Effects of acupuncture plus medication on hippocampus SIRT1 and FOXO3a expression, MDA content, and SOD activity of rats with Alzheimer disease
ZHAO Jian (赵健), DING Jian (丁见), WANG Lin (王林), LI Huaibin (李怀斌)
School of Basic Medical Science, Wannan Medical College, Anhui Province, Wuhu 241002, China
Abstract
Keywords: Acupuncture Therapy; Electroacupuncture; Acupuncture Medication Combined; Hippocampus; Superoxide Dismutase; Alzheimer Disease; Rats
Alzheimer disease (AD) is a neurodegenerative lesion that occurs most frequently in the elderly over 65 and currently cannot be completely cured with drugs, while certain curative effects can be achieved through combined therapies[1-2]. Electroacupuncture (EA) is a technique commonly used in the clinical practice of traditional Chinese medicine and has unique curative effects on many neurological diseases. Clinical evidence has shown that EA effectively improves the clinical symptoms of AD, and the total effective rate is significantly higher than that of Western medication treatment[3]. Resveratrol exists in many plants.Neurodegenerative disease treatment and anti-aging with resveratrol have been paid more and more attention in recent years. Resveratrol has certain effects on the prevention and treatment of AD[4]. Silent information regulator of transcription 1 (SIRT1) is a nicotinamide adenine dinucleotide (NAD)-dependent protein deacetylase that reduces acetylation of target proteins such as p53 and transcription factor forkhead box protein O3a (FOXO3a)[5], enhances the ability of glial cells to clear amyloid-β (Aβ), and promotes the process of amyloid precursor protein (APP) to reduce the production and toxicity of Aβ[6]. Forkhead box O(FOXO) is a member of the forkhead protein family,which regulates nerve cell survival, stress response, and neuron signaling, among which FOXO3a has a role in promoting the onset of AD[7]. The expression and deacetylation of FOXO3a are both regulated by SIRT1[8],and SIRT1 can antagonize the FOXO expression to protect neurons[9]. The lipid peroxide level may increase in the brain of AD patients, leading to pathological changes in nerve cells[10]. Both EA and resveratrol can regulate the oxidative stress response in the brain of AD rats[11-12], alter the levels of malondialdehyde (MDA)and superoxide dismutase (SOD), and affect the SIRT1 expression[4,13-15]. But whether combining acupuncture and medication can enhance the AD treatment effect has not been reported. In this study, the morphological changes of the hippocampal CA3 neurons, the expression of the hippocampal SIRT1 and FOXO3a proteins, and the changes in MDA content and SOD activity were determined after EA combined with resveratrol was used to treat AD rat models, thus to provide a theoretical basis for the possible mechanisms of acupuncture plus medication in improving AD-related neurological symptoms.
1 Materials and Methods
1.1 Laboratory animal
Sixty male Sprague-Dawley rats, weighing 220±20 g,3-month-old, were provided by the Experimental Animal Center of Hangzhou Medical College, Zhejiang Province, with the production license number SCXK(Zhe) 2019-0002. All rats were reared under a 12 h/12 h light/dark cycle, with a controlled room temperature of 20-25 ℃, the humidity of 60%-65%, and free access to food and water. All experimental processes were implemented following theGuiding Opinions on the Treatment of Experimental Animalsissued by the Ministry of Science and Technology of the People’s Republic of China in 2006. The rats were divided into 5 groups by the random number table method, a normal group, a model group, an EA group, a drug group, and an acupuncture-medication combined group,with 12 rats in each group.
1.2 Main reagents and instruments
Streptozotocin (STZ, Cat. No. S0130, Sigma, USA);resveratrol (Cat. No. R5010, Sigma, USA); SIRT1 (Cat. No.bs-0921R, Beijing Boaosen Biotechnology Co., Ltd.,China); FOXO3a (Cat. No. bs-1548R, Beijing Boaosen Biotechnology Co., Ltd., China); Nissl staining solution(Cat. No. C0117, Shanghai Biyuntian Biotechnology Co.,Ltd., China); HRP-labeled secondary antibody (Cat. No.P0615, Shanghai Biyuntian Biotechnology Co., Ltd.,China); GAPDH antibody (Cat. No. AF1186, Shanghai Biyuntian Biotechnology Co., Ltd., China); ultra-sensitive ECL chemiluminescence kit (Cat. No. P0018FS, Shanghai Biyuntian Biotechnology Co., Ltd., China); SDS-PAGE protein loading buffer (Cat. No. P0015L, Shanghai Biyuntian Biotechnology Co., Ltd., China); strept avidin-biotin complex kit (Cat. No. SA1022, Wuhan Boster Biotechnology Co., Ltd., China);diaminobenzidine color development kit (Cat. No.PA110, Beijing Tiangen Biochemical Technology Co., Ltd.,China); SOD detection kit (Cat. No. A001-1, Nanjing Jiancheng Bioengineering Institute, China); MDA detection kit (Cat. No. A003-1, Nanjing Jiancheng Bioengineering Institute, China).
Morris water maze (MWM) video analysis system(Beijing Zhongshi Dichuang Technology Development Co., Ltd., China); mouse brain stereotaxic instrument model 51600 (Shanghai Yuyan Scientific Instrument Co.,Ltd., China); SDZ-Ⅱ Hwato brand EA instrument(Suzhou Medical Products Factory Co., Ltd., China);Leica RM2235 paraffin microtome (Leica, Germany); Gel Doc XR gel imaging system (Bio-Rid, USA); BX51 microscope and Image-Pro Express C image analysis system (OLYMPUS, Japan).
1.3 AD modeling method
The AD rat model was prepared following the modeling method of YAO H B,et al[16]. The rats were anesthetized by intraperitoneal injection of 1% sodium pentobarbital at 4 mL/(kg·bw) and then immobilized on a rat brain stereotaxic apparatus. The top of the head was disinfected, the skin was incised along the midline,and the lateral ventricle was positioned according to
TheStereotaxic Atlas of the Rat Brain[17]: 0.9 mm posterior to the bregma, 1.4 mm lateral to the midline,and 3.6 mm deep. The calvarial bone was drilled and 10% STZ [diluted with artificial cerebrospinal fluid, at a dose of 30 μL/(kg·bw)] was injected into both lateral ventricles. The injection was 10 min/time with 5-minute needle retention. After the needle was withdrawn, the burr hole was closed with sterile bone wax, sterilized,and sutured. Repeated the same operation mentioned above on day 3. One week after the operation, the model was screened by the MWM test. The model was successfully produced if the escape latency was prolonged and the percentage of stay in the target quadrant was shortened compared with the normal group (P<0.05).
1.4 Groups and interventions
Rats in the normal group and model group were not given any intervention after intraperitoneal anesthesia.
Rats in the EA group were subjected to intraperitoneal anesthesia (same as the model group),and the left Zusanli (ST36) and Dazhui (GV14) were positioned according to theResearch and Production of Rat’s Acupoints Map[18]. Dazhui (GV14) is located between the 7th cervical vertebra and the 1st thoracic vertebra in the middle of the back. Zusanli (ST36) is located on the posterolateral side of the knee joint,about 5 mm below the fibula head. A needle of 0.32 mm in diameter and 25 mm in length was used for perpendicularly puncturing, with a 5 mm insertiondepth for Dazhui (GV14) and 7 mm for Zusanli (ST36).Then the needle handle at Dazhui (GV14) was connected to the positive pole, and the needle handle at Zusanli (ST36) on the left was connected to the negative pole. The sparse-dense wave with a frequency of 2 Hz/10 Hz, an intensity of 1 mA, and a wave width of 1 ms was used. Each stimulation lasted 30 min, once a day, for 4 consecutive weeks.
Rats in the drug group were administered resveratrol by gavage at 44 mg/(kg·bw), once a day for 4 consecutive weeks.
Rats in the acupuncture-medication combined group received EA and drug intervention at the same time for 4 consecutive weeks. The EA treatment was the same as in the EA group, and the resveratrol gavage was the same as in the drug group.
1.5 Observation indicators and detection methods
1.5.1 MWM test
The pool was 120 cm in diameter and 60 cm deep.The small platform for the experiment was about 9 cm in diameter and 23 cm high, and the water depth in the pool was about 1-2 cm. One day before the test, all rats were put into the pool and swam freely to find the platform for adaption. For the next 4 consecutive days,the rats were put into the water from different quadrants 4 times a day, and the time required for the rats to find the platform was recorded. Then the average value was used as the rat escape latency that day. If failed to find the platform within 90 s, the rat would be guided to the platform and stay for 10 s, and the escape latency was recorded as 90 s. After the platform was removed on day 5, the time required for the rat to find the platform spot and the percentage of stay in the target platform quadrant were recorded.
1.5.2 Tissue sampling and biochemical indicator detection
After the animal experiment, the rats were anesthetized by intraperitoneal anesthesia and flushed with normal saline through the left ventricle. The samples were quickly collected on a low-temperature platform, 6 pieces of tissue from the same part of the brain containing the hippocampus (2.0-5.5 mm after bregma) were selected from each group by the random number table method according to the rat brain atlas.The tissue sections were placed in ethanol with different concentrations, dehydrated in a gradient manner, transparentized in xylene, and embedded in paraffin for Nissl and immunohistochemical staining.Ten consecutive coronal sections at 5 μm were collected from each sample after staining. Two sections were randomly selected from each sample and observed under the field of view of a 400-fold optical microscope.The corresponding cell numbers were counted. Both sides of the cerebral cortices were bluntly dissected from the other 6 rats in each group with ophthalmic forceps and glass dissecting tools to expose the crescent-shaped hippocampus. About 180 mg of hippocampal tissue was isolated and stored at -80 ℃for Western blotting (WB) detection, and MDA content and SOD activity determination.
Nissl staining: Serial coronal sections of the paraffinembedded tissue blocks at 5 μm were prepared. The sections were dewaxed with xylene and rehydrated in an ethanol gradient, then placed in 0.1% tar violet staining solution, stained at a constant temperature of 37 ℃ for 10 min, rinsed with double-distilled water,then decolorized by 95% ethanol for 5 s, dried overnight in a 37 ℃ incubator, and cleared in xylene, mounted,and examined under microscope.
Immunohistochemical staining: The sections were routinely deparaffinized and rehydrated according to the instructions of the streptavidin-biotin complex kit.The dilution ratio of the primary antibody was: rabbit anti-SIRT1 (1:150) and FOXO3a (1:100). The slides were mounted with neutral gum. The cells were counted, and the morphology was observed with the image analysis system.
WB experiments: Tissue protein was extracted. The bicinchoninic acid method was used to determine protein concentration, followed by gel electrophoresis,membrane transferring, blocking, overnight incubation with primary antibody SIRT1 (1:400) or FOXO3a (1:400)at a low temperature, secondary antibody incubation,ECL luminescent solution exposure and development,film grayscale analysis by Image-Pro Express C image analysis system, and the obtained value was used as the integrated optical density (IOD) value.
MDA content and SOD activity determination: The thiobarbituric acid method and the xanthine oxidase method were used to determine the MDA content and
SOD activity, respectively. All the experimental steps were carried out according to the kit instructions.
1.6 Statistical methods
The SPSS version 26.0 software was used for data analysis. In the statistical results, the data of escape latency and SIRT1 protein expression conformed to normal distribution with heterogeneity of variance and were represented as the median (lower quartile, upper quartile [M (QL, QU)]. The Kruskal-Wallis test was used for comparison among multiple groups, and the Mann-WhitneyU-test was used for pairwise comparisons between groups. The rest indicators were in line with normal distribution and homogeneity of variance and were expressed as mean ± standard deviation (±s). One-way analysis of variance was used for comparisons among multiple groups, and the least significant difference test was used for pairwise comparisons between groups.P<0.05 indicated that the difference was statistically significant.
2 Results
2.1 Comparison of the MWM test results
Compared with the normal group, the escape latency was significantly prolonged (P<0.05), and the percentage of stay in the target quadrant was significantly reduced (P<0.05) in the model group.Compared with the model group, the average escape latency was shortened (P<0.05), and the percentage of stay in the target quadrant was increased (P<0.05) in the EA group and the drug group, and the acupuncturemedication combined group and showed more obvious efficacy (P<0.05). It is suggested that both EA and resveratrol improve the learning and memory ability of AD rats, and the combined application is more effective(Table 1).
Table 1. Comparing the Morris water maze test results of rats among groups ( ±s)
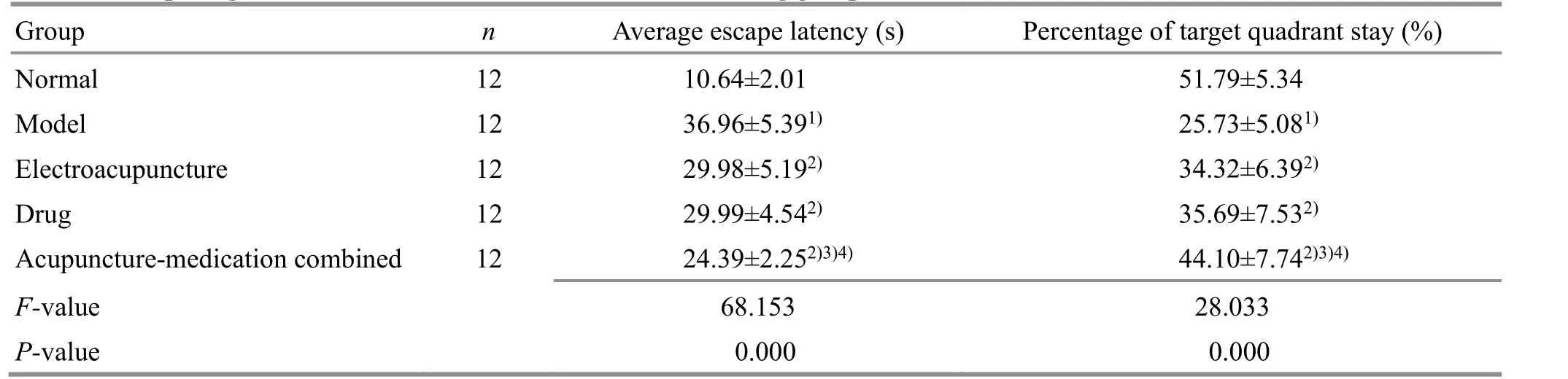
Table 1. Comparing the Morris water maze test results of rats among groups ( ±s)
Note: Compared with the normal group, 1) P<0.05; compared with the model group, 2) P<0.05; compared with the electroacupuncture group,3) P<0.05; compared with the drug group, 4) P<0.05
Group n Average escape latency (s) Percentage of target quadrant stay (%)Normal 12 10.64±2.01 51.79±5.34 Model 12 36.96±5.391) 25.73±5.081)Electroacupuncture 12 29.98±5.192) 34.32±6.392)Drug 12 29.99±4.542) 35.69±7.532)Acupuncture-medication combined 12 24.39±2.252)3)4) 44.10±7.742)3)4)F-value 68.153 28.033 P-value 0.000 0.000
2.2 Comparison of the Nissl staining results in rats’hippocampus among groups
Nissl staining in the normal group showed that the positive neuron number in the hippocampal CA3 area was greater with denser distribution. Most of the cell bodies were round with clear nucleoli and prominent protrusions. Compared with the normal group, the neuron number was reduced (P<0.05), showing morphological changes such as cell body shrinkage,unclear nucleoli, and reduced protrusions in the model group. Compared with the model group, the neuron number was increased significantly (P<0.05), the cell morphology was similar to that in the normal group,and there were more cell protrusions in the EA group,the drug group, and the acupuncture-medication combined group; while that in the acupuncture-medication combined group was more obvious (P<0.05). It is suggested that EA and resveratrol have neuroprotective effects on neurons in the hippocampal CA3 region of AD rats, and the combination has stronger neuroprotective effects(Figure 1 and Table 2).
2.3 Comparison of the immunohistochemical staining in the hippocampus of rats among groups
Compared with the normal group, the SIRT1 expression in rat hippocampal CA3 region of the model group was significantly decreased, the SIRT1 positive cell number was significantly decreased (P<0.05), while the FOXO3a expression was enhanced, and the number of FOXO3a immunopositive neurons was significantly increased (P<0.05). Compared with the model group,the SIRT1 expression in the EA group and the drug group was significantly increased, and the SIRT1 positive cell number was significantly increased (P<0.05), while the FOXO3a expression was significantly decreased, and the FOXO3a positive neurons were significantly decreased (P<0.05). The change in the acupuncture-medication combined group was most significant (P<0.05). See Table 2, Figure 2, and Figure 3.
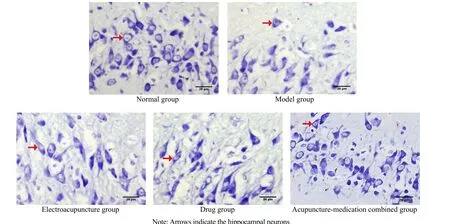
Figure 1. Neurons in the hippocampal CA3 region of rats in each group (Nissl staining, ×400)
Table 2. Comparing the hippocampal cell numbers among groups ( ±s, cells/high power field)

Table 2. Comparing the hippocampal cell numbers among groups ( ±s, cells/high power field)
Note: SIRT1=Silent information regulator of transcription 1; FOXO3a=Forkhead box protein O3a; compared with the normal group,1) P<0.05; compared with the model group, 2) P<0.05; compared with the electroacupuncture group, 3) P<0.05; compared with the drug group, 4) P<0.05
Group n Nissl staining Immunohistochemical staining SIRT1 FOXO3a Normal 6 29.83±2.04 25.17±1.17 9.83±1.83 Model 6 19.67±2.341) 14.83±1.171) 19.17±2.401)Electroacupuncture 6 23.67±1.032) 19.17±2.142) 15.17±1.942)Drug 6 23.33±1.862) 20.33±1.212) 14.67±1.632)Acupuncture-medication combined 6 27.33±2.162)3)4) 24.17±1.722)3)4) 10.33±1.632)3)4)F-value 24.504 44.020 24.337 P-value <0.001 <0.001 <0.001
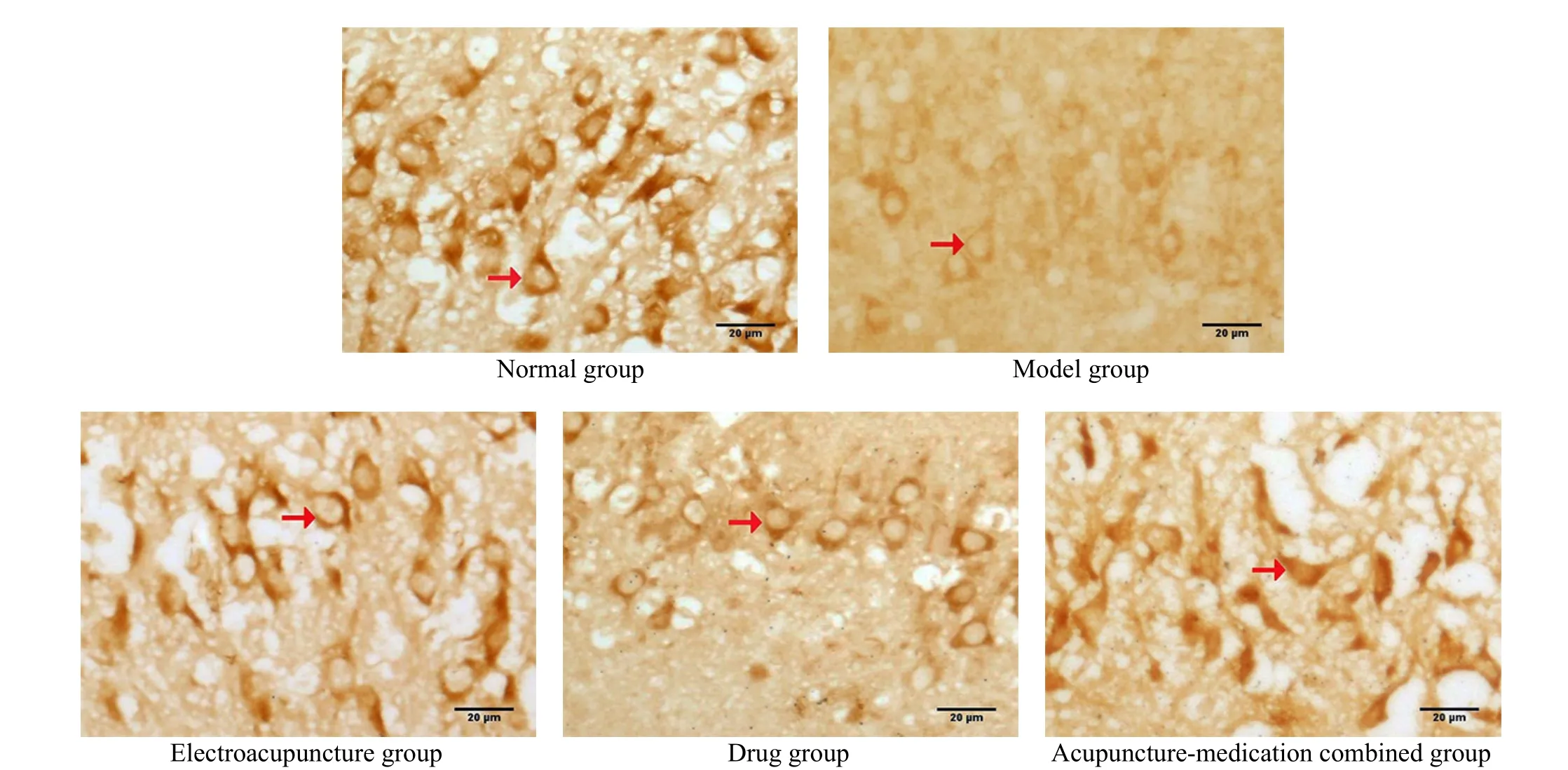
Figure 2. SIRT1-positive neurons in the hippocampal CA3 region of rats among groups (immunohistochemical staining, ×400)
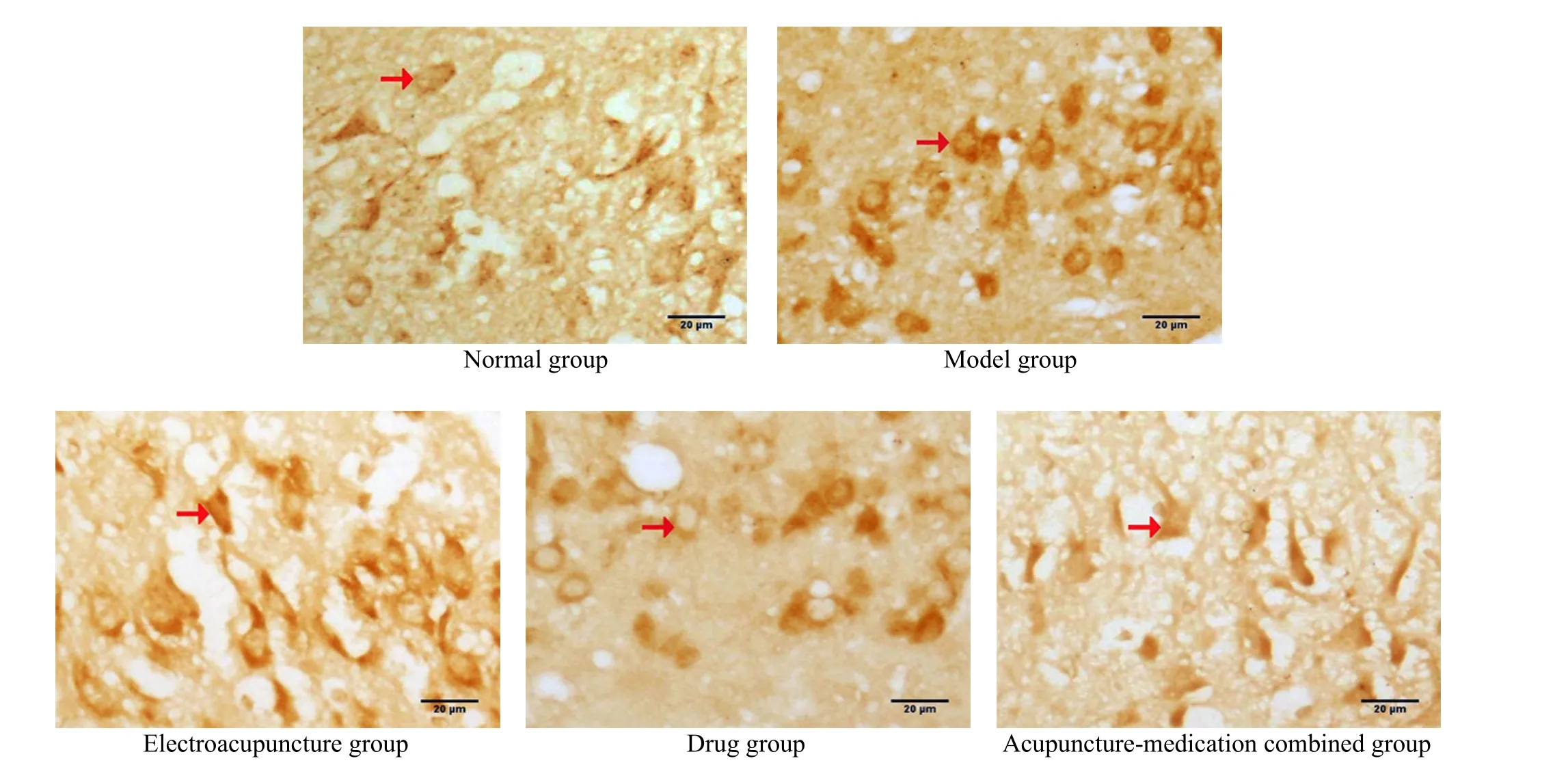
Figure 3. FOXO3a-positive neurons in the hippocampal CA3 region of rats in each group (immunohistochemical staining, ×400)
2.4 Comparison of the WB results in rats’ hippocampus among groups
Compared with the normal group, the SIRT1 protein expression was significantly decreased (P<0.05), and the FOXO3a protein expression was increased (P<0.05) in rat hippocampus in the model group. Compared with the model group, the SIRT1 protein expression was increased (P<0.05), and the FOXO3a protein expression was decreased (P<0.05) in the three intervention groups, suggesting that EA and resveratrol can upregulate the SIRT1 protein expression, down-regulate the FOXO3a protein expression in the hippocampus of AD rats. The regulation effect of EA combined with resveratrol was particularly significant (P<0.05). See Figure 4 and Table 3.
2.5 Comparison of the MDA content and the SOD activity in the hippocampus of rats among groups
Compared with the normal group, the MDA content was significantly increased (P<0.05), and the SOD activity was decreased (P<0.05) in the model group.Compared with the model group, the MDA content was decreased and the SOD activity was enhanced (P<0.05)in the EA group and the drug group; the effect of the acupuncture-medication combined group was more significant than that of the EA group (Table 4).
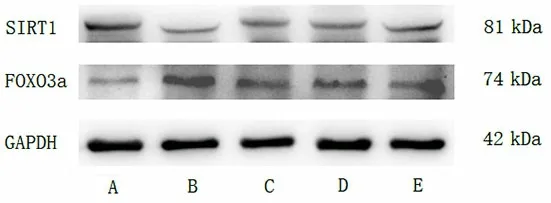
Figure 4. Expression of SIRT1 and FOXO3a proteins in the hippocampus of rats in each group
Table 3. Comparing IOD values of SIRT1 and FOXO3a proteins in the hippocampus of rats among groups ( ±s)
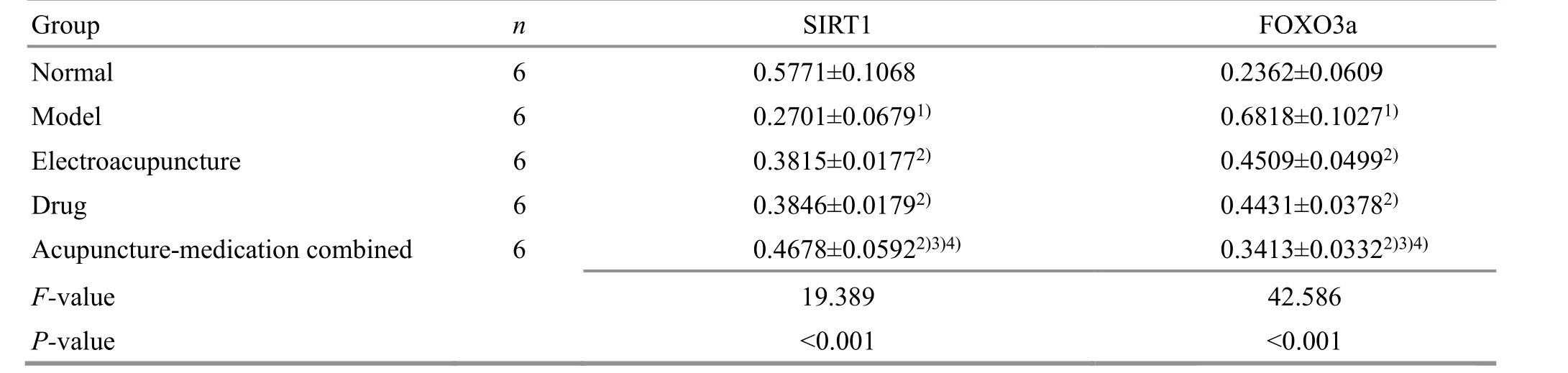
Table 3. Comparing IOD values of SIRT1 and FOXO3a proteins in the hippocampus of rats among groups ( ±s)
Note: SIRT1=Silent information regulator of transcription 1; FOXO3a=Forkhead box protein O3a; IOD=Integrated optical density;compared with the normal group, 1) P<0.05; compared with the model group, 2) P<0.05; compared with the electroacupuncture group,3) P<0.05; compared with the drug group, 4) P<0.05
Group n SIRT1 FOXO3a Normal 6 0.5771±0.1068 Model 6 0.2701±0.06791)Electroacupuncture 6 0.3815±0.01772)Drug 6 0.3846±0.01792)Acupuncture-medication combined 6 0.4678±0.05922)3)4)F-value 19.389 P-value <0.001 0.2362±0.0609 0.6818±0.10271)0.4509±0.04992)0.4431±0.03782)0.3413±0.03322)3)4)42.586<0.001
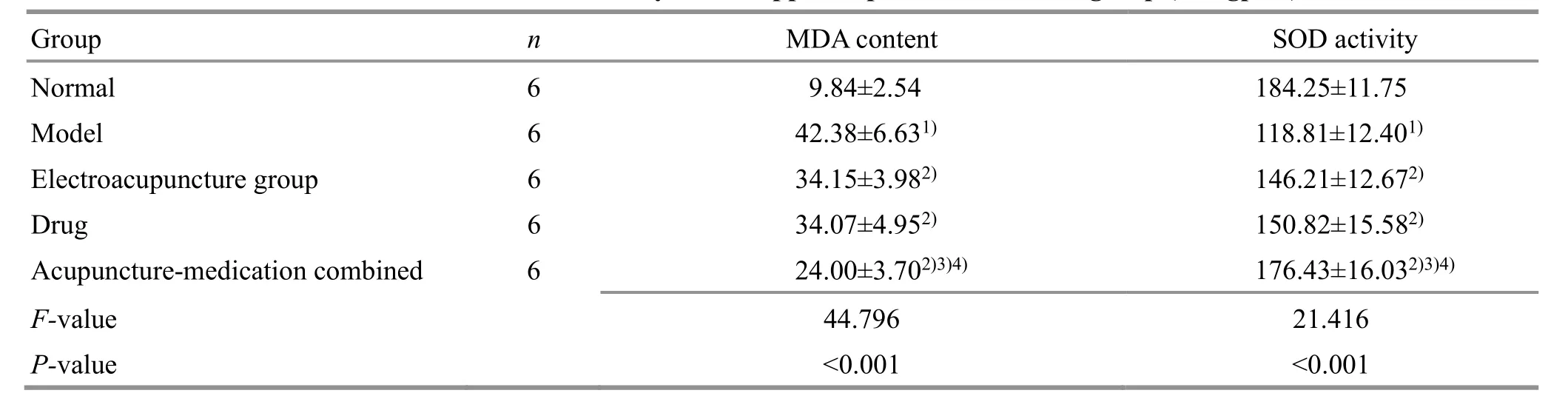
Table 4. The results of MDA content and SOD activity in the hippocampus of rats in each group (U/mgprot)
3 Discussion
Resveratrol is a natural polyphenol, mainly found in berries, nuts, and grapes[19], with natural antioxidant and free radical scavenging effects. To date, resveratrol is considered to be the strongest SIRT1 agonist among polyphenols. SIRT1 plays a key role in resveratrolmediated anti-inflammatory effect, anti-aging effect,and prevention of neurodegenerative diseases and cognitive dysfunction[20-21]. Activation of SIRT1 by resveratrol prevents Aβ-induced glial cell death and promotes Aβ metabolism by regulating APP, which improves cognitive function[21]. FOXO3a, one of the members of the FOXO family, promotes apoptosis.Studies have shown that SIRT1 can deacetylate FOXO3a and then degrade FOXO3a through ubiquitination,reduce the existing FOXO3a protein level, inhibit the ability of FOXO3a to induce cell death, and ultimately protect cells from oxidative stress damage[22].Conversely, when the level of SIRT1-deacetylated FOXO3a is reduced, FOXO3a accelerates the oxidative stress process and induces neuropathies. Therefore,activating the SIRT1-FOXO3a signaling pathway is considered an important mechanism for cells to resist oxidative damage, which also provides new ideas for enhancing the transduction of the SIRT1-FOXO3a signaling pathway improving the resistant ability against oxidative stress, thus intervening in AD. This study found that resveratrol improved the learning and memory function of AD rats, significantly reduced the damage of STZ on neurons in the CA3 region of the hippocampus, up-regulated the SIRT1 expression, and reduced the FOXO3a level.
The main pathological changes of AD are the deposition of Aβ protein and the tangles of nerve fibers.The high oxygen consumption in the brain and the lack of antioxidant enzymes lead to oxidative damage caused by free radicals during the pathogenesis of AD,which is an important factor in the induction of neurofibrillary tangles and senile plaques[10]. Under oxidative stress, oxygen free radicals attack various unsaturated fatty acids in biofilms, causing lipid peroxidation. Brain tissue is more sensitive to oxidative stress due to its greater oxygen consumption, a higher concentration of unsaturated fatty acids, and lower glutathione levels. MDA is the final product of lipid peroxidative decomposition, whose cytotoxic effect can induce neuronal degeneration and necrosis in AD[11].The MDA content can be used as an important detection indicator for the degree of lipid peroxidation.SOD is an oxygen-free radical scavenging enzyme and can reduce or eliminate the damage of superoxide anion free radicals to the body through disproportionation[12]. The ability of the body to scavenge free radicals is assessed by the SOD activityin vivoor in tissue. Our current results showed that the learning and memory abilities of rats were reduced.Meanwhile, the hippocampal neurons showed a significantly reduced number, shrunken cell body,reduced protrusions, increased MDA content, and significantly decreased SOD level in the model group.These results suggest that the increased peroxidation degree in AD rats results in the damage of hippocampal neurons, which is closely related to learning and memory dysfunction.
At present, there is no specific medicine for AD treatment. Acupuncture in traditional Chinese medicine has significant advantages in AD treatment, such as fewer adverse reactions and confirmed curative effects.Traditional Chinese medicine believes that AD is caused by kidney essence deficiency, phlegm coagulation, and blood stasis in the elderly. Acupuncture at Dazhui (GV14)can balance Yin and Yang, remove blood stasis, and resolve phlegm. Acupuncture at Zusanli (ST36) can regulate the spleen and stomach, invigorate the spleen and stomach to invigorate Qi, dredge the meridian passage, and dispel wind and dissolve dampness. PENG J,et al[23]treating AD via EA at Dazhui (GV14) with other points of the Governor Vessel significantly improved the overall intelligence and cognitive function of the patients. Based on clinical observation, BI D Y,et al[24]found that EA at Zusanli (ST36) and Fenglong (ST40)significantly increased the mini-mental state examination score and Barthel index score in senile dementia patients, which was more effective than the Western medication. The animal experimental results showed that EA at Dazhui (GV14) and Zusanli (ST36)reduced the production of APP and Aβ in the hippocampus, reduced the MDA content, enhanced the body’s antioxidant capacity, reduced the damage caused by oxidative stress to the body, and promoted the repair of neurons in the hippocampus, thereby preventing the occurrence of AD[25-27]. This study found that EA improved the learning and memory function of AD rats, improved the expression levels of SOD and MDA, and protected hippocampal neurons, which further verified the effectiveness of EA in AD treatment.
The high incidence and the unclear etiology of AD still plague the medical community. A single treatment method has a certain effect on delaying the pathogenesis of AD and improving clinical symptoms,but it is not ideal; combination therapy is gaining more and more attention[1-2]. In recent years, combined acupuncture-medication treatments have been widely used in AD treatment. Data analysis showed that acupuncture plus traditional Chinese medicine and acupuncture plus Western medicine had no statistical difference in reducing the scores of activities of daily living in AD patients, but the former was more effective in improving the mini-mental state examination score[28].
The current work revealed that both EA and resveratrol alone showed certain neuroprotective effects in treating AD, but their combined effect was more obvious. Together with the literature, we believe this may relate to the following reasons. First, EA improves the overall function of the body, enhances the gastrointestinal tract’s absorption of resveratrol, and increases the blood’s effective drug concentration.Second, EA promotes micro-angiogenesis, thereby increasing the blood flow of brain microcirculation,improving the oxygen supply to brain tissue[29], and promoting the further penetration of resveratrol through the blood-brain barrier[30], which is beneficial to the resveratrol absorption by the target organ of the brain. Third, EA and resveratrol share some common regulatory pathways in reducing Aβ production and inhibiting oxidative stress and inflammatory response,and the combination produces a superimposed synergistic effect. However, the exact mechanism still needs to be further explored.
The advantages of traditional Chinese medicine need to be continuously explored and carried forward.Although the combination of various methods in AD clinical treatment has achieved certain curative effects,there are still disputes on the specific implementation methods and mechanisms. With the advancement of science and technology and the development of medical undertakings, exploring a more effective comprehensive treatment strategy for AD prevention and treatment is the direction and goal for future research.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Project of National Natural Science Foundation of China (国家自然科学基金项目, No. 81901105); Leading Scientific Research Project of Wannan Medical College (皖南医学院重点科研基金项目, No. WK2020Z12); Cultivating Fund for Leading Scientific Research Project of Wannan Medical College (皖南医学院重点科研项目培育基金, No. WK2016Z09).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 18 June 2021/Accepted: 29 November 2021
 Journal of Acupuncture and Tuina Science2022年5期
Journal of Acupuncture and Tuina Science2022年5期
- Journal of Acupuncture and Tuina Science的其它文章
- Efficacy of mild moxibustion combined with surgery for meniscal injury and its effect on TGF-β1 and PDGF levels in the fluid of knee joint
- Improvement effect of acupuncture on locomotor function in Parkinson disease via regulating gut microbiota and inhibiting inflammatory factor release
- Effects of Mo-Rubbing abdomen manipulation on glucose metabolism and inflammatory factors in rats with type 2 diabetes mellitus
- Observation on the therapeutic efficacy of Tuina plus “three-bridge” exercise for non-specific low back pain
- Influence of herbal cake-partitioned moxibustion on lumbar functions and inflammatory factors in patients with lumbar disc herniation due to kidney deficiency and blood stasis
- Effect of acupuncture-like transcutaneous electrical nerve stimulation on labor pain in nulliparous women: a randomized controlled trial
