基于含双吡唑的四羧酸配体构筑的Fe(Ⅱ)/Co(Ⅱ)同构配合物的合成、晶体结构及磁性质
李芬芳 何 婧
(晋中学院化学化工系,晋中 030600)
0 Introduction
The prudent design of coordination polymers(CPs)by connecting the metal ions as single point nodes or secondary building units(SBU)with a variety of organic ligands as linkers has attracted immense interest in the past few decades[1].This is not only due to their fascinating network structures and novel functionalities but also due to the ease of tuning such structures with the change of linkers and metals.It has been observed CPs have great potential for multiple applications depending on their structure,chemical composition,particle size,etc.Thus,in the last decades,CPs have demonstrated usefulness in fields such as hydrogen and methane storage capture[2],separation of CO2[3],water adsorption[4],solvent sponge behavior[5],controlled drug entrapment and release[6],heterogeneous catalysis[7],and luminescence[8].While many of these applications are based on the framework porosity,CPs materials also exhibit physical properties traditionally associated with highly dense oxide systems.For example,CPs may also have interesting magnetic properties because the magnetic metal ions and their coupling can be tailored in the CP structure through the incorporation of magnetic moment carriers such as paramagnetic metals,open-shell organic ligands,or both[9].So the designed syntheses of CPs having attractive magnetic properties are immensely important among the ever-growing number of functional applications.Such a class of compounds was investigated to design magnetic materials because magnetic coupling can easily be tuned and controlled by altering the linkers and nodes[10-13].Side by side it is also very important to understand the magnetic exchange pathway to rationalize the exact design of magnetically functional CPs.
As magnetism is a cooperative phenomenon,a connection between moment carriers at distances within the interacting range is necessary;carboxylic-based and nitrogen-based ligands have proved to have good superexchange pathways for magnetic couplings[14].Especially,the carboxylate-based bridging ligand is one of the very popular choices for the execution of magnetic CPs.Thus,coordination architectures having carboxylate-donating linkers and paramagnetic metal ions have attracted the attention of contemporary research for understanding the magnetic exchange through the OCO bridges of the carboxylate ligands which show versatile binding abilities.In addition,flexible diamagnetic ligands are usually used to link magnetic d-or f-block metal ions into extended networks,facilitating magnetic exchange in one,two,and three dimensions[15-16].Paramagnetic transition metal elements allow the variation of spin quantum number and magnetic anisotropy,two important parameters in magnetism.Among these elements,Co(Ⅱ) and Ni(Ⅱ) appear as the preferred choice to develop magnetic CPs,because it provides the highest magneto-crystalline anisotropy,which results in record magnetic hardness[17].
In the CPs,there are self-assemblies of isomorphous or isotopologue compounds,which provide variations of magnetic anisotropy and spin quantum numbers that affect the magnetic behavior of such isomorphous systems,such as[M(L)2(CH3OH)2](M=Mn(Ⅱ),Fe(Ⅱ),and Co(Ⅱ),HL=2,6-bis(pyrazole-1-yl)pyridine-4-carboxylic acid),M(HCOO)2(4,4'-bpy)·nH2O(M=Co(Ⅱ)and Ni(Ⅱ));[M(L)(N3)]n·3nH2O(M=Mn(Ⅱ),Co(Ⅱ),and Ni(Ⅱ),L-=1-(4-carboxylatobenzyl-pyridinium-4-carboxylate),and[M(H2bpta)]n(H4bpta=2,2',4,4'-biphenyltetracarboxylic acid,M=Fe(Ⅱ),Ni(Ⅱ),Cu(Ⅱ),and Zn(Ⅱ))[18-20].The magnetic interactions between transition metal centers usually are mainly mediated through M-L-M super-exchange which is important in magnetic orbits of metal ions,in which the nd-orbits of metal ions are combined out of phase with the np-orbits of ligands.The orbital interaction plays a significant role in the spin Hamiltonian for a given magnetic system.Indeed,a substantial number of CPs with magnetic properties have been reported,due to the use of constitutive openshell transition metal ions within the framework of the structure.
With this in mind,we selected a ligand 1,1'-(1,4-phenylenebis(methylene))bis-(1H-pyrazole-3,5-dicarboxylic acid))(H4L,Scheme 1),succeeded in obtaining two new isomorphous 2D complexes{(NH2(CH3)2)2[Fe(L)]}n(1)and{(NH2(CH3)2)2[Co(L)]}n(2),and analyzed magnetic properties of the two complexes.The result of variable-temperature magnetic measurements exhibits antiferromagnetic exchange interactions in complexes 1 and 2.
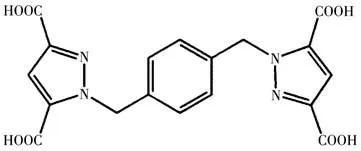
Scheme 1 Structure of H4L
1 Experimental
1.1 General methods and materials
H4L was purchased from Jinan Henghua Science&Technology Co.,Ltd.,China.All solvents and other reagents were commercially available and were used without further purification.Fourier transform(FT)IR spectra were taken on a BRUKER TENSOR27 spectrometer in a 4 000-400 cm-1region with KBr pellets.Elemental analyses of C,H,and N were recorded on a CHNO-Rapid instrument.Powder X-ray diffraction(PXRD)data were collected on a Bruker D8 Advance X-ray diffractometer with Cu Kα radiation(λ=0.154 18 nm)and the data were recorded within a 2θ range of 5°-50°.The working voltage and current were 60 kV and 50 mA,respectively.The calculated PXRD patterns were generated from the single-crystal X-ray diffraction data using PLATON software.Magnetic susceptibility data were obtained with a SQUID magnetometer(Quantum MPMS-VSM)in a temperature range of 1.8-300.0 K by using an applied field of 2 000 Oe.The magnetic susceptibility data were corrected for the diamagnetism of the samples using Pascal constants.Thermogravimetric analyses(TGA)were carried out with a Dupont thermal analyzer in a temperature range of 25-800℃under an N2flow with a heating rate of 5℃·min-1.
1.2 Preparation of complexes 1 and 2
A mixture of H4L(20.7 mg,0.05 mmol),FeSO4·7H2O(27.8 mg,0.10 mmol),SnCl2·2H2O(11.3 mg,0.05 mmol),and 10 mL of mixed solvents(acetonitrile/DMF/water,3∶3∶4,V/V)was placed in a 15 mL Teflonlined stainless steel autoclave.The mixture was heated under autogenous pressure at 160℃for 72 h and then cooled to room temperature naturally.Red block-shaped crystals of 1 were collected by filtration,washed with H2O,and dried in the air.Yield:70%.Anal.Calcd.for C22H26FeN6O8(%):C 47.1,H 4.63,N 14.9;Found(%):C 47.2,H 4.66,N 14.8.IR(KBr,cm-1):3 437s,3 130w,1 605s,1 533m,1 476m,1 362s,1 277m,1 241m,1 106 w,1 013s,821s,785w,764m,543m.
The preparation process of complex 2 was the same as that of 1,except that FeSO4·7H2O and SnCl2·2H2O were replaced by CoCl2·3H2O(23.8 mg,0.10 mmol).Pink block-shaped crystals of 2 were collected,washed with H2O,and dried in air.Yield:70%.Anal.Calcd.for C22H26N6CoO8(%):C 47.0,H 4.63,N 15.0;Found(%):C 46.8,H 4.64,N 14.8.IR(KBr,cm-1):3 419 w,2 802w,2 399w,1 922w,1 610s,1 525m,1 487m,1 355s,1 274m,1 245m,1 107w,1 024s,837s,792w,767m,550m.
1.3 X-ray crystallography
The data for complex 1 were collected using a SuperNova(Cu)X-ray Source diffractometer utilizing Cu Kα (λ=0.154 18 nm)radiation at 173(2)K.Singlecrystal X-ray diffraction data for complex 2 were collected in the Beijing Synchrotron Radiation Facility(BSRF)beamline 3W1A,which were mounted on a MARCCD-165 detector(λ=0.071 00 nm)with the storage ring working at 2.5 GeV.In the process,the crystal was protected by liquid nitrogen at 100(2)K.Data was collected by the program MARCCD and processed using HKL 2000.
All the structure was solved by direct methods employed in the program SHELXS-2014 and refined by full-matrix least-squares methods against F2with SHELXL-2016.The determination of cell parameters and data reduction was performed with SAINT Plus.Program SADABS was used for absorption corrections.After all non-H atoms were refined anisotropically,hydrogen atoms attached to C atoms were placed geometrically and refined using a riding model approximation,with a C—H length of 0.093 nm and Uiso(H)=1.2Ueq(C).A summary of the crystallographic data and data collection and refinement parameters for both complexes are listed in Table 1.
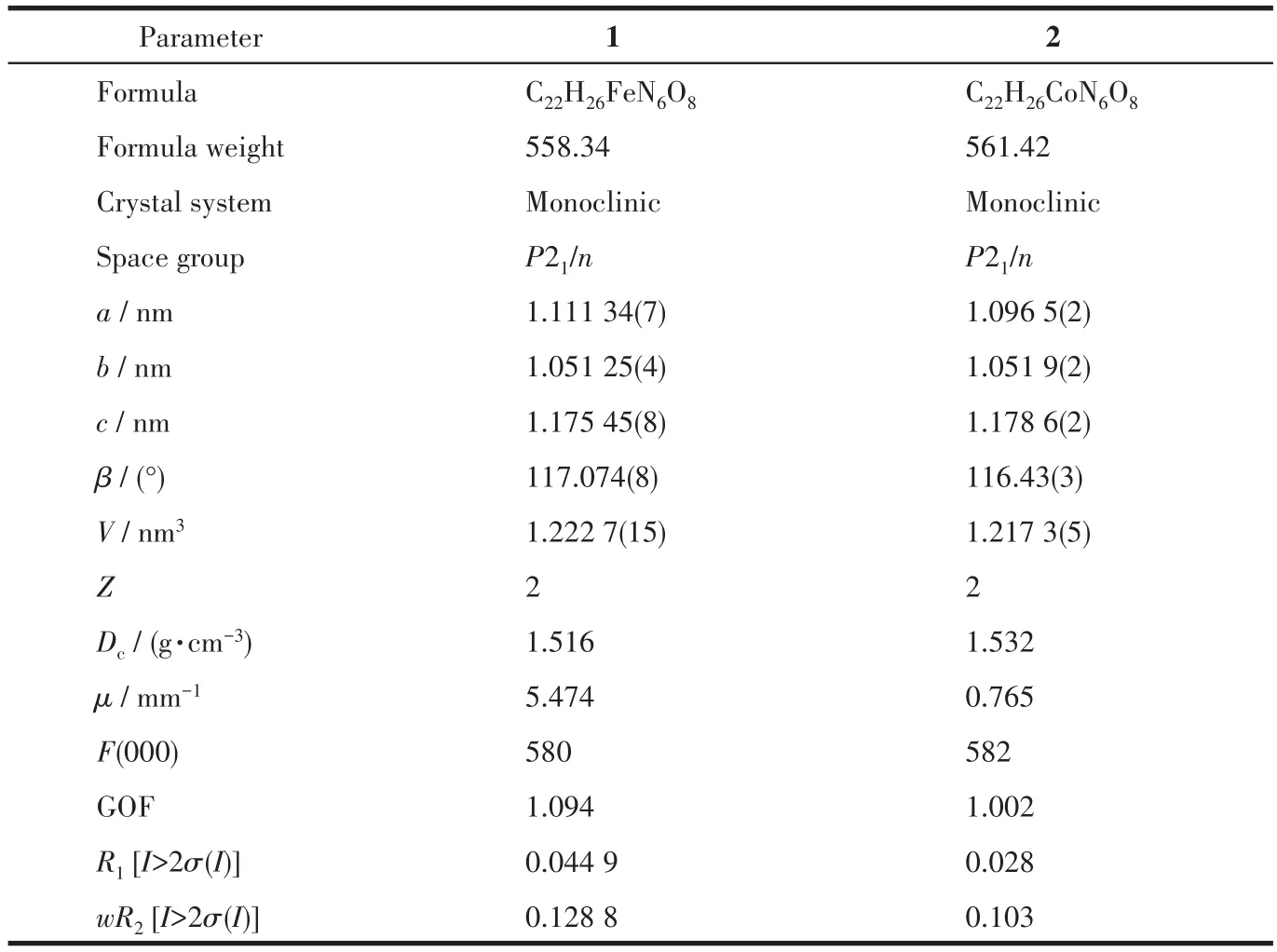
Table 1 Crystal data and structure refinement parameters for complexes 1 and 2
CCDC:1923316,1;1923318,2.
2 Results and discussion
2.1 IR characterization
The peaks of FT-IR indicate that the strong broad absorption bands in the range between 3 419 cm-1should be assigned to the characteristic vibrations of the νN—Hstretching frequencies.The absence of strong bands around 1 706 cm-1in the FT-IR spectra indicates that—COOH group has been completely deprotonated to generate L4-anions,which is in agreement with that from its X-ray single crystal structure,and the characteristic strong bands of the coordinated carboxylate groups appeared at 1 610-1 525 cm-1for the asymmetric stretching and 1 355-1 274 cm-1for the symmetric one(Fig.1).
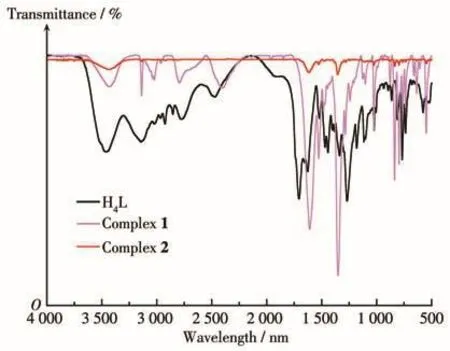
Fig.1 FT-IR spectra of H4L and complexes 1 and 2
2.2 Description of crystal structures
Since the two complexes are isomorphous,only the structure of complex 1 will be described here.Complex 1 crystallizes in the P21/n space group containing the anion framework.The asymmetric unit consists of a half Fe2+ion,a half of a fully deprotonated L4-ligand,and one non-coordinated protonated dimethylamine cation by hydrolysis of DMF(Fig.2).As can be deduced from the charge balance,the framework is anionic having a-2 charge,and the electroneutrality is achieved by the incorporation of the protonated amine in the voids of the net.In complex 1,the Fe(Ⅱ)ion is surrounded by four oxygen atoms(O1,O1iii,O3i,O3ii)from four L4-ions and two nitrogen atoms(N2,N2iii)of two different L4-ions to present an octahedron geometry where the cis-and trans-angles separate at the metal is 76.65(8)°-180.0°.The Fe—O and Fe—N bond lengths are in a range of 0.206 3(2)-0.216 5(2)nm and 0.216 7(2)nm,respectively,which are slightly shorter than those of Fe(Ⅱ)complexes[21].The bond lengths(M—O and M—N)for the two complexes decrease with the increase of the d-electronic number,in agreement with the radius variation of the metal ions(Table 2).

Fig.2 Atom labels and coordination environments of the Fe(Ⅱ)ions in complex 1 with displacement ellipsoids drawn at the 30% probability level
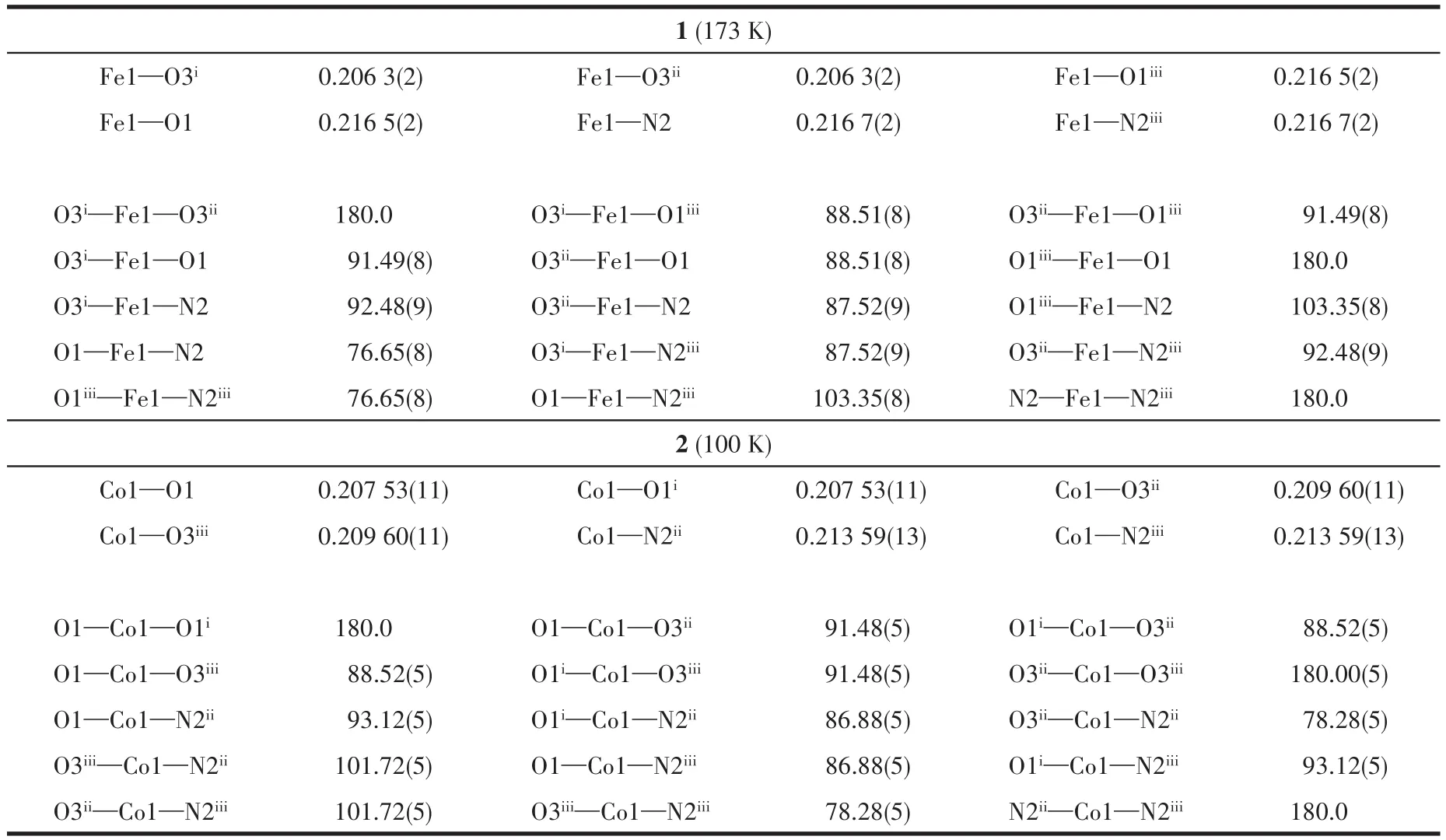
Table 2 Selected bond distances(nm)and angles(°)around metal centers in isomorphous polymers 1 and 2
As shown in Fig.3a,in complex 1,two carboxyl groups of pyrazole are fully deprotonated,each ligand bridges two Fe(Ⅱ)ions through chelating N,O atoms of the pyrazole ring and monodentate O atom of the same pyrazole ring,forming…Fe-L-Fe-L… chains parallel to the crystallographic b direction.The Fe…Fe distance separated by a μ1,5-pyrazole-carboxylate bridge is 0.795 7(1)nm(Co…Co 0.798 0(2)nm for 2).The dihedral angle between the planes of the benzene ring andpyrazole rings is 71.07(3)°.The 1D chains intersect to form an infinite 2D network that contains nearly square Fe4L4units.For each unit,four L4-anions act as the four edges,and four Fe(Ⅱ)ions represent the four vertices.The lengths of the diagonals are 1.051 3 and 1.194 8 nm,compared to 1.060 9 and 1.200 4 nm for 2,and the interior angles are 82.68°and 97.32°,compared to 82.46°and 97.54°for 2.
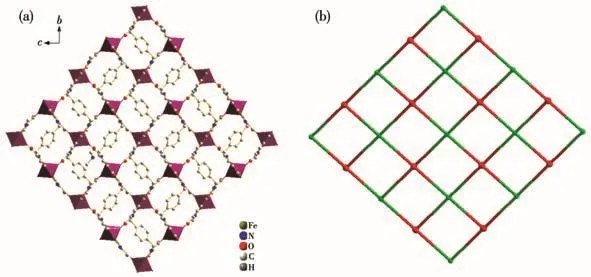
Fig.3 (a)Two-dimensional sheets extending in the b-axis and c-axis of complex 1;(b)Topology net of complex 1 with Schläfli symbol{312.414.52}
From the topological point of view,the frameworks of complexes 1 and 2 can be simplified by the application of a(4,4)-connected topological approach(Fig.3b)using TOPOS[22].Each ligand is linked to four Fe2+ions to act as a 4-connected node;each Fe2+ion is bound by four L4-ions to act as a 4-connected node.The topological notation is{312.414.52}from TOPOS program analysis.
To confirm that the phase of the bulk sample is pure and the crystal structures of 1 and 2 are truly representative of the bulk material,the PXRD experiments were carried out.As shown in Fig.4,PXRD patterns of 1 and 2 were determined at room temperature,which matched well with those simulated from their X-ray single crystal diffraction data,and the high purity of the complexes can be confirmed.In addition,TGA results indicate that complexes 1 and 2 were stable until about 604 K(Fig.5).
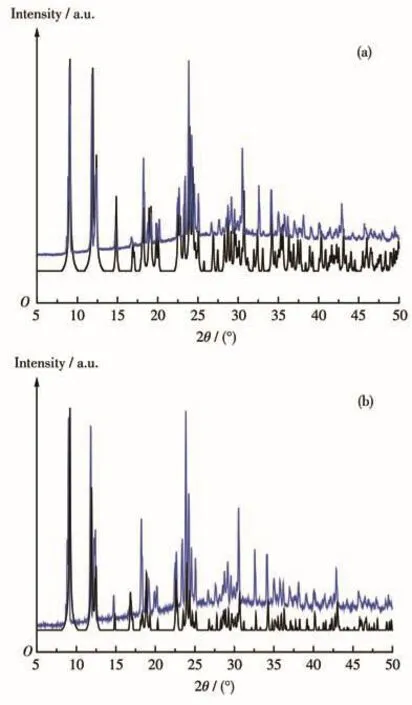
Fig.4 Simulated(bottom)and experimental(top)PXRD patterns of complexes 1(a)and 2(b)

Fig.5 TGA curves for complexes 1 and 2
2.3 Magnetic properties
To gain insight into magnetic changes in isomorphous polymers,magnetic measurements were carried out on the well-crushed crystalline samples.Variabletemperature magnetic susceptibilities of the two complexes were measured in a temperature range of 1.8-300.0 K with an applied magnetic field of 2 000 Oe.
As shown in Fig.6a,the χMT value of 1(3.07 cm3·mol-1·K)at 300.0 K was larger than the spin-only χMT of 3.00 cm3·mol-1·K expected for a single isolated highspin Fe(Ⅱ) ion(g=2.0 and S=2).Upon cooling,the χMT value decreased smoothly and reached a minimum of 1.67 cm3·mol-1·K at 2.0 K,which indicates a characteristic feature of antiferromagnetic coupling between Fe(Ⅱ) ions.The Curie-Weiss fit,namely χ=C/(T-θ),in the range of 1.8 to 300.0 K afforded a Curie constant of C being 3.11 cm3·mol-1·K and a Weiss constant of θ being-0.49 K(Fig.6a,Inset).
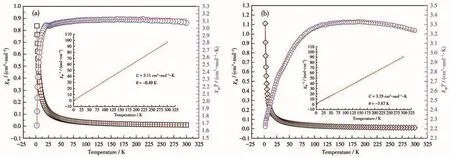
Fig.6 Temperature dependence of χMT and 1/χMcollected in an applied field of 2 000 Oe for complexes 1(a)and 2(b)
To further investigate the magnetic properties of 1,the data can be fitted upon 7.0 K by an expression(Eq.1)for S=2 systems[23]:

where J is the coupling constant between the neighboring Fe(Ⅱ) ions;N is Avogadro's number;β is the Bohr magneton;k is the Boltzmann constant;g is the Lande value.
The best fit well reproduced the experimental data over the entire temperature range with g=1.95,J=-0.181 cm-1with an agreement factor(R)of 5.7×10-4,where R=∑(χMTexp-χMTcal)2/∑(χMTexp)2.The negative θ and J values indicate the presence of weak antiferromagnetic interaction between adjacent Fe(Ⅱ)ions.
According to the literature[24],it can be deduced that the unpaired spin in egorbitals favor ferromagnetic interactions,whereas those in t2gorbitals favor stronger antiferromagnetic interactions,with only one unpaired electron in a t2gorbital being enough to dominate the overall superexchange.Therefore,Fe(Ⅱ) complexes should show antiferromagnetic.Our result is in good agreement with these expectations[25].
As shown in Fig.6b,the χMT value of 2(3.23 cm3·mol-1·K)at 300.0 K was larger than the spin-only value(1.88 cm3·mol-1·K,g=2.0 and S=3/2).Upon cooling,the χMT value slightly increased first,then decreased and reached 2.22 cm3·mol-1·K at 2.0 K,which indicates a characteristic feature of antiferromagnetic coupling between Co(Ⅱ)ions.The magnetic susceptibility obeys the Curie-Weiss law with a Curie constant C=3.35 cm3·mol-1·K,and a Weiss constant θ=-3.87 K(Fig.6b,Inset).We apply the following expressions(Eq.2 and 3)for a 1D Co(Ⅱ)chain to fit the data[26]:


where x=|J|kT;zj' represents the interaction between Co(Ⅱ)ions and J is the parameter of exchange interaction between the neighboring Co(Ⅱ)ions.The susceptibility values above 50 K were calculated,resulting in J=-0.11 cm-1,g=1.85,and the agreement factor defined by R= ∑ (χMTexp- χMTcal)2/∑ (χMTexp)2was 7.2×10-5.A negative J confirms a weak antiferromagnetic exchange that agrees with a negative θ value.
3 Conclusions
In summary,we have successfully constructed two new isomorphous coordination polymers from the 1,1'-(1,4-phenylenebis(methylene))bis-(1H-pyrazole-3,5-dicarboxylic acid))ligand under a similar synthetic procedure.The framework features anionic having a-2 charge and protonated dimethylamine cation by hydrolysis of DMF to maintain the electroneutrality of the complex.Magnetic studies indicate the presence of antiferromagnetic exchange for complexes 1 and 2.

