Incorporation of κ-carrageenan improves the practical features of agar/konjac glucomannan/κ-carrageenan ternary system
Dongling Qio,Ho Li,Ftng Jing,Siming Zho,Sheng Chen,Binji Zhng,*
a Chongqing Key Laboratory of Speciality Food Co-Built by Sichuan and Chongqing,College of Food Science,Southwest University,Chongqing 400715,China
b Glyn O.Phillips Hydrocolloid Research Centre at HBUT,School of Food and Biological Engineering,Hubei University of Technology,Wuhan 430068,China
c Group for Cereals and Oils Processing,College of Food Science and Technology,Key Laboratory of Environment Correlative Dietology (Ministry of Education),Huazhong Agricultural University,Wuhan 430070,China
d Yellow Crane Tower Science and Technology Park (Group) Co.,Ltd.,Wuhan 430040,China
Keywords:Agar/konjac glucomannan/κ-carrageenan ternary system Component interaction Multi-scale structure Practical features
ABSTRACT Three materials (agar,konjac glucomannan (KGM) and κ-carrageenan) were used to prepare ternary systems,i.e.,sol-gels and their dried composites conditioned at varied relative humidity (RH) (33%,54% and 75%).Combined methods,e.g.,scanning electron microscopy,small-angle X-ray scattering,infrared spectroscopy (IR) and X-ray diffraction (XRD),were used to disclose how κ-carrageenan addition tailors the features of agar/ KGM/κ-carrageenan ternary system.As aff irmed by IR and XRD,the ternary systems with κ-carrageenan below 25% (agar/KGM/carrageenan,50:25:25,m/m) displayed proper component interactions,which increased the sol-gel transition temperature and the hardness of obtained gels.For instance,the ternary composites could show hardness about 3 to 4 times higher than that for binary counterpart.These gels were dehydrated to acquire ternary composites.Compared to agar/KGM composite,the ternary composites showed fewer crystallites and nanoscale orders,and newly-formed nanoscale structures from chain assembly.Such multi-scale structures,for composites with κ-carrageenan below 25%,showed weaker changes with RH,as revealed by especially morphologic and crystalline features.Consequently,the ternary composites with less κ-carrageenan (below 25%) exhibited stabilized elongation at break and hydrophilicity at different RHs.This hints to us that agar/KGM/κ-carrageenan composite systems can display series applications with improved features,e.g.,increased sol-gel transition point.
1.Introduction
Encapsulation of food ingredients (such as nutrients and bioactive compounds) shows many advantages,e.g.,keeping away from unfriendly conditions,masking unpleasant f lavor,allowing controlled release,and increasing solubility and dispersibility [1-4].Among the encapsulation forms,core-shell capsules (having an external shell and an internal ingredient core) show regulatable structure/performance and thus attract huge interest in food ingredient encapsulation [5].
Proper materials such as alginate can be used as shells for the capsules;the shell material and the encapsulant solution firstly construct core-shell droplets,and then the shell material undergoes a hardening process to form capsules [6,7].The hardening of shell material is normally a gelation process,being previously realized by calcium ions induced formation of ionic network in alginate.Nonetheless,this gelation process shows inherent drawbacks such as the deformed shape of the capsules [8]and the instability in the present of chelating agents [9].Alternatively,the shell materials(e.g.,agar and gelatin) with cold-induced gelation ability could be practiced.The cold-induced gelling materials normally show advantages over previously used alginate material,since they need only one step to harden (without the step for ion induced network formation) and display well stability under chelating agentcontaining conditions.
Agar,derived from red algae,shows cold-induced gelation ability.This material contains agarose and agaropectin fractions and have been used as gelling,thickening,water-holding and stabilizing materials in pharmaceutical,cosmetic,food,medical,and biotechnology industries [10-13].Also,konjac glucomannan(KGM),made up of glucose and mannose units (mole ratio of 1:1.6)linked byβ-1,4-glycosidic bonds [14],can be blended with agar to obtain composites with enhanced tensile strength [15].For agar/KGM composite systems,the gel point (sol-gel transition for hardening)and strength evidently influence the product performance such as the shapes and loading amounts of related capsules.Moreover,the inherent hydrophilicity of agar and KGM often endows the composites with relatively high sensitivity to relative humidity (RH) [16],causing changes in the structure and performance.Therefore,it is significant to tailor the gel point (sol-gel transition temperature) and strength of agar/KGM systems and to enhance the stability of practical(mechanical and hydrophilic) features of the obtained composites under changed RH.
To address these concerns,different methods such as inclusion of other biopolymers can be practiced to improve the performance of agar/KGM composites [17-19].As a renewable biopolymer,κ-carrageenan is a linear polysaccharide with repeating disaccharide units of 3,6-anhydrogalactose and galactose linked by alternatingα-(1,3)andβ-(1,4) glycosidic bonds,accompanied by one anionic sulfate group per disaccharide unit [20]and a highly negative charge [21].κ-Carrageenan displays abilities to tailor the features of agar or KGM system.Specifically,κ-carrageenan has similar chain structure with that of agar and shows synergy effects with KGM to form gels [22].Incorporatingκ-carrageenan reduces agar gel rigidity [23];and the inclusion ofκ-carrageenan to KGM system initially increases its Young’s modulus,followed by a plateau and a prominent increase in the fracture strain and stress [24].However,to date,there are still limited studies on howκ-carrageenan inclusion tailors the gelation performance of agar/KGM system as well as the mechanical and hydrophilic features of the resultant composites under varied RH.
In this study,the aqueous mixtures of agar/KGM/κ-carrageenan were prepared;and the ternary composites were acquired by removing water from the mixtures and were conditioned at different RHs (33%,54%,75%).We hypothesize that the addition ofκ-carrageenan can improve the practical features of the ternary agar/KGM/κ-carrageenan system,by increasing the sol-gel transition point and gel strength and stabilizing the mechanical and hydrophilic features of the composites with RH.Then,the sol-gel transition and gel strength of the mixtures were measured using rheological and textural analyses;and the structural and mechanical/hydrophilic features of the ternary composites were inspected through combined methods such as X-ray diffraction.The results indicate that the above hypothesis could be well verified;and the links between the multiscale structures and the practical features were discussed.With these experiments and discussion,the main aim of this study is to rationalize howκ-carrageenan addition tailors the practical features of agar/KGM/κ-carrageenan ternary system.The present data would facilitate the rational production of biopolymer composites with tailored performance.
2.Materials and methods
2.1 Materials
Agar (gel strength: 750–1 000 g/cm2) was commercially supplied by Biofroxx (Einhausen,Hessen,Germany);konjac glucomannan(molecular weight: 9.67 × 105Da;viscosity: 30 000 mPa·s at 1 g/100 mL)was obtained from Hubei Konson Konjac Technology Co.,Ltd.(Wuhan,China).κ-Carrageenan was purchased from Shanghai Macklin Biochemical Co.,Ltd.(Shanghai,China).Glycerol (analytical grade) as well as magnesium chloride,magnesium nitrate and sodium chloride (chemical purity) were supplied by Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).
2.2 Preparation of agar/KGM/κ-carrageenan ternary composites
Agar/KGM/κ-carrageenan (5.0 g) with different mass ratios(75:25:0,60:25:15,50:25:25,40:25:35,and 30:25:45,m/m),0.5 g of glycerol and 194.5 mL of distilled water were added into a threeneck flask.This flask was incubated at 90 °C along with stirring at 800 r/min for 1 h to obtain an aqueous ternary mixture.Then,the mixture was placed into a plastic plate (radius: 15 cm) and stored at room temperature to form a gel.To acquire ternary composites,the gel samples on the plastic plates were further dehydrated in an oven at 60 °C for 8 h to obtain composite film materials.The prepared composites were collected and conditioned at different RH conditions at 25 °C for 14 days (RH: 33%,saturated magnesium chloride (MgCl2)solution;54%,saturated magnesium nitrate (Mg(NO3)2solution;75%,saturated sodium chloride (NaCl) solution).In this work,sample codes like “A30K25C45-33” will be used.
2.3 Sol-gel transition and gel hardness of ternary system
To probe the sol-gel transition,the aqueous ternary mixtures of agar/KGM/κ-carrageenan were evaluated by small-amplitude oscillatory shear tests performed on a rheometer (MCR92,Anton Paar,Graz,Austria).A strain sweep measurement was performed at first to determine the linear range of viscoelasticity.Then,the 0.1% strain was used,since 0.1% strain was in the linear range of viscoelasticity for all the aqueous mixtures.The temperature sweeps from 90 to 30 °C with a cooling rate of 2 °C/min were used to acquire the storage modulus (G’) and loss modulus (G’’) profiles,thus exploring the gelation temperature (sol-gel transition temperature) for the aqueous mixtures.
A Texture Analyzer (TA.XT Plus,Stable Microsystems,Surrey,UK) with a P/5 R probe was applied to determine the hardness of the composite gels induced by cooling [25].The gels were compressed at a compression degree of 75% with pre-test,test and post-test speed of 0.5,0.5 and 1.5 mm/s,respectively.
2.4 Scanning electron microscopy (SEM)
To observe the fracture surface of agar/KGM/κ-carrageenan composites,they were completely frozen in liquid nitrogen and fractured.Prior to the observation,the composites were cut into small pieces and coated using gold with a thickness of about 20 nm.A JSM 6390lv SEM system (JEOL,Tokyo,Japan) was used to observe the fracture morphology of the ternary composites.
2.5 Attenuated total reflectance Fourier-transform infrared(ATR-FTIR) spectroscopy
A Nicolet iS10 (Thermo Fisher Scientific,Waltham,MA,USA)spectrometer,equipped with Nicolet Smart Orbit ATR accessory,was applied to record the ATR-FTIR spectra for the agar/KGM/κ-carrageenan ternary composites.Before testing,these composites were cut into 2 cm × 2 cm.The measurements were performed in a wavenumber range of 4 000-600 cm-1at a resolution value of 4 cm-1with a total number of scans of 32 times at ambient temperature(26 °C).The air was used as the background whose spectrum was subtracted from the spectrum of each composite.
2.6 Small angle X-ray scattering (SAXS)
To probe the nanoscale structure of the composites,the BL19U2 SAXS beamline of the Shanghai Synchrotron Radiation Facility (Shanghai,China) was used.The agar/KGM/κ-carrageenan composites were placed on the sample stage,and were irradiated for 10 s to collect 2D scattering data.The 2D data were converted into SAXS patterns atqvalues of about 0.009 to 0.55 Å−1.The scattering vectorqis equal to 4πsinθ/λ,where 2θis the angle of X-ray scattering andλis the wavelength of X-ray source [26,27].The scattering data of an empty cell was recorded as the background.The SAXS profiles of the composites were background subtracted and normalized for further analysis.
2.7 X-ray diffraction (XRD)
A D8 advanced diffractometer (Bruker,Karlsruhe,Baden-Wuertenberg,Germany) was applied to probe the crystalline structure of the ternary composites.The equipment was operated at 40 kV and 30 mA and a copper target,with an X-ray wavelength of 0.154 7 nm [28].The XRD pattern of each composite was collected over a 2θrange of 4 to 45°,with the use of a step size of 0.02° and a step rate at 0.5 s per step [26].
2.8 Mechanical properties
The mechanical parameters of the composites were measured by a Texture Analyzer (TA.XT Plus,Stable Microsystems,Surrey,UK).The composites were cut into strips of 5 mm × 50 mm,and the thickness values of the strips were measured using a spiral micrometer.Miniature Tensile Grips for the TA.XT Plus were used to load the sample trips.Then,the tensile strength (σt) and the elongation at break (εb) for the composites were measured with a 50 mm initial grip length and a 0.5 mm/s cross-head speed.Then,a method reported in our previous work was used to calculate the results ofσt(MPa) andεb(%) [15].
2.9 Water contact angle
A goniometer (OCA15EC,Dataphysics,Filderstadt,Germany)was applied to determine the water contact angle for the composite materials with time.The samples were placed on a horizontal sample stage.2.0 μL of deionized water was dropped on the surface of one composite;the water contact angle was measured every 1 s toin situmonitor its evolutions as the time rose.
2.10 Statistical analysis
The data were expressed in the format of mean ± standard deviation.A statistical difference level was used atP<0.05.Statistical analysis of the data was performed using Microsoft excel 2010 (Redmond,Washington,USA).
3.Results and discussion
3.1 Sol-gel transition temperature and gel hardness of ternary composite systems
The storage modulus (G’) and loss modulus (G’’) curves of agar/KGM/κ-carrageenan ternary mixtures accompanying temperature change are included in Fig.1a.It is seen that theG’ andG’’ curves for the aqueous binary system (A75K25) crossed at 48 °C (the sol-gel transition temperature).The addition ofκ-carrageenan increased the sol-gel transition temperature.A higherκ-carrageenan proportion further increased the sol-gel transition temperature for the aqueous ternary mixture.Regarding this,theκ-carrageenan has a similar molecular structure with that of agar and synergistic effects with KGM to form gels [22];the hydrated random coils of agar andκ-carrageenan should reassemble into helical aggregates during cooling,which then aligned with the KGM chains to form gel structures.Therefore,the use ofκ-carrageenan facilitated the formation of gel structure for the ternary system,making it suitable for preparation of shell materials of core-shell capsules.
The hardness results of the formed agar/KGM/κ-carrageenan ternary gels are presented in Fig.1b.The inclusion ofκ-carrageenan evidently enhanced the hardness for the ternary gels,and the ternary composites could show hardness about 3 to 4 times higher than that for the binary sample.This hardness enhancement should be mainly associated with the component interaction events within the gels.That is,theκ-carrageenan formed brittle gels containing helical aggregates,assembling with helical aggregates of agar having similar chain structure withκ-carrageenan.On the other hand,KGM possessed side chains of acetyl groups occurred every 10-19 units on the carbons of mannopyranose.The distance of those acetyl groups was close to the length ofκ-carrageenan helical aggregates,resulting in strong hydrogen bonding andκ-carrageenan/KGM synergy effects [22].An earlier investigation confirmed the role ofκ-carrageenan in strengthening KGM gels especially with mass ratio ofκ-carrageenan/KGM was 60:40 [22].These two events of component interactions could be responsible for the hardness increase for the agar/KGM/κ-carrageenan gels.However,the highestκ-carrageenan content (45% for A30K25C45) less effectively increased the hardness for ternary gels,as the high amount ofκ-carrageenan should be difficult to uniformly distribute in the ternary gel and probably caused phase separations.
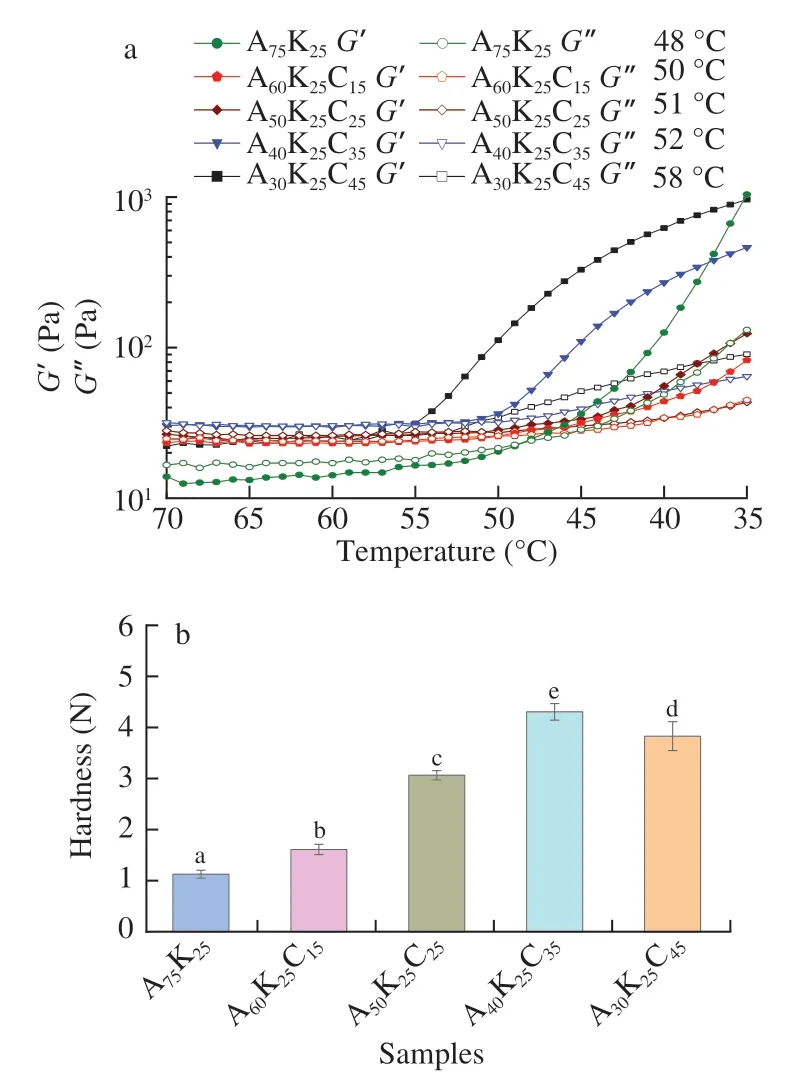
Fig.1 Storage (G’) and loss modulus (G’’) profiles of aqueous agar/KGM/κ-carrageenan ternary mixtures (a) (solid symbol,G’;open symbol,G’’),and the hardness of the formed ternary gels (b).
3.2 Fracture surface morphology of ternary composites
The fracture surface of agar/KGM/κ-carrageenan ternary composites conditioned at different RHs (33%,54% and 75%) are displayed in Fig.2.Apparently,under different RHs,a uniform and smooth cross section could be seen for the Agar/KGM binary composite (A75K25).The addition ofκ-carrageenan allowed a rough and uneven fracture surface for the ternary composite.This phenomenon was much obvious at highκ-carrageenan contents such as 45% (mass ratio: 30:25:45 agar/KGM/κ-carrageenan),presumably due to the helical aggregation of agar andκ-carrageenan.Besides,a higher RH further increased the roughness and looseness of the fracture surface,especially for A30K25C45and A40K25C35.Accounting for this,the less uniformly distributedκ-carrageenan in ternary composites allowed inhomogeneous distribution of water molecules absorbed into the composite matrices at high RHs such as 75% .Consequently,a rougher and looser fracture surface could be seen.

Fig.2 SEM images of cross-section for agar/KGM/κ-carrageenan ternary composites conditioned at various RHs (33%,54% and 75%).
3.3 ATR-FTIR analysis of ternary composites
The ATR-FTIR spectra of agar/KGM/κ-carrageenan ternary composites are presented in Fig.3.The composites displayed characteristic peaks of agar (black arrows in Fig.3a),including 3 286 cm−1(stretching of–OH group),2 926 cm−1(C–H stretching related to the ring methine hydrogen bond in agar) and 1 372 cm−1(ester sulfate group) [29].The peaks at around 1 068,1 035 and 930 cm−1for C–O stretching of 3,6-anhydro-galactose were related to both of agar andκ-carrageenan with repeating disaccharide units of galatose and 3,6-anhydrogalactose [22].The peaks at 845 and 1 260 cm-1were ascribed to C-O-SO4on C4ofD-galactose-4-sulfate onκ-carrageenan(red arrows in Fig.3a).There were also infrared absorption peaks for KGM in the composites (blue arrows in Fig.3a),e.g.,2 881 cm−1(stretching of methyl C–H),1 724 cm−1(acetyl group),as well as 871 and 808 cm−1(mannose of KGM) [30].Therefore,the preparation process did not substantially alter the chemical structures of agar,KGM andκ-carrageenan components.

Fig.3 ATR-FTIR spectra of agar,KGM and κ-carrageenan as well as their ternary composite (a),and full (b,d and f) and enlarged (c,e and g) ATR-FTIR spectra of agar/KGM/κ-carrageenan composites.
In Figs.3b-g,as theκ-carrageenan content rose,the characteristic peaks ofκ-carrageenan (1 260 and 845 cm-1) became more intense and that for agar (1 372 cm-1) became less visible.Besides,the stretching peak of–OH group shifted slightly to lower wavenumbers when an increased content ofκ-carrageenan was used (Figs.3b,3d and 3f).This shift suggested that the addition ofκ-carrageenan resulted in changes in the molecular interaction events between components in the ternary composites.
3.4 Nano-structural features of ternary composites
Fig.4 includes the SAXS patterns of agar/KGM/κ-carrageenan ternary composites subjected to conditioning at varied RHs.Among the composites,the binary composite had a more visible scattering shoulder at about 0.15 Å-1related to the presence of molecular orders [31].Hence,a higher amount of nanoscale molecular orders existed in the binary system than in the ternary composites,consistent with the XRD results that the binary composite contained more crystalline components.Furthermore,the inclusion ofκ-carrageenan induced an increase in the scattering intensity atqvalues belowca.0.07 Å-1,especially when we increased the percentage ofκ-carrageenan component.This result was ascribed to the generation of nanoscale structures having length scales higher than about 9 nm as calculated with Woolf-Bragg equation [32].Such structure probably corresponded to the aggregation and/or assembly ofκ-carrageenan and agar/KGM chains in the ternary composites.In addition,an increase in RH caused fluctuations of the overall scattering intensity but did not alter the changing trend of scattering intensity bellowca.0.07 Å-1as induced by the increase inκ-carrageenan content.Accounting for this,the X-ray scattering intensity for the samples was positively associated with the density difference between the ordered and amorphous regions.A higher RH could facilitate the penetration of water molecules into the matrices (especially amorphous regions)of the composites,and thus fluctuations in the scattering intensity could be observed while changing RH.

Fig.4 SAXS patterns of agar/KGM/κ-carrageenan composites conditioned at different RHs (33% (a),54% (b) and 75% (c)).
3.5 Crystalline features of ternary composites
The XRD patterns of neat agar,KGM andκ-carrageenan films as well as their ternary composites are presented in Fig.5.The agar or KGM film material had two broaden peaks at 2θof 13.4° and 19.7° or 12.1° and 20.8°,indicative of the existence of agar or KGM ordered structure [15].Moreover,theκ-carrageenan film material showed sharp peaks at 2θof 11.7°,20.8°,23.6°,28.5°,29.2°,31.2°,33.4°and 40.5°,related to metal elements presenting during extraction ofκ-carrageenan from algae and during growth of algae [33].
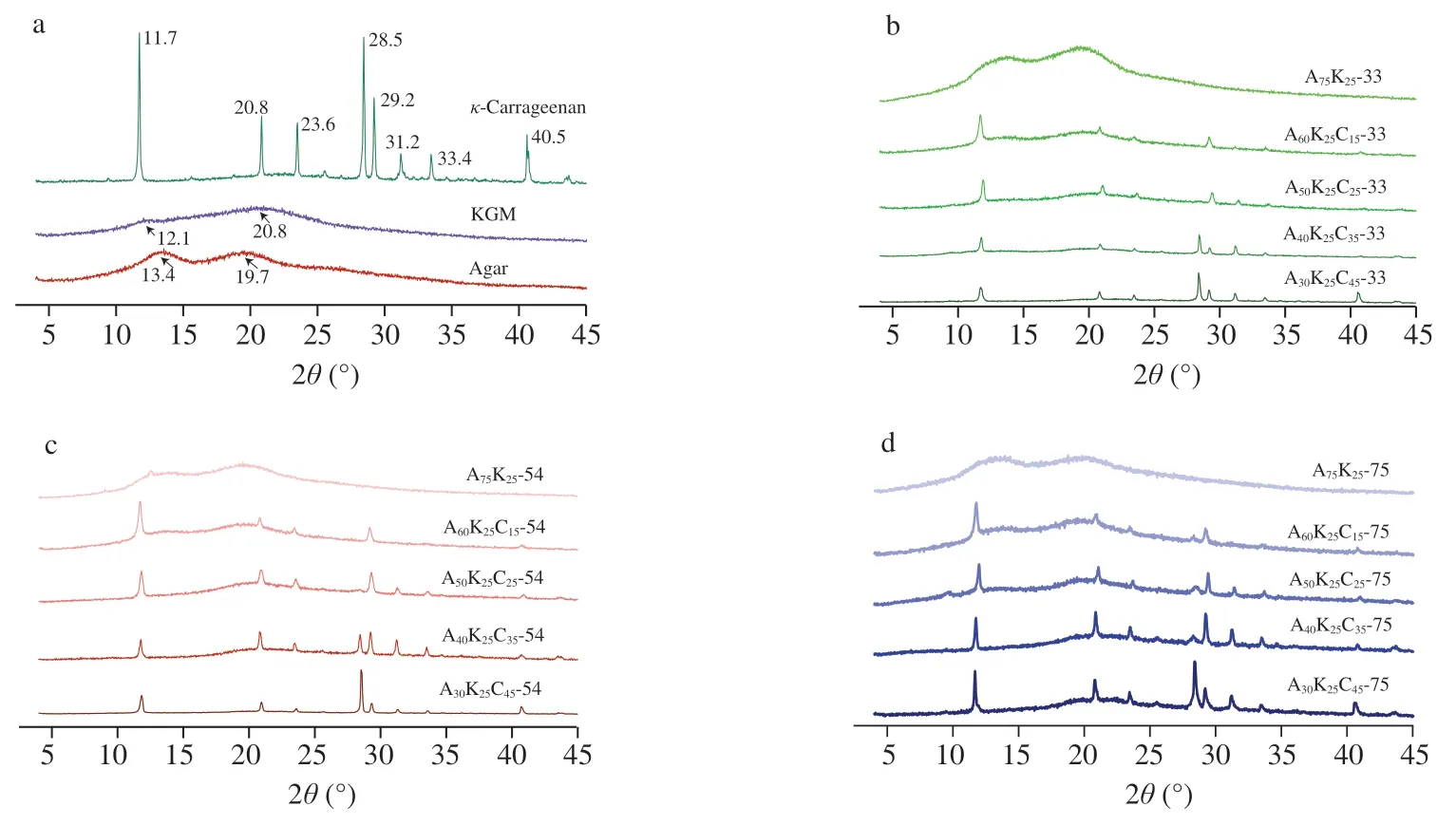
Fig.5 XRD patterns of agar,KGM or κ-carrageenan film material conditioned at RH of 75% (a),and ternary composites conditioned at different RHs (33% (b),54% (c) or 75% (d)).
For the binary composite (A75K25),two diffraction peaks at about 13° and 20° appeared on the XRD pattern.With the increase ofκ-carrageenan content,those two peaks became less prominent irrespective of the RH.This suggests that theκ-carrageenan interacted with agar and KGM molecules (confirmed by FTIR),thus suppressing the assembly of agar and KGM molecules.An increase in RH negligibly altered the diffraction intensities for the ternary composites with lessκ-carrageenan (e.g.,A50K25C25and A60K25C15) but strengthened the two diffractions for the composites rich inκ-carrageenan (e.g.,A40K25C35and A30K25C45).That is,the ternary composites with lessκ-carrageenan probably possessed proper component interactions and thus exhibited weakened chain reassembly during conditioning at varied RHs.Nonetheless,at higherκ-carrageenan contents (35% or above),the less uniformly distributedκ-carrageenan,probable phase separations,tended to weaken the interactions ofκ-carrageenan with agar/KGM.Hence,an observed level of agar/KGM chain reassembly during conditioning occurred.
3.6 Mechanical features of ternary composites
Theσtandεbfor the composites following conditioning at different RHs are displayed in Fig.6.Regardless of RH,the binary composite (A75K25) had the highestσt.The inclusion ofκ-carrageenan decreased theσtof the ternary composites,and theσtshowed a further decrease with the increase ofκ-carrageenan content.It is known that the crystalline structure in materials could weaken the movability of molecular chains and allow the increase of the strength and rigidity of materials [34].Thus,this reduction inσtshould be ascribed to the reduced proportion of crystalline components and nanoscale orders (XRD and SAXS results) accompanying the inclusion ofκ-carrageenan.In addition,the uneven distribution ofκ-carrageenan in the material matrices played some role in reducing the strength for the ternary composites rich inκ-carrageenan (A30K25C45and A40K25C35).As for theεb,an initial increase inκ-carrageenan content lead to a higherεb,but a further increasedκ-carrageenan proportion reduced theεb.That is,at lowκ-carrageenan contents,the occurrence ofκ-carrageenan/agar/KGM interactions (see FTIR and XRD) and the reduction in ordered components contributed to increasing the elongation at break.Also,the formation of nanoscale structures from chain aggregation and assembly could play a role in increasing the elongation at break.While using high amounts ofκ-carrageenan such as that for A30K25C45,the less uniformly distributedκ-carrageenan and emergence of probable phase separations (see gel hardness) could allow occurrence of stress concentration and therefore a decrease in the elongation at break for the composites.

Fig.6 Tensile strength (σt) and elongation at break (εb) of agar/KGM/κ-carrageenan composites conditioned at RHs of 33% (a1 and a2),54% (b1 and b2) and 75% (c1 and c2).
Compared to the composites at RH of 33%,an increase in RH tended to decrease theσtbut increase theεbfor the ternary composites.Note that an increased amount of water molecules could be absorbed into the composite system,eventually enhancing the mobility of molecular chains within the composite matrices.This could soften the composite matrices (lowerσt) and loosen the connections between component chains (rougher fracture surface and higherεb).It is worth mentioning that those ternary composites with lessκ-carrageenan displayed only slight changes in the value ofεbwith the increased RH(indicative of stable mechanical features),which could be associated with proper interactions between components without notable uneven component distribution in the ternary composite system.
3.7 Contact angle of ternary composites
Fig.7 shows the changing curve of water contact angle as a function of time for the composites after equilibrium under different RHs (33%,54% and 75%).It is seen that the water contact angle for all composites decreased as a function of time,related to the hydrophilic nature of the original materials including agar,KGM andκ-carrageenan.At RH of 33%,the contact angle showed a gradual decrease as theκ-carrageenan proportion rose,revealing reduced hydrophobicity for the ternary composites.When the RH was increased,the contact angle plots for the binary composite withoutκ-carrageenan (A75K25) displayed a more evident shift to lower values than did the ternary composites.This indicates that the incorporation ofκ-carrageenan could enhance the hydrophilicity stability for the ternary composite system.Especially,a less apparent shift of the contact angle profile could be observed for the ternary composites containing proper levels ofκ-carrageenan such as A60K25C15and A50K25C25.This could be related to the presence of proper component interactions related toκ-carrageenan/agar helical aggregation andκ-carrageenan/KGM alignment,without notable unevenκ-carrageenan distribution in the ternary composites.
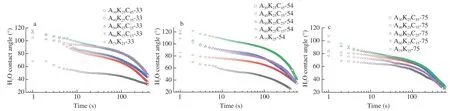
Fig.7 Water contact angle of agar/KGM/κ-carrageenan composites conditioned at different RHs of 33% (a),54% (b) and 75% (c).
4.Conclusions
This work discloses the influence ofκ-carrageenan addition on the application-related properties of agar/KGM/κ-carrageenan ternary system,especially by inspecting the microstructural evolutions of resultant ternary composites under different RHs.In particular,the inclusion ofκ-carrageenan at lower mass ratios,e.g.,15% and 25% (60:25:15 and 50:25:25 agar-KGM-κ-carrageenan),could allow agar/KGM/κ-carrageenan interactions.Such features caused increases in the sol-gel transition temperature and the hardness of the ternary gel.For instance,the ternary gel containing 25%κ-carrageenan displayed hardness three times higher than that of agar/KGM binary gel.Then,for the ternary composites via dehydration of the gels,the incorporation ofκ-carrageenan (15% to 25%) led to reduction in crystallites and nanoscale orders as well as formation of nanoscale structures related to chain assembly.Also,only slight changes occurred in the micron scale morphology without notable phase separations,as compared to the ternary composites possessing moreκ-carrageenan such as 35% or higher.These structural features spanning different length scales endow the ternary composites with enhanced stabilities of elongation at break and water contact angle(hydrophilicity) for the resultant ternary composites under different RHs.
The current results hint to us that adding certain amounts ofκ-carrageenan into agar/KGM would generate ternary composite systems with tailored features,e.g.,increased sol-gel transition point and gel hardness,and stabilized mechanical/hydrophilic features under varied RH.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to acknowledge the National Natural Science Foundation of China (32172240).Also,we thank the staffs from BL19U2 beamline of National Facility for Protein Science in Shanghai (NFPS) at Shanghai Synchrotron Radiation Facility,for their assistance during data collection.
- 食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil

