäkelä &Hobbs,2019;Xie et al.,2020).In vitroculture systems provide the opportunity to explore the genetic and environmental factors affecting germ cell development and the regulatory mechanisms underlying gametogenesis.Many culture systems have been developed to mimic the germ cell niche and provide various supplementary factors to produce haploid sperm from SSCs (Xie et al.,2020).In mice,in vitro-derived gametes from pluripotent stem cells have been established by stepwise differentiation after exposure to morphogens and hormones in co-culture systems (Hamazaki et al.,2021;Hayashi et al.,2011;Ishikura et al.,2021;Zhou et al.,2016).In the teleost medaka (Oryzias latipes),long-term cultured SSCs are capable of differentiation into motile sperm (Hong et al.,2004).However,stable SSC lines in other fish species have not been reported due to the lack of an efficient culture system supporting continuous proliferation of SSCsin vitro(Kawasaki et al.,2012;Lacerda et al.,2010;Shikina et al.,2008).Various culture systems applied in mammals and birds have been adopted to test if they supportin vitrodifferentiation of fish germ cells from disassociated testicular tissue containing SSCs.In medaka,round spermatids have been generated from the division of meiotic spermatocytes without direct contact with somatic cells (Saiki et al.,1997).In zebrafish,functional sperm have been generated via coculture of early stage spermatogonia and Sertoli-like cells in adherent culture systems (Kawasaki et al.,2016;Sakai,2002).In the cyprinid honmoroko (Gnathopogon caerulescens),functional sperm have been generated from disassociated testicular cells in suspension culture (Higaki et al.,2017).The testis can be viewed as a special type of threedimensional (3D) structure.In this context,3D models have been widely exploited to enhance communication between SSCs and scaffold material to promote SSC adhesion,proliferation,and differentiation.3D culture systems have been applied for spermatocytes and SSC differentiation in mammals (Lee et al.,2006).Furthermore,using different 3D culture methods and material,spermatozoa have been successfully generated from murine premeiotic male germ cells (Abu Elhija et al.,2012),rat spermatogonia (Reda et al.,2016),and cells isolated from the seminiferous tubules of azoospermia patients (Mohammadzadeh et al.,2019).In contrast,3D models for fish germ cell culture have not yet been reported.Therefore,establishing a culture system for the differentiation of male germ cells into functional sperm in aquaculture fish remains a key challenge.
Four-eyed sleepers (Bostrychus sinensis),a burrowing fish species long regarded as a promising nutritious food for accelerating wound healing after surgery,are widely cultured in China and Southeast Asia (Dong et al.,2021).In culture,about 10% of males develop infertile or ovotesticular gonads(Hong et al.,2006),leading to the loss of germ resources.In this study,we established a 3D culture system for four-eyed sleepers in which functional sperm were generated from premeiotic spermatogonia.We identified melatonin as a key factor in promoting functional sperm production and demonstrated that melatonin acts through extracellular signalregulated kinase 1/2 (ERK1/2) signaling.
MATERIAL AND METHODS
Ethics statement
All procedures using four-eyed sleepers were approved by the Ethics Committee of Sun Yat-Sen University(2018YFD0901205).
Fish and in vitro fertilization assay
Five-month-old and one-year-old four-eyed sleepers (over 1 000 individuals) were provided by the Longhai Ruiquan Aquaculture Cooperation Group (Fujian,China).The fish were maintained in circle tanks containing 500 L of filtrated seawater (salinity=12‰,oxygen level=3 mg/L) at 28 ℃ under a 14 h/10 h light-dark photoperiod.The fish were fed Chironomidae larvae twice a day.
During the breeding season,four-eyed sleepers (n=20)were selected and injected with 13 μg/kg luteinizing hormone releasing hormone A2 (LHRHA2,110 252 087,Shusheng,China) and maintained in the dark for 72 h.The fish were reinjected with 5 000 units/kg human chorionic gonadotropin(hCG,110 251 282,Shusheng,China) and 13 μg/kg LRHA2(Liu et al.,2019).After the second injection,spawned eggs were collected forin vitrofertilization with fresh sperm andin vitro-derived sperm,respectively.All experiments were performed independently and repeated at least five times.
Immunofluorescence
Testes or spherical clumps were fixed in 4% (w/v)paraformaldehyde (PFA) for 2 h,washed in phosphatebuffered saline (PBS) three times (5 min each),immersed in 25% sucrose,and embedded in frozen section compound (4 853,Sakura,Japan).Samples were sectioned at 10 μm with a cryostat (CM1900,Leica,Germany).Immunofluorescence was performed as described previously (Xu et al.,2005).Briefly,slides were air dried at 37 °C for 1 h,rehydrated in PBS (3×5 min),blocked in 4% normal goat serum for 30 min,then incubated with anti-Vasa antibody (1:400;ab209710,Abcam,UK) at 4 °C overnight.Residual antibodies were washed in PBS (3×10 min),then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit immunoglobulin G (IgG) (H+L) (1:400;A11070,Invitrogen,USA) and 6-diamidino-2-phenylindole (DAPI,1 μg/mL diluted in PBS) for 1 h at room temperature.Images were captured using a Zeiss confocal laser scanning microscope (LSM800,Zeiss,Germany).
Isolation of spermatogenic cells
Testes were dissected from 5-month-old males,sterilized with 70% ethanol for 30 s,washed in PBS (3×5 min),and minced in PBS containing 200 U/mL penicillin-streptomycin (15 140-122,Gibco,USA).The minced testes were digested in Leibovitz’s L-15 medium (41 300 070,Gibco,USA)supplemented with 4 mg/mL collagenase type IV (17 104-019,Gibco,USA),10% fetal bovine serum (FBS) (10 099 141,Gibco,Australia),0.25% trypsin (T6325,Macklin,China),and 0.05% DNase I (D807125,Solarbio,China) at 37 °C for 1 h.After digestion,the cells were filtered with a 75 μm nylon mesh filter (YA0949-1EA,Solarbio,China) to obtain a single-cell suspension.The single-cell suspension was transferred to a Percoll (17 089 109,GE Healthcare,USA) gradient consisting of 1.5 mL of 25% Percoll and 1.5 mL of 40% Percoll in a 15 ml centrifuge tube and centrifuged at 120 ×gfor 20 min at 28 ℃.After centrifugation,the cells in different layers were collected for immunofluorescence and RNA isolation.
Cell culture and in vitro spermatogenesis
After density gradient centrifugation,the cells in the middle Percoll layer were collected and plated on 24-well plates or 6.5 mm transwell-COL permeable supports (3 422,Corning,USA) in Dulbecco’s Modified Eagle Medium (DMEM)supplemented with 10% FBS,2% fish serum (Yi et al.,2010),100 ng/mL epidermal growth factor (EGF,AF10015,PeproTech,USA),10 ng/mL basic fibroblast growth factor(bFGF,10 014-HNAE,SinoBiological,China),100 ng/mL insulin-like growth factor-I (IGF-I,AF11011,PeproTech,USA),and 0.1 mmol/L β-mercaptoethanol (M6250,Sigma,USA) at 28 °C in humidified air.Half of the culture medium was replaced with fresh medium every 3 days.
In vitrospermatogenesis was induced by supplementation of sex hormones as described previously (Higaki et al.,2017),with several modifications.Briefly,the sex hormones contained 10 U/mL hCG,10 U/mL pregnant mare’s serum gonadotropin (P9970,Solarbio,China),100 ng/mL 11-ketotestosterone (K98340,Acmec,China),100 ng/mL testosterone (IT0110,Solarbio,China),100 ng/mL 17βestradiol (E2758,Sigma,USA),and 50 ng/mL 17α,20βdihydroxy-4-pregnen-3-one (16 146,Cayman,China).Dynamic changes in spermatogenic cells were monitored and captured periodically.Cells were collected for RNA isolation,flow cytometry,and immunofluorescence at 0,1,2,3,4,and 5 weeks after culture (WAC).
To assess its effects on spermatogenic cell development,various concentrations (0,0.1,1,and 10 μm) of melatonin were added to the medium,and half of the medium was replaced by fresh medium containing melatonin every 3 days.After 1 week of exposure to melatonin,10 μmol/L 5-fluoro-2'-deoxyuridine (EdU,C10310,Ribobio,China) was added.The cells were fixed in 4% PFA at 48 h after EdU supplementation.The spermatogenic cells were then cultured in a 3D+Hormore(Hor) culture systems exposed to various stimuli:i.e.,1 μmol/L melatonin (M5250,Sigma,USA) (3D+Hor+Mel),1 μmol/L melatonin and 1 μmol/L non-selective MT inhibitor luzindole(HY-101 254,MCE,USA) (3D+Hor+Mel+Luz),1 μmol/L melatonin and 1 μmol/L MT2-specific inhibitor 4-PP (HY-100 609,MCE,USA) (3D+Hor+Mel+4-PP),1 μmol/L MT1-specific activator 2-Iod (HY-101 176,MCE,USA) (3D+Hor+2-Iod),and 1 μmol/L melatonin and 1 μmol/L ERK inhibitor ravoxertinib(HY-15 947,MCE,USA) (3D+Hor+Mel+RX),respectively.The cells were collected for analysis of germ cell proliferation and differentiation after 1 and 4 weeks of exposure,respectively.
EdU staining
After culture in medium supplemented with 10 μmol/L EdU for 48 h at 28 °C,the cells were fixed in 4% PFA for 15 min and neutralized by 2 mg/mL glycine for 5 min.After neutralization,the cells were permeabilized in 0.5% triton X-100 for 10 min,blocked with 2% normal goat serum for 1 h,and incubated with anti-Vasa antibody at 4 °C overnight.To visualize Vasa,EdU,and nuclei,the cells were stained with Alexa Fluor 567-conjugated goat anti-rabbit IgG,apollo staining buffer(Ribobio,China),and DAPI for 45 min,respectively.Images were captured using a microscope (Cytation 1,BioTek,USA).
Total RNA extraction and polymerase chain reaction(PCR)
Total RNA was extracted using an RNA Extraction Kit(Promega,USA) according to the manufacturer’s instructions.Total RNA quality and amount were measured using a Nanodrop One Spectrophotometer (Thermo Scientific,USA).First-strand cDNA was synthesized using Moloney murine leukemia virus (MMLV) reverse transcriptase with oligo-dT(RP1105,Promega,China).
Semi-PCR was performed to examine the expression of different types of cell markers in cells from different Percoll layers and cells in the 3D+Hor culture systems at 0,1,2,3,4,and 5 WAC.Cell markers included stem cell markers (oct4,plzf,andly75),spermatogenic cell markers (Vasa,dnd,andpiwi),testicular somatic cell markers (fshr,sox9,andamh),and meiosis markers (dmc1,sycp3,rec8,andacorsin).The cycling conditions were:98 °C for 2 min,followed by 35 cycles of 98 °C for 10 s,59 °C for 10 s,and 72 °C for 10 s.β-actinwas used as an internal control.The PCR products were electrophoresed on 1% agarose gel,and images were captured using a gel imager (Sage Creation,China).
Quantitative PCR (qPCR) was performed to examine gene expression in cells cultured in the 3D+Hor culture systems exposed to various stimuli on a thermal cycler (LightCycle 480 II,Roche,Switzerland),as described previously (Liu et al.,2020;Zhang et al.,2020).Genes included melatonin receptors (mt1,mt2,mt3,androrα),cyclins and cyclin dependent kinases (cyclin A/B/D/Eandcdk1/2/4),mitosis-and apoptosis-related genes (pcna,bcl2andcaspase 3),and spermatogenesis-related genes (dmrt1,amh,andfshr).Relative expression levels were calculated with the 2-Δctmethod usingβ-actinas an internal reference (Liu et al.,2019).Primer information is listed in Supplementary Table S1.
Flow cytometry analysis
Flow cytometry was performed as described previously(Higaki et al.,2017).In brief,the single-cell suspension was fixed in 70% ice-cold ethanol for 1 h,washed twice in PBS,resuspended in PBS containing 0.1% bovine serum albumin(BSA,ST023,Beyotime,China) (2×5 min),and treated with 1 mg/mL RNase A (RP1105,Promega,USA) to remove RNA for 30 min at 37 °C.After digestion,the cells were stained with 10 μg/ml propidium iodide (PI,81 845,Sigma,USA) for 30 min,and DNA content was tested by flow cytometry.
Western blot analysis
Protein was extracted from cells in the 3D culture systems after 4 weeks of exposure to different stimuli,including 3D control,3D+Hor,3D+Mel,3D+Hor+Mel,3D+Hor+2-Iod,and 3D+Hor+Mel+Luz.ERK1/2 (ET160129,Huabio,China),p-ERK1/2 (ET160322,Huabio,China),DMC1 (ab11054,Abcam,UK),andβ-actin(T56715S,Abmart,China) were examined by western blotting as described previously (Jia et al.,2013).
Statistical analysis
All values are shown as mean±standard deviation (SD) from at least three independent experiments.Two groups were analyzed by Student’st-test and multiple groups were evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s test.Results were considered statistically significant atP<0.05.
RESULTS
Isolation and characterization of premeiotic spermatogonia
To establish the culture system forin vitrospermatogenesis,we isolated spermatogenic cells from the testes of 5-monthold males (Figure 1A).Immunofluorescence staining showed that the testes contained large single spermatogonia with a strong Vasa signal,a cluster of primary spermatocytes with a modest Vasa signal,and secondary spermatocytes with a weaker Vasa signal,as well as many spermatids with a very weak Vasa signal and condensed spermatozoa and somatic cells with no Vasa signal (Figure 1B,C).Different types of cells were then isolated by Percoll density gradient centrifugation to separate cells into top,middle,and bottom layers (Figure 1D,E).Cells in the middle layer exhibited classic characteristics of spermatogonia:spherical nucleus,transparent cytoplasm,and high nucleus/cytoplasm ratio(Figure 1F).Compared to the disassociated testicular cell suspension,the proportion of Vasa-positive (Vasa+) cells,representing spermatogenic cells,increased from 25.7% to 84.3% in the middle layer after centrifugation (Figure 1G−I).Next,we defined cell types in different layers using various cell markers,including stem cell markersoct4,plzf,andly75(Hayashi et al.,2011;Panda et al.,2011;Zhao et al.,2018),spermatogenic cell markersVasa,dnd,andpiwi(Houwing et al.,2007;Li et al.,2016;Liu et al.,2019),meiosis markersdmc1,sycp3,rec8,andacorsin(Deng et al.,2016;Reda et al.,2016;Xie et al.,2020),and somatic cell markersfshr,sox9,andamh(Lin et al.,2017;Xie et al.,2020).Spermatogenic cell markers were detected in all layers,whereas germ stem cell markers were only detected in the middle-layer cells.In contrast,meiosis markers were only detected in the bottom-layer cells.Somatic cell markers were detected in both top-and middle-layer cells,albeit with markedly lower expression in the middle layer.Therefore,cells in the middle layer were mainly comprised of premeiotic spermatogonia.
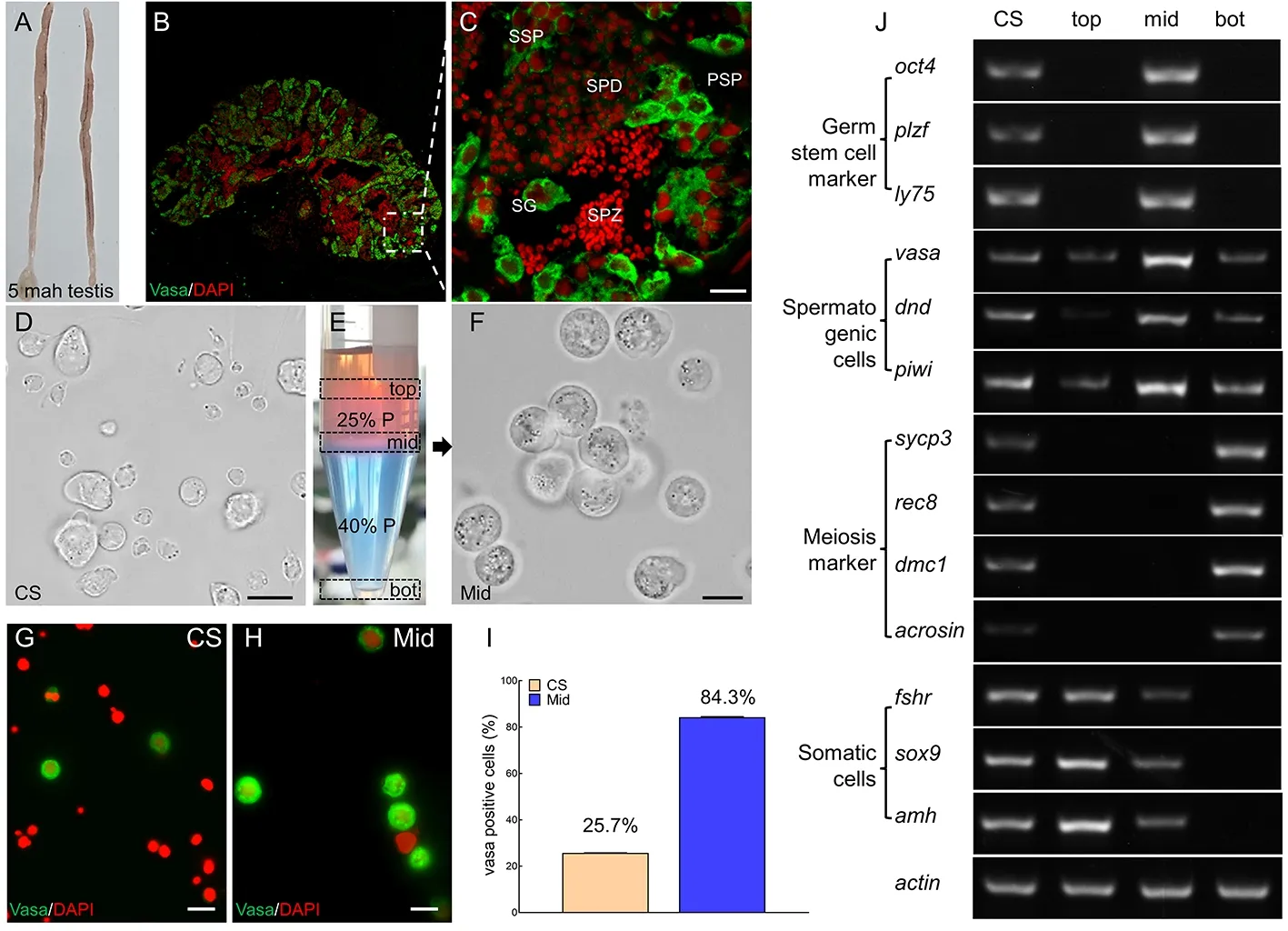
Figure 1 Isolation and characterization of premeiotic spermatogonia by Percoll density gradient centrifugation
In vitro spermatogenesis and dynamic changes in spermatogenic cells
The isolated premeiotic spermatogonia were transferred to a 24-well plate for adherent 2D culture or transwell-COL permeable supports for 3D culture.In the 2D culture,the cells did not adhere and only a few fiber-like somatic cells proliferated by 3 WAC (Figure 2A1–A4).Compared to 2D culture,the 3D culture system facilitated germ cell adherence and aggregation (Figure 2B1–B4).Subsequently,sex hormones were supplemented in both the 2D (2D+Hor) and 3D (3D+Hor) culture systems to inducein vitrospermatogenesis,as reported previously (Higaki et al.,2017;Kawasaki et al.,2016;Miura et al.,1991;Sakai,2002).As expected,hormone supplementation obviously promoted germ cell adherence and aggregation,especially in the 3D culture system (Figure 2C1–D4).In response to sex hormone exposure,germ cells in the 3D culture system formed spherical clumps at 2 WAC,which became larger in subsequent culture (Figure 2D2–D4).Spherical clump formation is a hallmark of spermatogenesisin vitro(Ishikura et al.,2021;Lee et al.,2006).To confirm the occurrence of spermatogenesis,we analyzed the expression of meiosis markers and DNA content in cells in cultures at 4 WAC.Results showed that the transcription of meiosis markersdmc1andacrosinwas remarkably higher in the 3D+Hor culture system (Figure 2E).Haploid cells were detected in both the 2D+Hor (4.08%) and 3D+Hor (9.49%) culture systems,but the proportion was obviously higher in the 3D+Hor culture system (Figure 2F,G).Therefore,premeiotic spermatogonia can enter meiosis and reproduce haploid cells in both the 2D+Hor and 3D+Hor culture systems,and the 3D+Hor culture system has the advantage of promotingin vitroproduction of haploid cells.
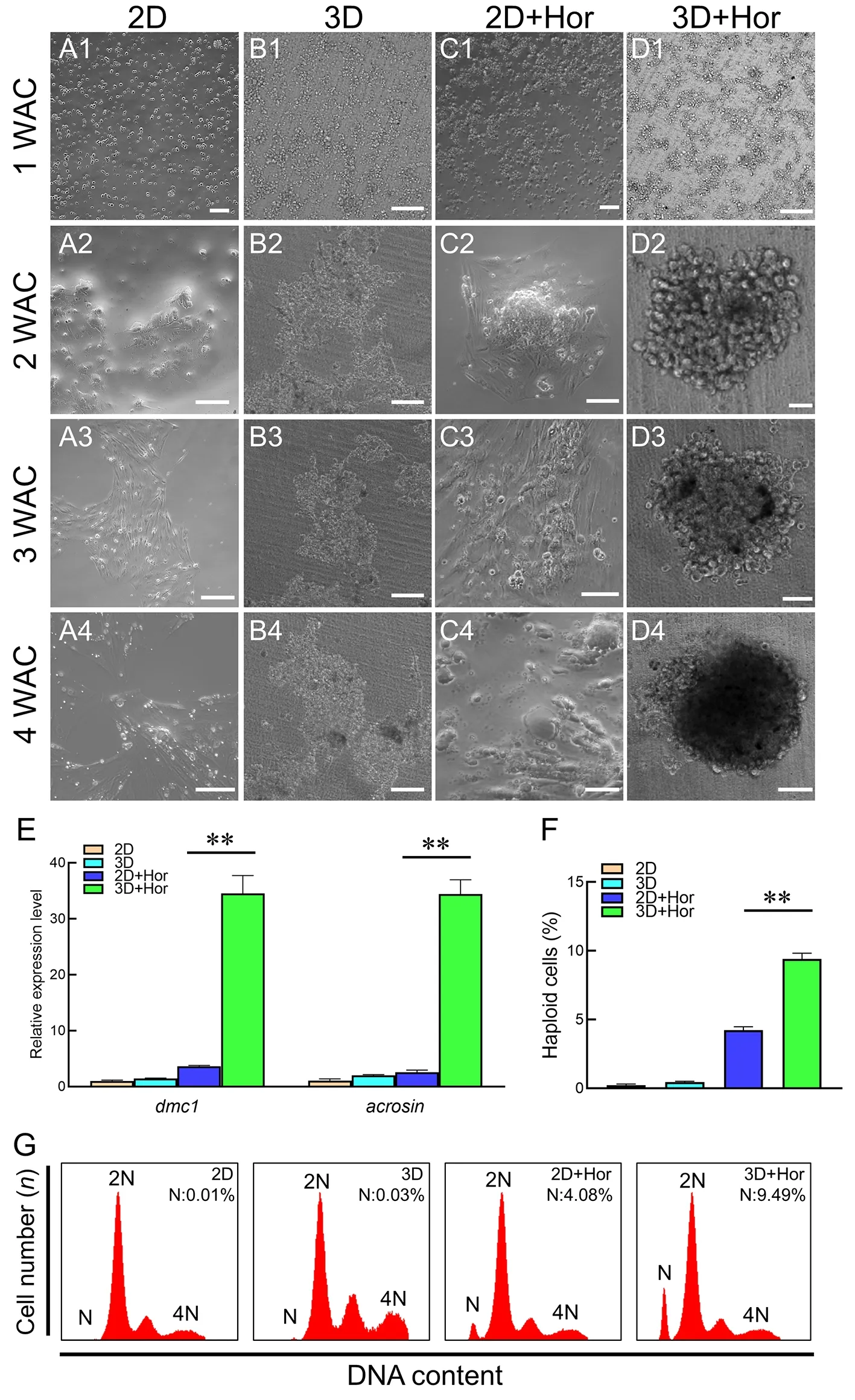
Figure 2 In vitro spermatogenesis under different culture conditions
Subsequently,we monitored the dynamic changes in spermatogenic cells in the 3D+Hor culture system.Vasa+cells began to cluster at 1 WAC,leading to the formation of spherical clumps at 2 WAC (Figure 3A,B).The spherical clumps continued to grow until the cell clusters reach diameters of 300 and 500 μm at 3 and 4 WAC,respectively(Figure 3C,D).Of note,the proliferating spermatogonia at the edge of the clump strongly expressed the proliferation marker EdU at 4 WAC (Figure 3E).Furthermore,the spherical clumps shared similar structural patterns and cellular distributions as mature testes (Figure 3F).To trace meiosisin vitro,we examined meiosis markers and DNA content.Transcription of meiosis markersacrosin,dmc1,sycp3,andrec8was not detectable until 3 WAC,when the testicular cells formed testislike spherical clumps (Figure 3G).Consistently,we observed a small proportion of haploid cells (4.87%) at 3 WAC,which increased to 7.46% and 12.29% at 4 and 5 WAC,respectively(Figure 3H,I).In addition,motile flagellated sperm were observed in the medium at 4 WAC (Figure 3J;Supplementary Movie S1).Therefore,the premeiotic spermatogonia form testis-like clumps and undergo meiosis to produce flagellated sperm in the 3D+Hor culture system at 3 WAC.
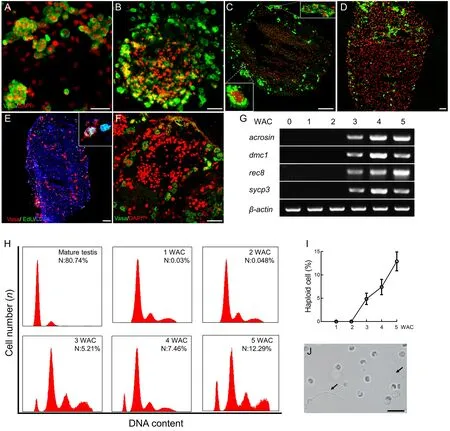
Figure 3 Dynamic changes,expression patterns,and DNA content in spermatogenic cells in 3D+Hor culture system
Confirmation of fertility of in vitro-derived sperm
We subsequently performed artificial insemination to assess the fertility of thein vitro-derived sperm by calculating the proportion of mature oocytes that developed into cleavageand hatching-stage embryos,representing the fertilization and hatching ratios,respectively.Fresh sperm yielded high fertilization (84.72%) and hatching ratios (55.09%) (Table 1).When the mature oocytes were mixed with the cell suspension at 3 WAC,several oocytes were fertilized and the proportion of eggs that developed to the hatching stage was 3.50%±0.36%,which increased to 26.88%±4.13% and 19.65%±2.31% at 4 and 5 WAC,respectively (Table 1).No significant morphological differences were observed between the embryos generated from fresh sperm andin vitro-derived sperm (Figure 4).Collectively,these results demonstrated thatin vitro-derived sperm from premeiotic spermatogonia in 3D culture can fertilize eggs and subsequently develop into normal larvae.
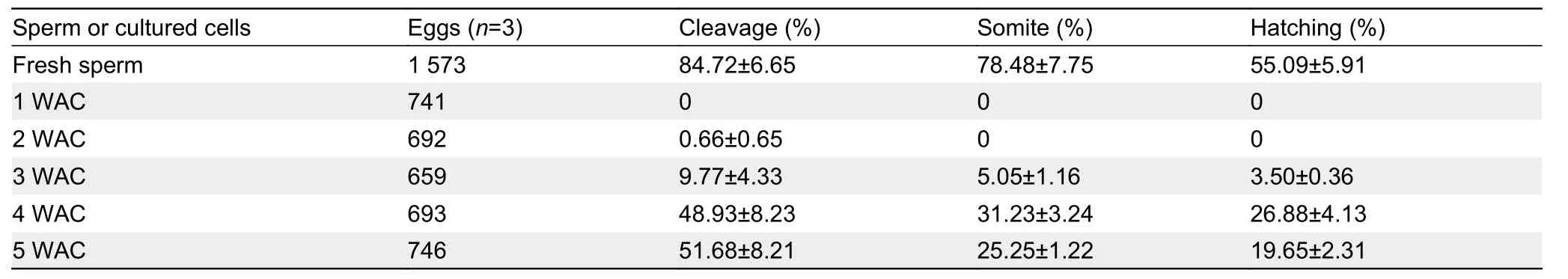
Table 1 Proportion of embryos at different stages generated from mature oocytes mixed with fresh sperm or cultured cells in 3D+Hor culture system.
Melatonin promotes proliferation of spermatogonia
Despite showing competent fertilization,egg development was not satisfactory.Thus,we optimized conditions by supplementation of other factors in the 3D culture system.The pleiotropic molecule melatonin promotesin vitrodifferentiation of sperm from early developing spermatogonia (Deng et al.,2016;Reda et al.,2017).Therefore,to investigate the effects of melatonin on spermatogenic cell development in four-eyed sleepers,we added increasing amounts of melatonin to the culture media.EdU-staining analysis indicated that 1 μmol/L melatonin significantly promoted testicular cell proliferation.This phenomenon was most evident in spermatogonia positive for both EdU and Vasa (EdU+/Vasa+).In sharp contrast,the proportion of EdU+/Vasa−cells,representing proliferating somatic cells,was not affected under the same conditions(Figure 5A–P).To explore the potential mechanisms underlying melatonin-mediated spermatogenic cell proliferation,we compared the transcription levels ofmt1/2/3,rorα,cyclinA/B/D/E,andcdk1/2/4,mitosis markerpcna,and apoptosis-related markersbcl2andcaspase 3in 3D+Hor systems in the absence or presence of melatonin.Results showed that melatonin significantly elevated the transcription ofmt1/2,cdk1/2/4,cyclin A/B/D/E,andpcna,but notmt3,rorα,bcl2,andcaspase 3(Figure 5Q),suggesting that melatonin may promote the proliferation of spermatogonia through the up-regulation of cyclin proteins and cyclin-related kinases.
Melatonin regulates spermatogenic cell proliferation via activation of melatonin receptor 1 signaling
Melatonin exerts biological functions through plasma membrane receptors MT1/2 to initiate hierarchical signal transduction (Boutin et al.,2020;Juszczak et al.,2014).Consistently,we observed an increase in the transcription ofmt1andmt2in premeiotic spermatogonia supplemented with melatonin (Figure 5Q).To identify the receptor related to melatonin signaling,we blocked MT signaling with either luzindole (Luz,non-selective inhibitor of both MT1 and MT2)or 4-phenyl-2-propionamidotetralin (4-PP,MT2-specific inhibitor).Interestingly,supplementing the culture medium with Luz rather than 4-PP significantly suppressed melatonininitiated elevation in the proportion of Vasa+cells and the expression ofcdk2,cyclin E,andpcna(Figure 6A–D,F,G),implying that MT1 may play an important role in promoting spermatogenic cell proliferation.To test this hypothesis,we initiated MT1 signaling with 2-iodomelatonin (2-Iod,MT1-specific activator).Results showed that 2-Iod significantly enhanced the proportion of Vasa+cell and the transcription ofcdk2,cyclin E,andpcna(Figure 6E–G),consistent with the melatonin-induced promotion of germ cell proliferation(Figure 6).Thus,these results suggest that melatonin promotes spermatogenic cell proliferation by activating MT1 signaling in the 3D culture system.
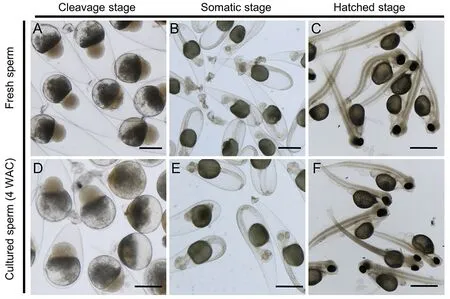
Figure 4 Representative images of embryos generated from mature oocytes fertilized with fresh sperm or in vitro-derived sperm at 4 WAC (Scale bar:1 mm)

Figure 5 Melatonin promoted spermatogonia proliferation
Melatonin promotes in vitro production of functional sperm
To determine whether continuous exposure to melatonin would affect spermatogenic cell differentiation in the 3D+Hor culture system,we examined the transcription of spermatogenesis-related genes and meiosis markers.As expected,4 weeks of exposure to both melatonin and 2-Iod significantly enhanced the expression of spermatogenesisrelated genes,includingdmrt1,amh,andfshr,as well as meiosis markersacrosin,dmc1,rec8,andsycp3(Figure 7A,B).Consistently,supplementation of melatonin or 2-Iod increased the proportion of haploid cells to 17.13% and 19.46% (Figure 7C),and the proportion of hatched-stage embryos increased to 38.56% and 39.74%,respectively(Table 2).These results suggest that both 2-Iod and melatonin favor the differentiation of spermatogenic cells into functional sperm in the 3D+Hor culture system.
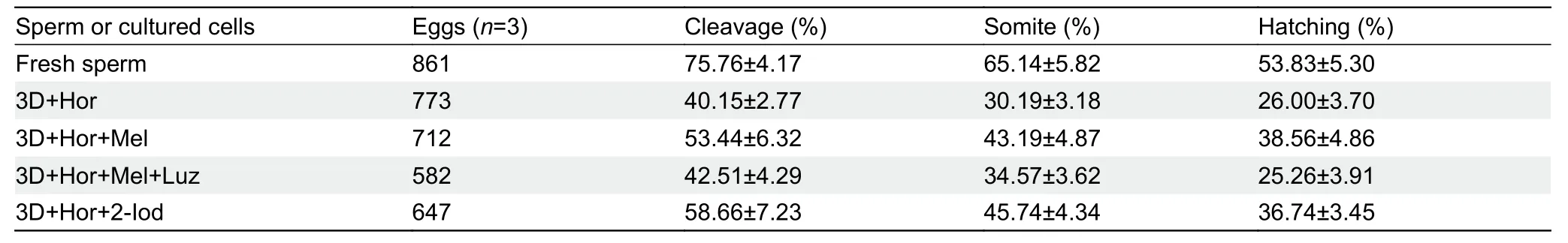
Table 2 Proportion of embryos at different stages generated from mature oocytes mixed with fresh sperm or cultured cells in 3D+Hor culture system exposed to different stimuli
Active ERK signaling is required for in vitro spermatogenesis
As a classical G protein-coupled receptor (GPCR),the melatonin receptor regulates germ cell development through several factors such as cyclic adenosine monophosphate(cAMP) and reactive oxygen species (ROS) to activate downstream ERK1/2 (Cao et al.,2021;Chen et al.,2020).ERK1/2 is a signaling hub that integrates different signals from a variety of GPCRs to regulate SSC self-renewal and differentiation as well as sperm maturation in mammals (Niu et al.,2017;Sun et al.,2021).However,whether melatonin can activate the ERK1/2 signaling pathway to regulate fish spermatogenic cell development remains unclear.To check this possibility,we examined the expression of ERK and DMC1 in testicular cells after exposure to various stimuli.Results indicated that both melatonin and 2-Iod enhanced the levels of ERK1/2 phosphorylation (pERK1/2) and DMC1 in the 3D culture system,and selective blockade of MT2 did not interfere with the melatonin-mediated increase in pERK1/2 and DMC1 (Figure 8A;Supplementary Figure S1),suggesting a correlation between melatonin-MT1 signaling and ERK1/2 activation in promoting meiosis in four-eyed sleepers.We also studied the role of ERK1/2 in the melatonin-initiated promotion of spermatogenic cell development using the ERK-specific inhibitor RX (Zhu et al.,2021).Results showed that RX interfered with the melatonin-mediated elevation in the transcription of mitosis markerscdk2,cyclin E,andpcnaand meiosis markersrec8,dmc1,acrosin,andscyp3(Figure 8B,C).Notably,the transcription of meiosis markers was markedly inhibited when the cells were exposed to RX,with lower expression than that in the 3D or 3D+Hor cultures in the absence of melatonin (Figure 8C).However,no significant changes were observed in the transcription of apoptosisrelated genescaspase3andbcl2after inhibition of ERK1 activity by RX compared to the 3D+Hor+Mel culture system(Figure 8D).Furthermore,RX decreased the proportion of haploid cells to 1.11%,implying that ERK1/2 signaling was required for meiosisin vitro(Figure 8E).Therefore,these results indicate that melatonin promotes spermatogenic cell proliferation and differentiation via the ERK1/2 signaling pathway.

Figure 6 Melatonin promoted spermatogonia proliferation via melatonin receptor 1 (MT1)
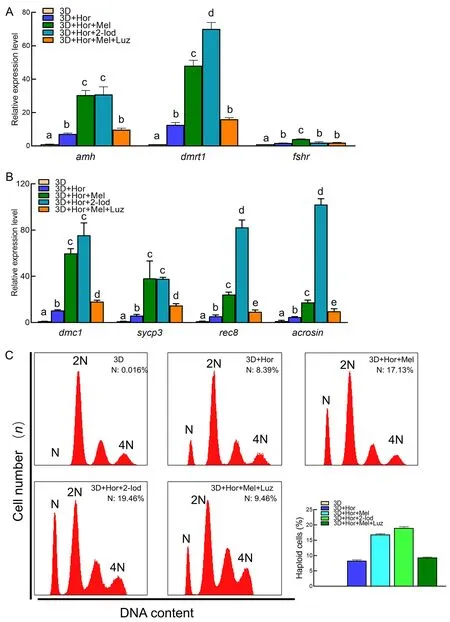
Figure 7 Melatonin promoted differentiation of spermatogonia into haploid cells
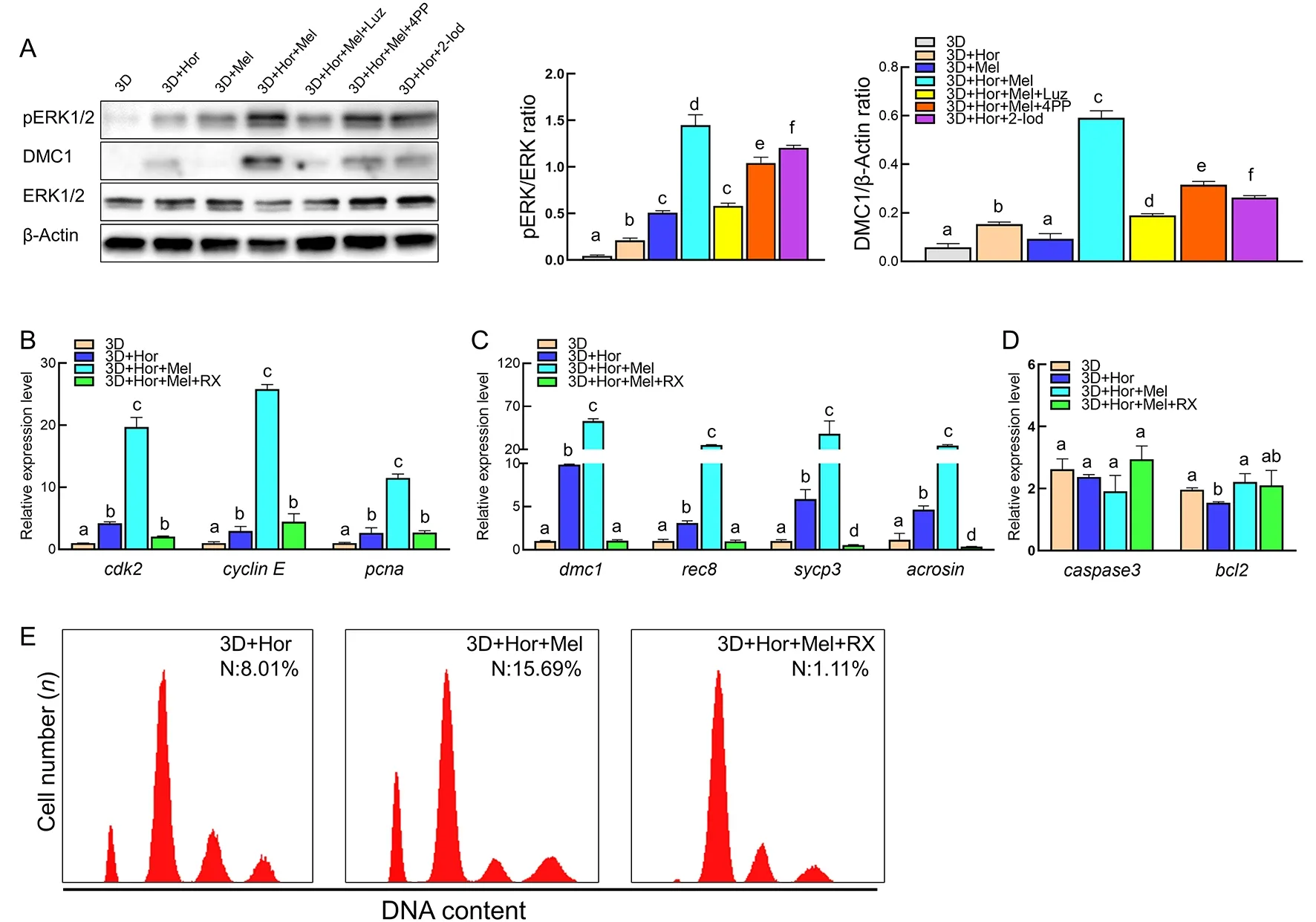
Figure 8 Activation of ERK1/2 signaling pathway is required for spermatogenesis in vitro
DISCUSSION
In this study,we isolated premeiotic spermatogonia via density gradient centrifugation.The isolated spermatogonia were then cultured using a 3D system,resulting in their aggregation into testis-like spherical clumps followed by meiosis to generate functional sperm.Based on this 3D culture system,melatonin and 2-Iod efficiently promoted the production of functional sperm via activation of the ERK1/2 signaling pathway.Importantly,thein vitro-derived sperm were capable of fertilizing mature oocytes and developing into healthy larvae.Thus,we successfully established a novel 3D culture system to produce functional sperm,offering a potential strategy to accelerate the genetic breeding of fish and improve the aquaculture seed industry.
The first obstacle for spermatogenic cell culture is the isolation of SSCs.Available methods include magneticactivated cell sorting with SSC-specific surface antigens,fluorescence-activated cell sorting using germline-specific transgenic fish strains,density gradient centrifugations,and different plating (Xie et al.,2020).SSC-specific surface antigens have been identified in mammals (Kanatsu-Shinohara et al.,2004;Panda et al.,2011;Shinohara et al.,1999;Zhao et al.,2018),but most fish species,including foureyed sleepers,do not share these antigens.Although germline transgenic strains have been successfully established in zebrafish,medaka,and cyprinid honmoroko in the laboratory (Higaki et al.,2021;Iwasaki-Takahashi et al.,2020;Li et al.,2009;Ye et al.,2019),they have not been established in aquaculture fish species (except rainbow trout)due to their long generation intervals and unclear genetic background.Therefore,in the current study,we used Percoll density gradient centrifugation to isolate SSCs,despite limitations in terms of purity and specificity.Immunostaining and qPCR analysis revealed that 84.3% of cells in the middle layer were Vasa+cells,which showed high expression of germline stem cell markers but not meiosis markers(Figure 1),suggesting that density gradient centrifugation may be an attractive strategy for isolation of spermatogonia in aquaculture fish.
Male germ cells usually do not attach to gelatin-coated dishes but can attach to somatic cells after they have attached to the dish (Kawasaki et al.,2016;Miura et al.,1991;Sakai,2002).The co-culture of male germ cells and somatic cells was first established in Japanese eel (Miura et al.,1991),and later in zebrafish (Kawasaki et al.,2016;Sakai,2002) and rainbow trout (Iwasaki-Takahashi et al.,2020).However,given the failure to derive proliferating cells from normal testes after several passages in most fish species,stable fish testicular somatic cell lines have only been reported in zebrafish and rainbow trout (Kawasaki et al.,2016;Wolf &Quimby,1962).In addition,the effects of feeder cells on the development of spermatogonia differ among species (Nasiri et al.,2012),thereby restricting co-culture application in many fish species.In this study,we established a 3D culture system for spermatogenic cell development in four-eyed sleepers.Compared to the 2D culture,spermatogonia attached to the 3D supports,formed spherical clumps,initiated meiosis,and produced functional sperm (Figure 2-4).Formation of testislike spherical clumps is a critical step and could be considered as an important index to assess culture systems forin vitrospermatogenesis (Sakai,2002).In zebrafish,spherical clumps disappear after~20 days and SSCs stop proliferating and differentiating in co-culture systems (Sakai,2002).In our 3D culture system,the spherical clumps contained proliferating spermatogonia and were maintained for at least 4 weeks(Figures 2,3),demonstrating that the 3D culture system promoted spermatogenesis.To the best of our knowledge,this is the first study to report on a 3D culture system for male germ cells in fish.
Our 3D culture system yielded a higher production (12.3%)of haploid cells than the 2D culture system,but a lower production than previous suspension cultures of cyprinid honmoroko (34.2%) (Higaki et al.,2017).We speculate that the reason for this may be the cell population used for cell culture,i.e.,84.3% premeiotic spermatogonia in the 3D culture system and~50% meiotic spermatocytes in the suspension culture of cyprinid honmoroko.Positive correlation between total Sertoli cell number and daily sperm production has been reported in several species (Meroni et al.,2019).In mice,complete meiosis can be reconstituted by the co-culture of primordial germ cell-like cells (10 000) and fetal testicular somatic cells (20 000) (Ishikura et al.,2021).In zebrafish,functional sperm can be derived from spermatogenic cells by co-culture with ZtA6 Sertoli-like cells (5×105) (Kawasaki et al.,2016;Sakai,2002).We speculated that the reconstitution of male germ cell development requires a soma/germline ratio ≥1.In our 3D culture system,the soma/germline ratio was~0.186,far lower than that used for mice and zebrafish.In the future,we will investigate whether the soma/germline ratio affects the efficiency of functional sperm production in 3D culture models.
Melatonin plays different roles in the regulation of reproductive hormones in long-day and short-day breeders(Yu et al.,2018).Melatonin inhibits spermatogenesis by suppressing the synthesis of testosterone in long-day breeders,such as rodents (Ahmad &Haldar,2010;Forger &Zucker,1985),but promotes germ cell differentiation by increasing luteinizing hormone (LH) and follicle-stimulating hormone (FSH) secretion as well as testosterone concentration in short-day breeders,such as sheep and foxes(Forsberg &Madej,1990;Lincoln &Clarke,1997).Melatonin in sheep also promotes the differentiation of spermatogonia into spermin vitro(Deng et al.,2016).As a burrowing and nocturnal fish,the four-eyed sleeper is considered a short-day breeder (Dong et al.,2021).In this study,melatonin promoted spermatogenic cell proliferation and differentiation into functional sperm,thus demonstrating the conserved role of melatonin in promoting spermatogenesis in short-day fish breeders.
Fish,amphibians,and birds have three plasma membrane receptors (MT1,MT2,and MT3) and a nuclear receptor(RORα) (Li et al.,2013).However,in most fish species,the relationships among these receptors in response to melatonin remain unclear.In this study,melatonin significantly increased the transcription of MT1 and MT2 but not MT3 or RORα,and selectively inhibiting or activating MT1/2 demonstrated that melatonin promoted spermatogenic cell proliferation and differentiation via MT1 rather than MT2 (Figures 5–7,8A).These results suggest functional differences in melatonin receptors in regulating spermatogenic cell development.To further elucidate the mechanisms by which MT1 and MT2 differentially respond to melatonin in spermatogenic cell development,we will identify the G proteins that binds with MT1/2 and how they activate downstream ERK signaling.
ERK signaling is a master regulator of various evolutionarily conserved cellular processes by phosphorylating diverse substrates (Lavoie et al.,2020).Previous studies have shown that pERK1/2 is required for both SSC and Sertoli cell proliferation (Meroni et al.,2019;Niu et al.,2015;Tassinari et al.,2015;Zhang et al.,2021b).However,in our 3D+Hor culture system,melatonin-activated ERK signaling promoted the proliferation of premeiotic spermatogonia but not of somatic cells (Figure 5).We speculated that this may be due to the divergent effects of hormones on spermatogenic and somatic cell development.On the one hand,hormone supplementation decreased the proportions of spermatogenic cells and proliferating spermatogonia to 20% and 4% at 1 WAC,respectively (Figure 1I,5N,5P).On the other hand,hormone supplementation activated ERK signaling,which was enough to promote somatic cell proliferation at 1 WAC(increase in proportion from 15.7% to 80%) (Figures 5N,8A).Therefore,we hypothesized that the 3D+Hor culture system promoted somatic cell proliferation and induced differentiation of germ cells into sperm.Furthermore,supplementation with melatonin and 2-Iod enhanced the proliferation and number of spermatogonia,which then underwent meiosis to produce functional sperm.
CONCLUSIONS
We successfully established a novel 3D culture system for spermatogenic cell culture and differentiation in an aquacultural fish species (four-eyed sleeper).Furthermore,we optimized the system using melatonin,which significantly promoted spermatogenic cell proliferation and differentiation into functional sperm via the ERK1/2 signaling pathway.This study provides a new strategy forin vitropropagation of functional gametes,holding great promise for improving genetic breeding in the aquaculture industry.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
W.L.and M.S.Y.contributed to project conception.C.Y.M.and M.D.D.collected the materials.H.Z.,W.W.Z.,and K.T.J.conducted the experiments.H.Z.and W.L.conducted data analysis and prepared the manuscript.H.Z.,W.L.,and M.S.Y.critically reviewed the manuscript.All authors read and approved the final version of the manuscript.
- Zoological Research的其它文章
- Zoological Research call for papers of Cavefish Special Issue
- Fuel source shift or cost reduction:Context-dependent adaptation strategies in closely related Neodon fuscus and Lasiopodomys brandtii against hypoxia
- Ecological study of cave nectar bats reveals low risk of direct transmission of bat viruses to humans
- Population and conservation status of a transboundary group of black snub-nosed monkeys (Rhinopithecus strykeri) between China and Myanmar
- Nucleus accumbens-linked executive control networks mediating reversal learning in tree shrew brain
- Europe vs.China:Pholcus (Araneae,Pholcidae) from Yanshan-Taihang Mountains confirms uneven distribution of spiders in Eurasia

