UPLC-Q-TOF/MS-based metabolomics reveals modulatory effects of Mesona chinensis Benth polysaccharide in liver injury mice induced by cyclophosphamide
Yuzhen Hong,Mingyue Shen,Qiang Yu,Yi Chen,Jianhua Xie*
State Key Laboratory of Food Science and Technology,Nanchang University,Nanchang 330047,China
Keywords:Metabolomics Polysaccharide Liver injury Cyclophosphamide
ABSTRACT The main purpose of this study was to investigate the improvement effect of Mesona chinensis Benth polysaccharide (MP) on cyclophosphamide (CTX) induced liver injury in mice.To explore metabolic prof ile of liver tissue and feces among normal group,CTX-induced group and MP management group based on metabolomics method by using UPLC-Q-TOF/MS.The results showed that MP could alleviate liver injury and promote the production of short chain fatty acids (SCFAs),with the best dose of 200 mg/kg·body weight (bw).The principal component analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLSDA) scores plots of the liver and feces samples showed a clear separation among normal,model and highdose of MP (MPH).There were 18 endogenous metabolites in liver and 29 endogenous metabolites in feces,which were mainly involved in 8 metabolic pathways: taurine and hypotaurine metabolism,phenylalanine metabolism,α-linolenic acid metabolism,tricarboxylic acid (TCA) cycle,phenylalanine,tyrosine and tryptophan biosynthesis,arachidonic acid metabolism,sphingolipid metabolism as well as tryptophan metabolism.Moreover,a common metabolite arachidonic acid was observed in liver and feces samples.These endogenous metabolites may be considered to be MP’s response to liver protection.It will help to further understand the mechanism of MP and provide a basis for further research.
1.Introduction
Liver is an important hub of metabolism in our organisms,responsible for the main synthesis,decomposition,transformation,excretion and other metabolic processes [1].Therefore,the liver is one of the organs most vulnerable to toxic damage.Once damaged,it will cause a serious disorder of systemic metabolism,which seriously threatens human health.Moreover,most drugs undergo biotransformation in the liver.In the process,the toxic damage of drugs and their metabolites to the liver leads to various types of drug-induced liver injury (DILI),which is also the most common cause of acute liver failure [2].At present,DILI has risen to the f ifth highest mortality rate of liver diseases in the world [3].Meanwhile,cyclophosphamide (CTX) is widely used clinically in various cancers chemotherapy drugs,and is also typical drugs that cause DILI.They can trigger and aggravate liver toxicity through a variety of ways,including direct cytotoxicity,oxidative stress and inf lammation [4],and its clinical application was limited by severe hepatotoxicity.Therefore,it is of great signif icance to f ind natural active substances that can protect against DILI.
In recent years,natural polysaccharides have attracted more and more attention due to their low toxicity,safety and high efficiency,and have been widely studied and applied in the field of food and traditional medicine because of their antioxidant,antibacterial,hepatoprotective,immunomodulatory and other biological activities [5-9].As a medicinal and edible plant resource,Mesona chinensisBenth,also known as Hsian-tsao,is an annual herb with a unique flavor [10].It is widely planted in Jiangxi,Fujian,Zhejiang,Taiwan and Guangdong provinces in China.Polysaccharide is the main active substances inM.chinensisBenth,and previous studies have shown thatM.chinensisBenth polysaccharide (MP) showed a protective effect on CTX-induced oxidative damagein vivo[11].Its biological activity and functional properties are inseparable from the polysaccharide active substance it contains.
Metabolomics is a novel and effective method of systems biology following genomics,transcriptomics and proteomics [12].It is a quantitative measurement of the dynamic multi-parameter response of biological systems to pathophysiological stimuli or genetic modification,mainly studying endogenous metabolites of low molecular weight (Molecular weight (Mw) <1 000) in biological samples [13].The detection techniques currently applied in metabolomics research include nuclear magnetic resonance(NMR),gas chromatography/mass spectrometry (GC/MS) and liquid chromatography/mass spectrometry (LC/MS),and ultrahigh performance liquid chromatography tandem quadrupole timeof-flight mass spectrometry (UPLC-Q-TOF/MS) in LC/MS was widely used due to its high sensitivity and high precision [14,15].At present,metabolomics has been widely used in many fields,including disease diagnosis,drug toxicity,nutritional intervention and pharmacodynamic mechanism research.It also has been successfully applied to CTX-induced liver injury animal models,non-alcoholic fatty liver and so on.These studies provide a new direction for the evaluation and prediction liver toxicity [16,17].In addition,compared with representative biological fluids representing the whole body,tissue samples extracted from the expression phenotype tend to produce more meaningful results [18].The evaluation of tissue metabolites is of great value in metabolomics research,and it can provide more direct metabolic information.However,there is no metabonomic analysis reported on the liver protection of MP and its mechanism so far.
Our previous studies have shown that MP relieves liver damage and explored the beneficial effects of MP on the balance of intestinal microbiota through 16S rRNA,but its regulation of metabolism is still unclear [19].Therefore,UPLC-Q-TOF/MS was used to analyze the metabolic profile of liver and feces of mice with liver damage caused by CTX after MP intervention,and explored from the perspective of affecting metabolic pathways to further clarify the protective effect of MP on CTX-induced liver injury.This may provide a new metabolomics perspective to support the value of MP in the treatment of liver injury.
2.Materials and methods
2.1 Chemicals and reagents
CTX was provided by Sigma-Aldrich (Sigma,St.Louis,MO,USA),which also supplied butyric acid standard and highperformance liquid chromatography (HPLC) grade formic acid.The aspartate aminotransferase (AST) and alanine aminotransferase (ALT)assay kits were produced by Nanjing Jiancheng Bioengineering Institute (Nanjing,Jiangsu,China).Acetic acid,valeric acid standard,HPLC grade methanol,acetonitrile as well as analytical grade chloroform were purchased from Merck (Darmstadt,Germany).Propionic acid standard was purchased from Janssen Chimica(Belgium).Ultrapure water was obtained from a Milli-Q water purification system (Millipore Corp.,Billerica,MA,USA).
2.2 Preparation and characterization of MP
DryM.chinensisBenth was purchased from Jiangxi,China.A MP was obtained by using water extraction,alcohol precipitation and deproteinized by Sevag method,which was according to our previous studies [20].The neutral sugar,uronic acid and protein contents of MP were 39.01%,29.30% and 27.30%,respectively,which had an average molecular weight 157 kDa.Compared with the monosaccharide standards,MP was composed of glucose,xylose,galactose and galacturonic acid in a molar ratio of 1.49:2.54:0.68:6.33 [20].
2.3 Animal experiments and sample collection
Animal experiments were conducted according to our previous study [19].Briefly,male BALB/c mice weighing approximately(20 ± 2) g were commercially obtained from Hunan Slake Scene of Laboratory Animal (SJA) Co.,Ltd.(Changsha,China;certificate number: SCXK (Xiang) 2016-0002).All animal guidelines and protocols were in compliance with Nanchang University Medical College Animal Care Review Committee.The mice were housed in a condition room with temperature of (22 ± 1) °C and a light/dark cycle of 12 h.Throughout the experimental period,the mice freely ingested food and water.
After 7 days of acclimatization,all mice were randomly divided into five groups (10 in each group).The normal control group(NC) treated with physiological saline (0.9% NaCl) only.The model control group (MC) were intraperitoneally injected with 100 mg/kg·bw CTX for 3 consecutive days,followed by intragastric administration of 0.9% NaCl for the next 7 days.The low-dose of MP(MPL) group were intraperitoneally injected with 100 mg/kg·bw CTX for 3 consecutive days,followed by intragastric administration of 50 mg/kg·bw MP for the next 7 days.The medium-dose of MP (MPM)group were intraperitoneally injected with 100 mg/kg·bw CTX for 3 consecutive days,followed by intragastric administration of 100 mg/kg·bw MP for the next 7 days.The high-dose of MP (MPH)group were intraperitoneally injected with 100 mg/kg·bw CTX for 3 consecutive days,followed by intragastric administration of 200 mg/kg·bw MP for the next 7 days.Fecal samples were collected before animals were sacrificed and liver tissue was removed quickly.All collected samples were immediately stored at −80 °C for further analysis.
Add 100 mg of liver samples to cold phosphate buffer (1:9) and homogenate to obtain 10% liver homogenate,then centrifuge at 10 000 ×gfor 10 min at 4 °C to obtain the supernatant.The activities of AST and ALT in liver were determined using a commercial kit.
2.4 Measurement of short chain fatty acids (SCFAs) in fecal
The method for determining SCFAs from fecal was based on previous study [21].Briefly,100 mg feces of mice were diluted with ultrapure water in a ratio of 1:9,then vortex sufficiently to mix evenly and ultrasound for 5 min at 4 °C.Afterwards,centrifuge twice at 13 000 ×gfor 15 min at 4 °C to collect the supernatants.Finally,the supernatants were filtered with a 0.22 μm hydrophilic membranes and analyzed by Agilent 6890 GC system.
2.5 Sample preparation for metabolomics analysis
Liver samples preparation was conducted according to the method of [22]with slightly modified.Add 3 mL of pre-chilled mixed solution to 100 mg of thawed liver sample.The mixed solution was composed of methanol,chloroform and water (5:2:2,V/V).Then it was fully homogenized and stand at 4 °C.After 30 min,the supernatants was extracted by centrifugation at 13 000 ×gfor 15 min at 4 °C and passed through a 0.22 μm filter membrane to the sample bottle for further UPLC-Q-TOF/MS analysis.
Fecal samples were thawed at room temperature prior to analysis.Add 600 μL of the pre-cooled mixed solution to 80 mg stool sample and vortexed thoroughly.The mixed solution was composed of ultrapure water,methanol and acetonitrile (1:1:1,V/V).Finally,centrifugation was performed at 13 000 ×gfor 15 min at 4 °C and repeated twice.The obtained supernatants were passed through a 0.22 μm filter membrane to the sample bottle for further UPLC-Q-TOF/MS analysis.
2.6 UPLC-Q-TOF/MS analysis
Chromatographic conditions: chromatographic separation of liver was performed on an Agilent ZORBAX Eclipse Plus C18column(100 mm × 2.1 mm,1.8 μm) using UPLC 1290 system (Agilent Technologies,USA).The column temperature was maintained at 35 °C and 3 μL of each sample solution was injected.The mobile phases consisted of a gradient elution with phase A (water with 0.1% formic acid) and phase B (100% acetonitrile) with eluted at a flowing rate of 0.3 mL/min.The gradient elution conditions were set as follows: 0-3 min,5% −8% B;3-4 min,8% −9% B;4-11 min,9% −35% B;11-15 min,35% −60% B;15-21 min,60% −70% B;21-40 min 70% −100% B.Each sample drawed 10 μL of supernatant and add into the same sample bottle as a quality control (QC) sample.In the UPLC-QTOF/MS analysis process,a QC sample was inserted every 10 samples to check the stability and repeatability of the analysis system.
Metabolite separation of fecal was also conducted on Agilent UPLC 1290 system equipped with ZORBAX Eclipse Plus C18column (100 mm × 2.1 mm,1.8 μm).The mobile phase A was water with 0.1% formic acid,while the mobile phase B was acetonitrile modified with 0.1% formic acid.The gradient elution conditions were set as follows: 0-5 min,2% −3% B;5-6 min,3% −50% B;6-11 min,50% −60% B;11-17 min,60% −80% B;17-18 min,80% −98% B;18-22 min 98% B;22-25 min,98% −3% B;25-27 min,3% B.The column temperature,injection volume,flow rate and the preparation of QC sample were the same as liver.
Mass spectrometry analysis of liver and fecal samples were performed by using an Agilent 6538 series Q-TOF equipped with an electrospray ionization ion source in the positive (ESI+) and negative(ESI−) ion mode.The detection conditions of the mass spectrometer were set as follows: the drying gas temperature was 350 °C and rate was 10 L/min;the fragment voltage was 120 V;the capillary voltages were 3 500 V/(−3 500 V);the nebulizer pressure was 275.8 kPa;the mass spectrum scanning range wasm/z50−1 200.
2.7 Multivariate analysis and identification of potential biomarkers
The raw data generated by UPLC-Q-TOF/MS was converted into (.mzData.xml) format by using Mass Hunter software (Agilent technologies,USA).Then upload to the XCMS Online platform(https://xcmsonline.scripps.edu) for normalization,peak alignment and peak extraction,and the obtained sample information,ion information (retention time (RT) and mass-to-charge ratio (m/z))and a multivariate data matrix of ion intensity.The parameters of XCMS Online were set as default.The obtained data was imported into SIMCA-P14.0 (Umetrics AB,Umea,Sweden) for unsupervised principal component analysis (PCA) and supervised orthogonal partial least squares discriminant analysis (OPLS-DA).The importance of variance (variable projected importance,VIP) value of the candidate metabolite was greater than 1,and thePvalue of the analysis of variance was less than 0.05,which were considered as a potential biomarker.Online databases: HMDB (https://hmdb.ca/),METLIN(https://metlin.scripps.edu/),and KEGG (http://www.genome.jp)were used to identify and explain all potential marker metabolites.Metaboanalyst 4.0 (https://www.metaboanalyst.ca/) was used for relative intensity analysis,clustering heatmap analysis,correlation analysis and pathway analysis.
2.8 Statistical analysis
All data were performed at least in duplicate,and the results were expressed as “mean ± SEM (standard error of mean)” using SPSS software version 21 (IBM software,NY,USA).The difference among various groups was performed by one-way analysis of variance(ANOVA) using Duncan’s test and a value ofP<0.05 were regarded as statistically significant.
3.Results
3.1 Effects of MP on the markers of liver function
To investigate the effect of MP on CTX-induced liver function in mice,the activities of AST and ALT in the liver were measured in this study.In the MC group,CTX significantly increased the activity of AST and ALT in the liver when compared with the NC group (P<0.05) (Fig.1).It showed that CTX seriously causes liver damage.When compared with the MC group,the activity of ALT was significantly reduced in the MPL group,MPM group,and MPH group (P<0.05).In particular,the activities of AST and ALT in the MPH group were significantly different from those of the MC group(P<0.05),indicating that high-dose MP had a better improvement on CTX-induced liver injury in mice.Therefore,MPH group was selected for subsequent metabolic profile studies.
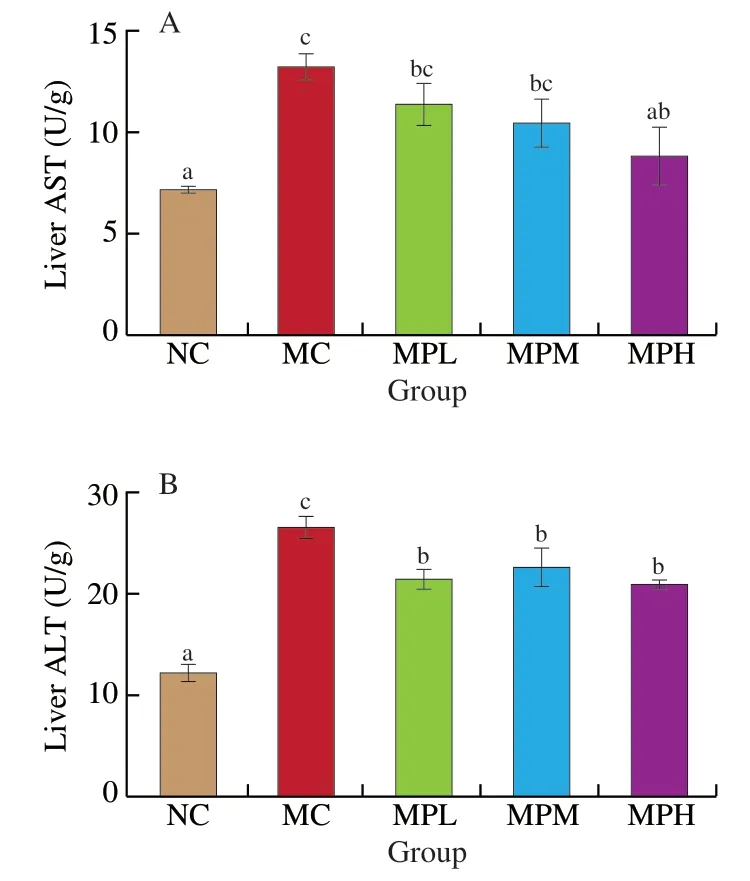
Fig.1 Effect of MP on liver damaged in mice induced by CTX.(A) and (B)represent the activities of AST and ALT in liver tissue,respectively.Different letters (a−c) in the same column represent significant differences among treatments when P <0.05.
3.2 Effect of MP on SCFAs of fecal in mice
Undigested polysaccharides can be used by intestinal microbes,and SCFAs are the main metabolites of natural polysaccharide produced by intestinal microbiota fermented in the distal intestine,which regulate host physiological processes,and their confusion plays an important role in the occurrence of many diseases [23,24].SCFAs mainly included acetic acid,propionic acid,butyric acid and valeric acid.The concentrations of acetic acid,propionic acid,butyric acid,valeric acid and total SCFAs in the CTX-treated group were significantly reduced compared with the normal group (P<0.05)(Fig.2).However,after intragastric administration of MP,the concentration of the above four total SCFAs was evidently increased and showed a dose-dependent relationship,and the MPH group returned to the concentration of the NC group (P<0.05).These results further revealed that MP was beneficial to promote the production of SCFAs.
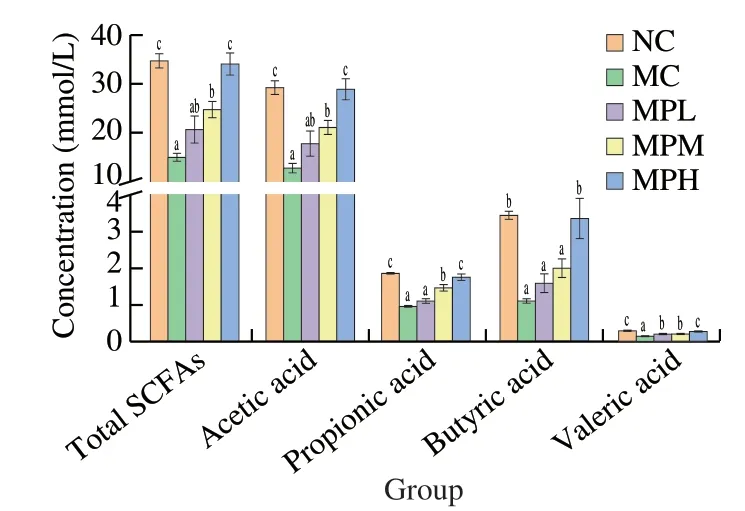
Fig.2 The concentration of total SCFAs,acetic acid,propionic acid,butyric acid and valeric acid in fecal sample.Different letters (a−c) in the same column represent significant differences among treatments when P <0.05.
3.3 Liver metabolomics analysis
As shown the 3D score plot of PCA in Figs.3A−B,the QC samples were closely clustered in the score plot both in the positive and negative ion models,respectively.This showed that the system was provided with good stability and repeatability to ensure the reliability of the experiment.Subsequently,the liver sample PCA score plot was used to distinguish the difference between the NC group,MC group and MPH group.The 3D score plot of PCA demonstrated that the three groups were clustered separately with theof positive and negative ion models were 0.555 and 0.491,and the MC group had a tendency to deviate from the normal group,suggesting alters in endogenous small molecules metabolites.What’s more,the MPH group was separated from the CTX-treatment group significantly in the positive ion mode.After that,a supervised OPLS-DA was applied for further distinguished the differences of the metabolite profiles between the two groups both in the positive and negative ion models,respectively.The score plots of the OPLS-DA model are present at Figs.3C-F,the NC group could be separated from MC group apparently (ESI+:=0.989,Q2=0.703;ESI−:=0.980,Q2=0.716).Similarly,the MPH group could be obviously separated from the MC group (ESI+:=0.998,Q2=0.961;ESI−:=0.984,Q2=0.820).This showed that the OPLS-DA model was reliable,and also suggested that mouse liver metabolomics had obvious characteristics in the NC group,CTX treatment group and MP treatment group.In addition,200 permutation tests were performed to verify whether the OPLS-DA model was overfitted.AllR2andQ2values of the models were lower than the original value,whileQ2value was close to zero,indicating that the OPLS-DA model did not show overfitting and the obtained results were highly reliable (Fig.S1).
According to the above results,the selection of latent variables as biomarkers was based on the value of VIP greater than 1 in the OPLS-DA model,and thePvalue of Student’sttest was less than 0.05.The results showed that there were 12 different metabolites between the NC group and the MC group,and 13 different metabolites between the MC group and the MPH group were identified in the liver samples (Table 1).The alters in differential metabolites caused by CTX-induced liver injury mainly involve a significant increase the level of LysoPC (18:2),LysoPC (20:4),LysoPC (18:1),LysoPE(18:0),L-phenylalanine,PE (18:1),LysoPI (18:0),arachidonic acid,dihomo-γ-linolenic acid,oleic acid and 16-hydroxyeicosatetraenoic acid (HETE),and significantly reduced the level of octadecanamide.However,with the intervention of MP,the different metabolites between MC group and MPH group wereL-valine,sphinganine,LysoPC (20:4),LysoPE (18:0),linoleamide,octadecanamide,L-phenylalanine,L-tryptophan,flavin adenine dinucleotide,docosahexaenoic acid,arachidonic acid,oleic acid.Furthermore,LysoPC (20:4),LysoPE (18:0),octadecanamide,L-phenylalanine,arachidonic acid and oleic acid coexist in the NC group,MC group,and MPH group.All these altered different metabolite,LysoPE(18:0) was significantly up-regulated in the MC group,while it was effectively reversed to normal levels in the MPH group.
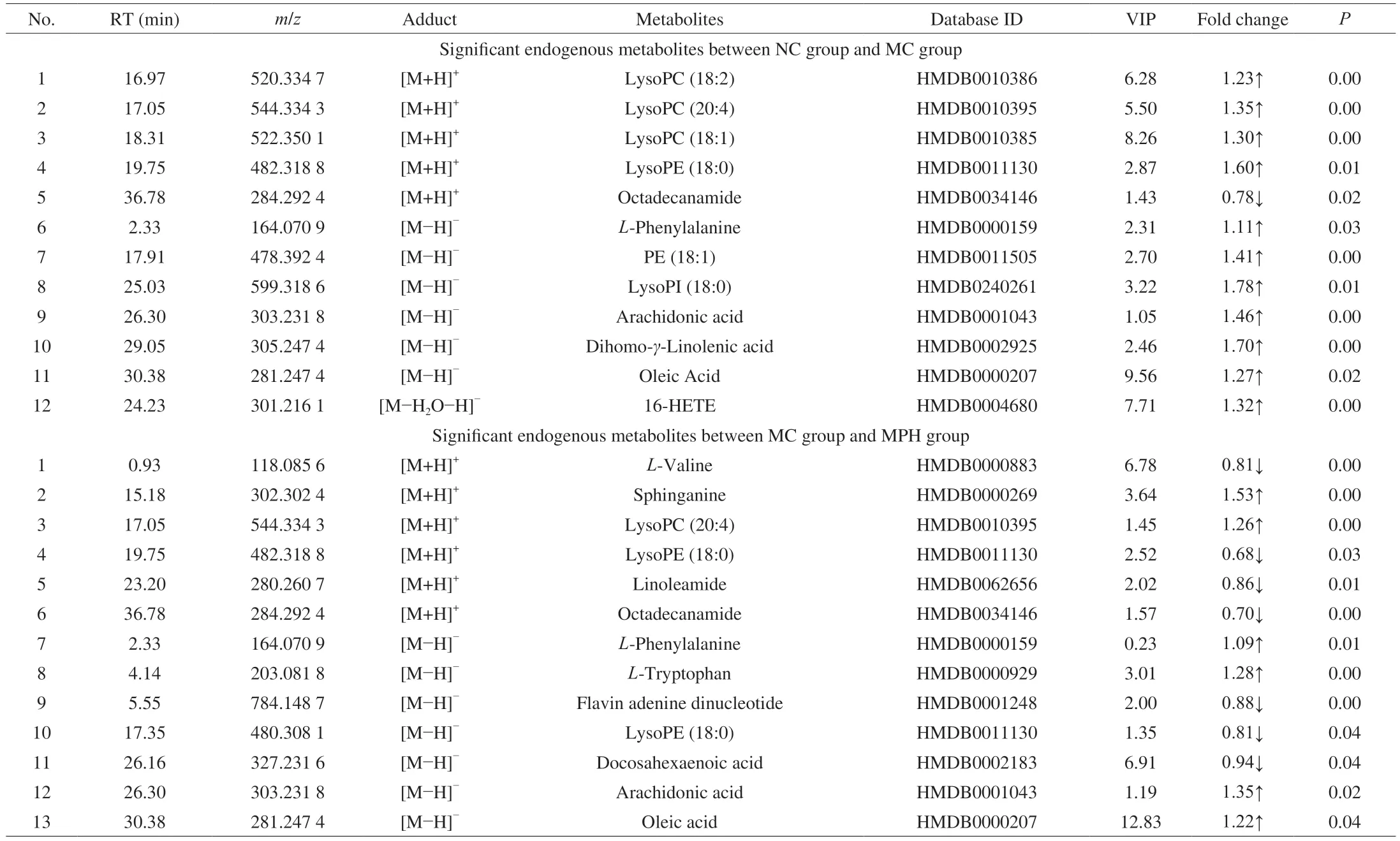
Table 1 The endogenous metabolites among groups in the liver samples of mice.
3.4 Fecal metabolomics analysis
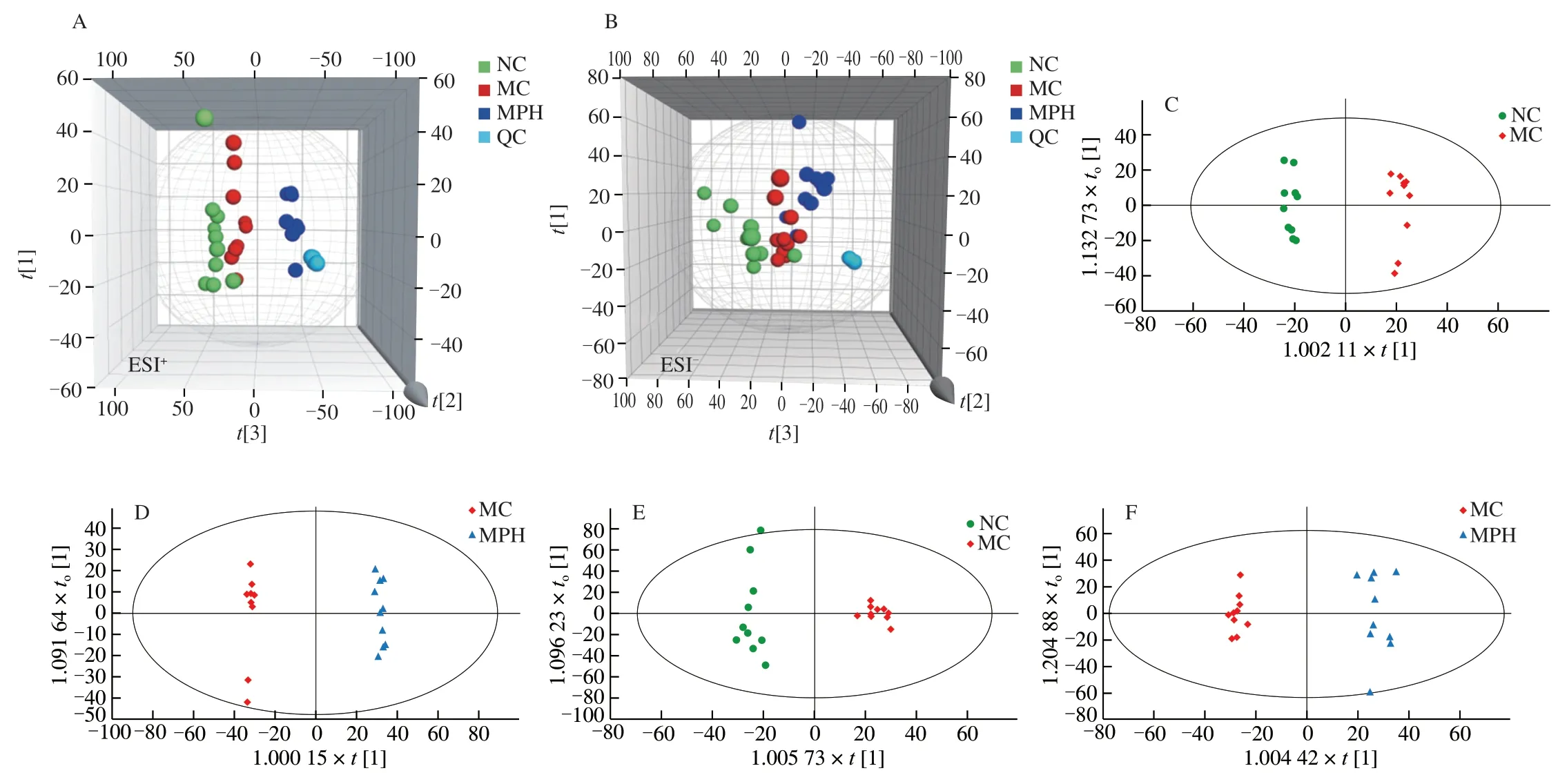
Fig.3 Multivariate statistical analysis of liver in each groups based on UPLC-Q-TOF/MS.(A) and (B) Non-supervised principal component analysis,PCA score plots between the NC,MC and MPH groups both in positive ion and negative ion modes,respectively;(C−F) Orthogonal partial least squares discrimination analysis,OPLS-DA score plots between the three groups,(C) NC group vs MC group in positive ion modes;(D) MC group vs MPH group in positive ion modes;(E) NC group vs MC group in negative ion modes;(F) MC group vs MPH group in negative ion modes.
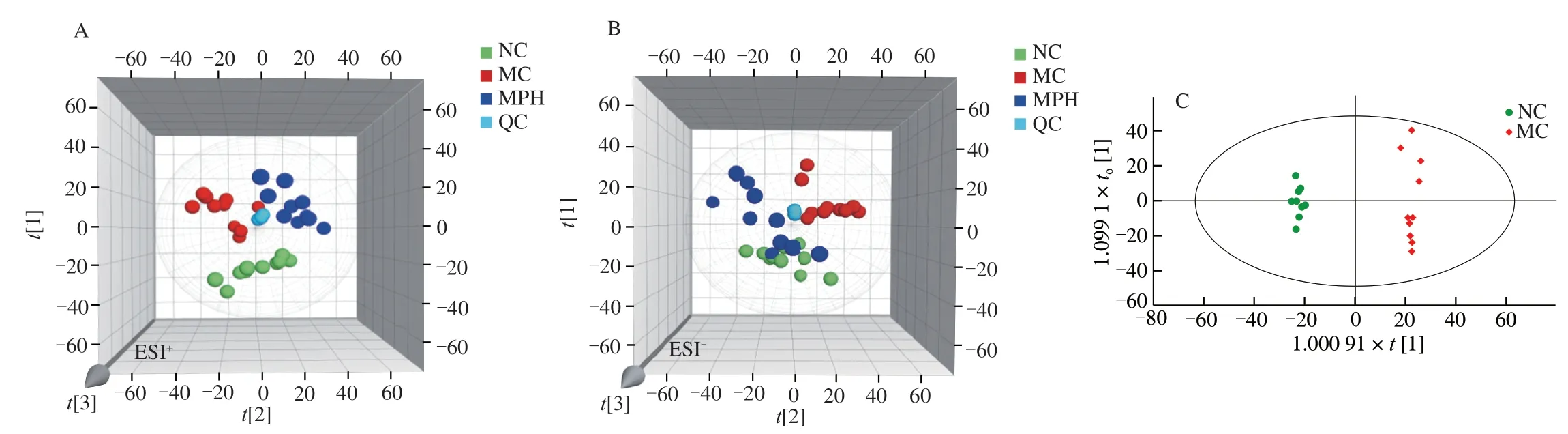
Fig.4 Multivariate statistical analysis of fecal in each groups based on UPLC-Q-TOF/MS.(A) and (B) Non-supervised principal component analysis,PCA score plots between the NC,MC and MPH groups both in positive ion and negative ion modes,respectively;(C−F) Orthogonal partial least squares discrimination analysis,OPLS-DA score plots between the three groups,(C) NC group vs MC group in positive ion modes;(D) MC group vs MPH group in positive ion modes;(E) NC group vs MC group in negative ion modes;(F) MC group vs MPH group in negative ion modes.
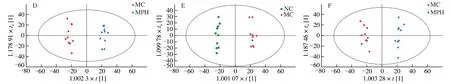
Fig.4 (Continued)
The results of fecal metabolomics were similar to those of the liver,confirming the improvement effect of MP on CTX-induced liver injury in mice.First of all,QC samples were also obviously gathered together to verify the stability of the system as shown in Figs.4A−B.Moreover,the PCA analysis results of fecal showed that the three groups clustered independently both in positive and negative ion modes,respectively (ESI+:=0.585;ESI−:=0.611).At the same time,the MC group was significantly different from normal mice,further indicating that the endogenous metabolites caused by CTX had changed significantly,and the model was successfully established.Interestingly,after MP intervention,the MPH group approached the NC group,which revealed that MP treatment changed the metabolic response of fecal to CTX exposure.For the purpose of revealing the beneficial effects of MP on mice,the OPLS-DA model was used to analyze the fecal sample data.The score plots of the OPLS-DA model are shown in Figs.4C−F,NC group,MC group and MPH group could be separated in pairs.Analogously,separated apparently between the NC group and the MC group (ESI+:=0.993,Q2=0.934;ESI−:=0.992,Q2=0.716),and obviously separated between the MC group and the MPH group (ESI+:=0.985,Q2=0.883;ESI−:=0.978,Q2=0.883).These results indicated that CTX and MP could cause metabolic changes.Similarly,the results of 200 permutation tests found that all OPLS-DA models did not overfit(Fig.S2).
After that,metabolites with VIP >1 andP<0.05 in OPLS-DA model were selected as fecal potential biomarkers for identification.The MC group significantly changed 21 endogenous metabolites in fecal sample when compared with the NC group,of which 13 endogenous metabolites were up-regulated and 8 endogenous metabolites were down-regulated (Table 2).Among them,the levels of MG (18:2),linoelaidic acid,oleic acid,linoleoyl ethanolamide,palmitoyl ethanolamide,chenodeoxycholic acid,ursodeoxycholic acid,L-malic acid,N-acetyl-L-glutamic acid,leucinic acid,phenyllactic acid,ricinoleic acid and arachidonic acid were significantly increased.On the contrary,the levels of guanosine,guanine,stearidonic acid,octadecanamide,deoxyguanosine,sebacic acid,α-linolenic acid and inosine were significantly reduced.After intervention of MP,19 endogenous metabolites were significantly changed compared with MC group,of which 8 endogenous metabolites were up-regulated and 11 endogenous metabolites were down-regulated (Table 2).MP management significantly risen the level of hippuric acid,citric acid,guanosine,inosine,cinnamoylglycine,sebacic acid,dodecanedioic acid and 12,13-DiHOME,while decreased the level of linoelaidic aid,oleic aid,linoleoyl ethanolamide,palmitoyl ethanolamide,taurocholic acid,ursodeoxycholic acid,taurine,N-acetylneuraminic aid,phenyllactic acid,ricinoleic acid and arachidonic aid.Moreover,linoelaidic acid,oleic acid,linoleoyl ethanolamide,palmitoyl ethanolamide,ursodeoxycholic acid,guanosine,inosine,phenyllactic acid,sebacic acid,dodecanedioic acid and arachidonic aid were found in all three treatment groups.Fortunately,MP alleviated the metabolic disorder caused by CTX and reversed the level of linoelaidic aid,oleic aid,ursodeoxycholic acid,phenyllactic acid,sebacic acid,inosine,guanosine,ricinoleic acid and arachidonic aid which were close to the level of the NC group.
3.5 Heatmap analysis
The identified data was subjected to clustering heatmap analysis and correlation analysis in order to further understand the metabolic differences of mice in different treatment groups (Fig.5).The heatmap directly reveals the changes of each biomarker,indicating the relative increase or decrease in value among the NC group,MC group and MPH groups.
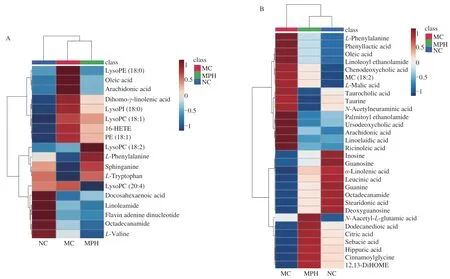
Fig.5 Heat maps of differential metabolites in liver (A) and feces (B),Rows: samples;Columns: biomarkers;(C) represented the correlation analysis of the metabolites in three groups both in liver and fecal.Red represented upward adjustment and blue represented downward adjustment.
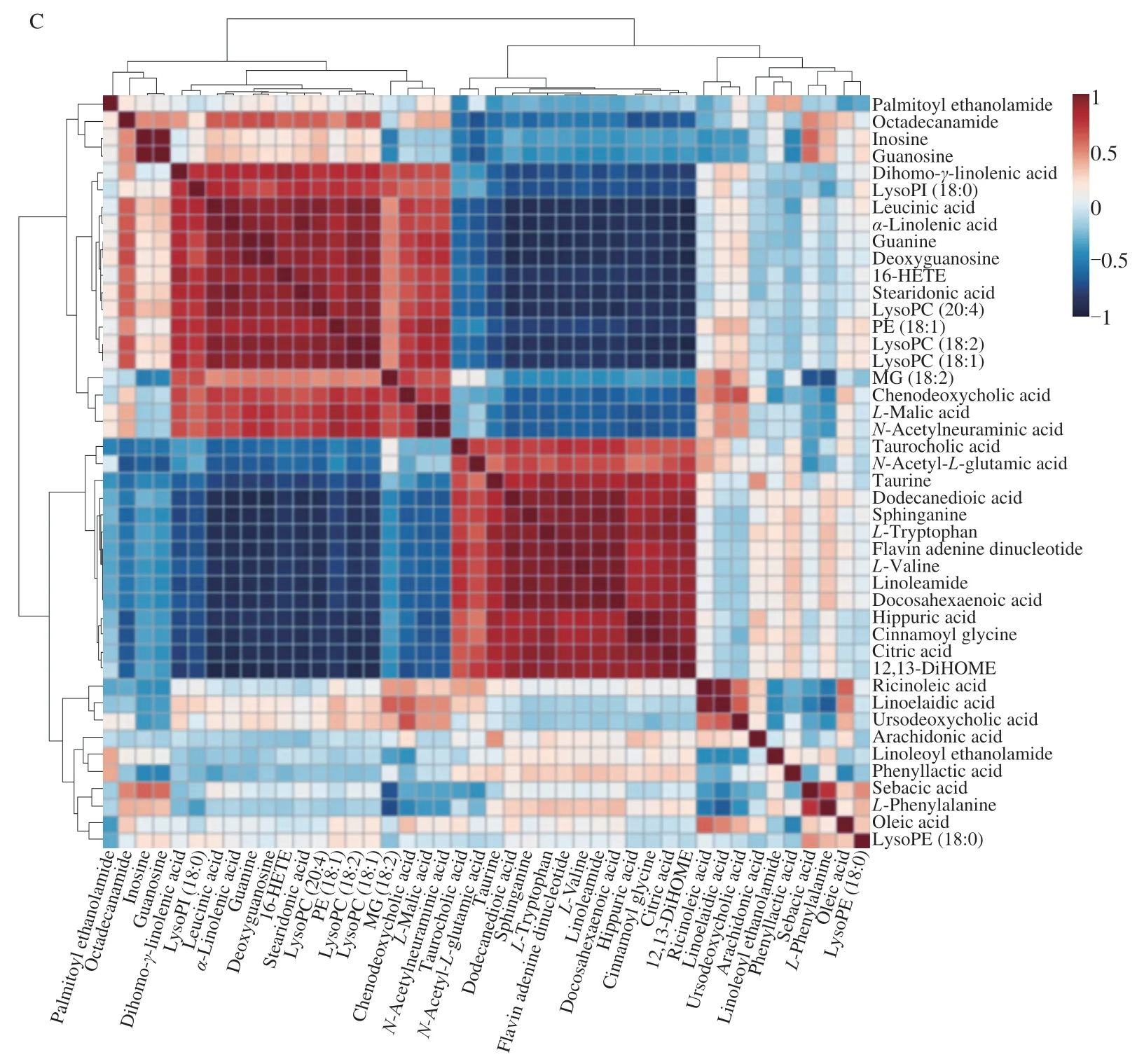
Fig.5 (Continued)
3.6 Biochemical interpretation and pathways analysis
In order to identify and visualize the possible metabolic pathways that were affected by MP on the liver injury caused by CTX,MetaboAnalyst 4.0 was used for metabonomic pathway analysis.In this study,the impact-value threshold calculated by the path topology analysis was set to 0.10,and the impact-value threshold above 0.10 was considered as a potential metabolic pathway.Liver metabolism results showed that the interference of CTX and MP treatment mainly included the following five metabolic pathways:sphingolipid metabolism,phenylalanine,tyrosine and tryptophan biosynthesis,arachidonic acid metabolism,phenylalanine metabolism and tryptophan metabolism (Fig.6A).Similarly,Fig 6B showed the effect of CTX and MP on fecal metabolism,mainly involved six metabolic pathways: taurine and hypotaurine metabolism,phenylalanine metabolism,α-linolenic acid metabolism,citrate cycle (tricarboxylic acid (TCA) cycle),phenylalanine,tyrosine and tryptophan biosynthesis,and arachidonic acid metabolism.Among them,phenylalanine metabolism,arachidonic acid metabolism and phenylalanine,tyrosine and tryptophan biosynthesis jointly participate in the metabolism of liver and feces.The information of important metabolic pathways was summarized in Table 3,and the metabolic pathways were constructed accordingly Fig.7.The results showed that these pathways exhibit significant disturbances in CTX-induced injury mice,and can be regulated by MP to inhibit the occurrence of liver injury.
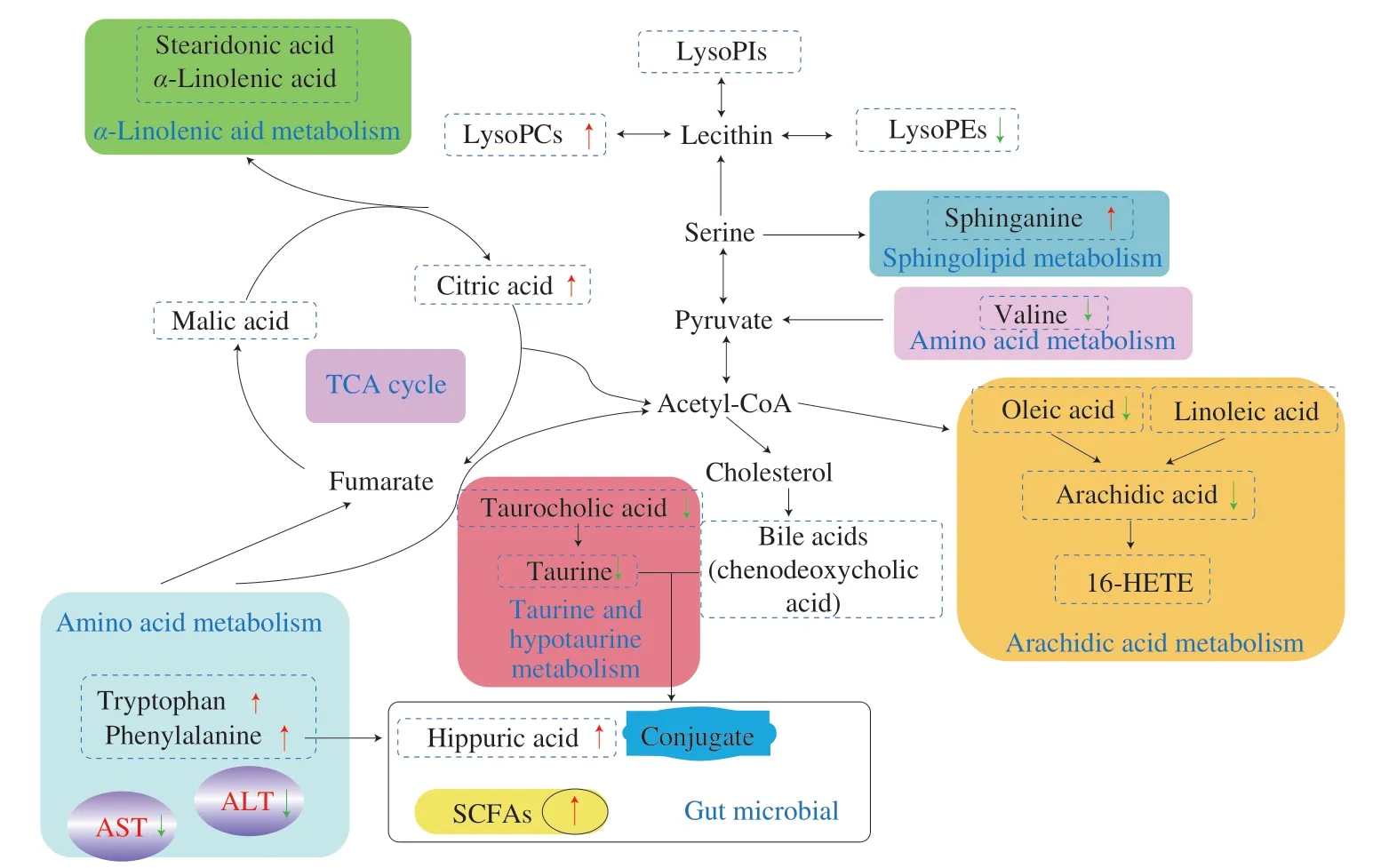
Fig.7 Analysis of related metabolic pathways and crucial biomarkers of hepatoprotective effect of MP on liver damaged in mice induced by CTX.Compared with the MC group,the red arrows and green arrows next to the metabolites represented the relative increase and decrease after MP treatment,respectively.
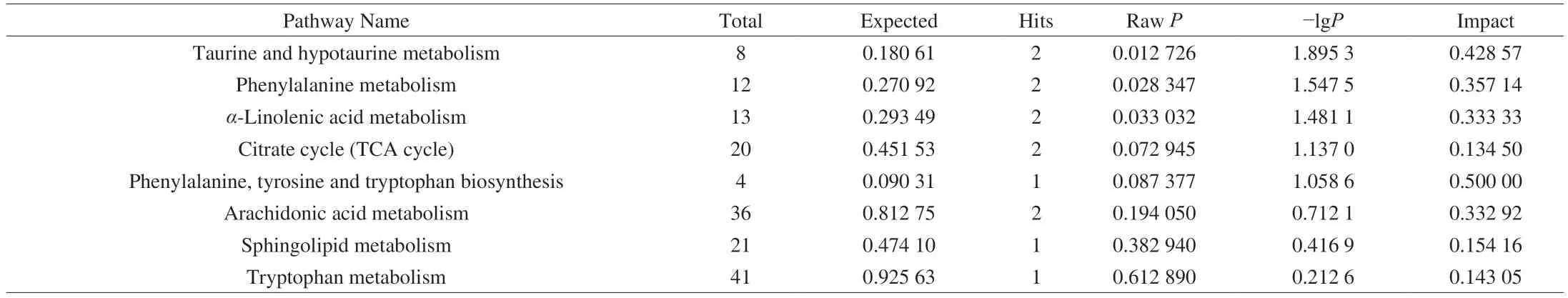
Table 3 Pathway analysis result with MetaboAnalyst 4.0.
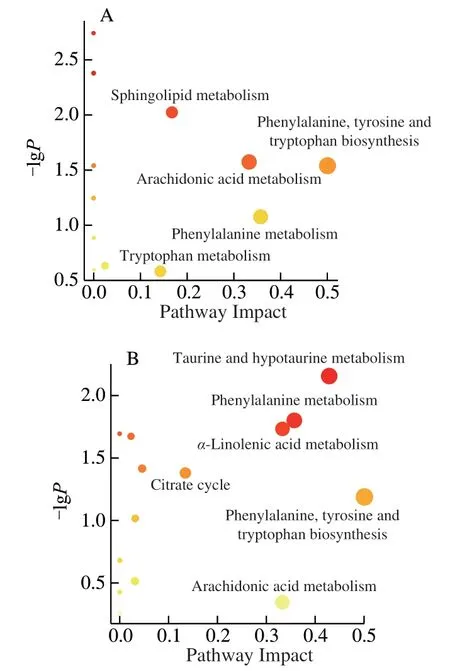
Fig.6 Summary of pathways analysis with MetaboAnalyst 4.0 in liver (A)and feces (B).
4.Discussion
Liver,an important organ of amino acid metabolism,plays an irreplaceable role in maintaining the balance of amino acid metabolism.Previously,it had been reported that when the liver was damaged,hormones that promote protein catabolism increase and inactivation decreases,leading to increased levels of protein catabolism and some amino acids in the systemic circulation [25].In the current study,the liver metabolomics results showed that the content of phenylalanine in the liver of mice with liver injury increased significantly when compared with the NC group (P<0.05),suggesting metabolic disorder [26].
AST and ALT are two important enzymes located in hepatocytes,which are indicators of liver damage.When lipid peroxidation of the liver cell membrane occurs,these two enzymes are easily released.In this study,these two important enzymes in the liver were simultaneously tested and found that CTX treatment did significantly increase the activities of AST and ALT (P<0.05),and the differences between the NC group and the MC group were observed,indicating that the liver injury model was successfully established,which were consistent with previous studies [4].Fortunately,gavage of MP for 7 consecutive days could reduce the release of AST and ALT toalleviate liver damage.Specifically,MP at 200 mg/kg·bw has a better hepatoprotective effect.
Phenylalanine was involved in phenylalanine metabolism as well as phenylalanine,tyrosine and tryptophan biosynthesis,indicating that the metabolism of phenylalanine was severely affected by CTX.On the one hand,abnormal amino acid metabolism leads to decreased amino acid metabolism and bring about increased levels of amino acids;on the other hand,the body’s ability to absorb amino acids is reduced.Since many amino acids were metabolized by enzymes in the liver,they were directly related to the activity of liver enzymes,which was consistent with the results of AST and ALT activities in the liver mentioned above.Tryptophan is one of the eight essential amino acids for the human body and participates in tryptophan metabolism.However,the levels of phenylalanine and tryptophan still increased after MP treatment,and this abnormal change may indicate dysfunction of amino acid metabolism.Interestingly,the level of valine,an important branched-chain amino acid,was significantly reduced after MP treatment (P<0.05).The above results could be inferred that MP may improve CTX-induced liver damage by adjusting the metabolism of branched-chain amino acids.
A large number of reports have proved that lipid metabolism plays a key role in the physiology and pathophysiology of human life systems,such as inflammation,cancer,vascular endothelial function and alcohol-induced liver injury [27].The glycerophospholipid is one of the most abundant and complex types of phospholipids in the human body,which is widely present in all tissues.In addition to participating in the synthesis of membrane surfactants and other substances,it also involve in bile synthesis,cell signal transduction and maintenance of the body’s metabolism,so as to ensure that the body’s energy and various metabolisms are normal [22,28].Therefore,many interference pathways have been reported in tissue metabolomics research.Phosphatidylcholine (PC),phosphatidylethanolamine (PE) and phosphatidylinositol (PI) are the main biological forms,and lysopho-phatidylcholines (LysoPCs) are produced by the hydrolysis of PC [29].LysoPCs are good marker for assessing the severity of fulminant liver failure.Generally speaking,their content in cells or tissues is very low,and it will cause damage to the membrane system of cells and serious body dysfunction if the concentration is too high.The results of this study show that the content of LysoPC (18:2),LysoPC (20:4),LysoPC (18:1),LysoPE(18:0),PE (18:1) and LysoPI (18:0) in the MC group was significantly increased in the liver (P<0.05).It is suggested that CTX treatment leads to a serious disorder of glycerophospholipid metabolism.The changes of these glycerophospholipids may be a choice as potential markers.Furthermore,LysoPE (18:0) level in the liver of MPH group mice were inversely correlated with MC group,and MP treatment significantly reversed CTX-induced abnormal changes,suggesting that MP may relieve glycerophospholipid metabolism disorders to some extent by regulating LysoPE (18:0).
In this study,it was also found that CTX-induced liver damage was closely related to arachidonic acid metabolism.A small differential molecule in the arachidonic acid metabolic pathway,arachidonic acid,is a precursor of pro-inflammatory metabolites such as HETEs,which can accelerate the progression of liver toxicity [30].Previous studies have proved that when the liver was damaged,arachidonic acid is broken down into free form by phospholipase A2 and released,and then a series of metabolic enzymes produce a variety of active substances [31].Consistently,our data showed that arachidonic acid levels in the liver and feces were significantly increased in the CTX-induced liver injury group when compared with the NC group (P<0.05).At the same time,the inflammatory metabolite 16-HETE in the MC group was also significantly increased in the liver (P<0.05),indicating that the imbalance of arachidic acid metabolism may be a sign of CTX-induced liver injury.Although MP treatment did not reduce the level of arachidonic acid in the liver,it significantly returned to the normal level in the feces(P<0.05),inhibiting metabolic disorders.Additionally,there were similar metabolites in liver tissue and fecal metabolomics,suggesting that MP treatment may influence the interaction between liver and fecal.The liver is most closely related to the intestine,where it circulates through the portal vein to protect poor bacterial composition and metabolites [32].
Moreover,oleic acid and linoleic acid can be metabolized into arachidonic acid [33].In this study,the content of oleic acid was significantly increased in liver homogenate and feces of mice in the liver injury group,and the content of linoelaidic acid was also significantly increased in feces (P<0.05).After the administration of MP,the content of oleic acid and linoelaidic acid in feces decreased sharply (P<0.05),which further indicated that MP could alleviate the abnormal metabolism of arachidonic acid.MP also mediate sphingosine metabolism.Sphingosine is an amino alcohol with a long unsaturated hydrocarbon chain and is one of the components of cell membranes.Its metabolic abnormalities are closely related to various diseases and have important physiological functions in regulating cells [34].Our results suggested that MP may affect sphingosine metabolism by regulating the content of sphingosine.The levels of stearidonic aid andα-linolenic aid in mice with liver injury were significantly lower than those in NC group (P<0.05),indicating that CTX severely interfered withα-linolenic acid metabolism.
Malic acid is the intermediate metabolite of the TCA cycle.As we all know,the TCA cycle is an important hub for the metabolism of the three major substances of carbohydrate,fat and protein,and provides energy sources for mammals [35,36].When compared with the NC group,the MC group had a significant increase in malic acid(P<0.05),revealing that CTX may significantly interfere with the TCA cycle.After the liver was injured by CTX,the energy metabolism of TCA slows down,and the same phenomenon was observed in CCl4induced liver injury mice [25].Citrate synthesis is an important part of the TCA cycle,the MPH group found a significant increase in biomarker citric acid when compared with the MC group(P<0.05).The above studies may provide a potential relationship between CTX and MP regulatory metabolites,MP has effect on energy metabolism and more research is needed in the future.
According to the structure,bile acids can be divided into free bile acids,such as cholic acid and deoxycholic acid,and bound bile acids,including the above mentioned free bile acids combined with glycine and taurine.First of all,the abnormal increase of bile acid causes the production of oxygen free radicals and endoplasmic reticulum stress,and finally induces cell apoptosis,which could be used as a biomarker of liver damage [37].CTX causes a significant increase in chenodeoxycholic acid and ursodeoxycholic acid,while MP restored the increased of ursodeoxycholic acid to normal level(P<0.05).Second,taurocholic acid was hydrolyzed to form taurine,which is then decarboxylated by cysteine to form taurosulfonic acid,which is further oxidized and participates in taurine and hypotaurine metabolism [38].Elevated taurocholic acid content can cause intrahepatic cholestasis,leading to intrahepatic bile acid metabolism disorder,and then causing liver toxicity.The hepatoprotective effect of MP on CTX may be realized by relieving the disturbed bile acid metabolism.Since primary bile acids are directly synthesized by liver cells using cholesterol as raw materials,they are secreted from the liver and enter the intestinal lumen.Under the action of some enzymes and intestinal microorganisms,they become secondary bile acids.Therefore,the hepatic protective effect of MP on CTX was closely related to intestinal flora [39].
The level of hippuric acid reflects the dysfunction of liver mitochondria,and the decrease of its content is a common indicator of inducing toxic compounds.After the application of MP,hippuric acid levels can be detected in the stool significantly increased,suggesting that MP has a protective effect on liver damage.On one hand,hippuric acid participates in phenylalanine metabolism.On the other hand,hippuric acid is a co-metabolite of gut microbes,which is largely related to the activity of intestinal microorganisms [40].Intestinal microbes play an important role in the digestion and absorption of many nutrients and the prevention of diseases.SCFAs are the main products of intestinal microbial fermentation,and their confusion can lead to a variety of diseases,such as inflammation,liver fibrosis,metabolic diseases,etc.[41,42].The SCFAs concentration of mice in the liver injury group was significantly lower than that in the NC group.MP treatment obviously returned to the level of NC group.Our previous studies also proved that MP can play a beneficial effect on the intestinal flora [19].The above results further confirmed that MP could regulate the activity of intestinal microbes and alleviate CXT-induced liver injury.
5.Conclusion
In summary,this study combined biochemical indicators and metabolomics methods to evaluate the effects of MP on CTXinduced liver injury in mice.MP reduces the levels of AST and ALT in the liver,and the dose at 200 mg/kg·bw is the best.The UPLC-QTOF/MS liver and fecal metabonomics method was established,18 potential biomarkers were found in liver homogenate and 29 potential biomarkers were found in feces.These metabolites are mainly related to 8 metabolic pathways.In addition,MP promotes the production of SCFAs in feces,which may be related to the metabolism of intestinal microbes.These results not only indicated that MP may play a role in hepatoprotective by regulating endogenous metabolites,but also provide a scientific basis for the treatment of liver injury.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgement
This study was supported by grants from the National Natural Science Foundation of China (21566024).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.1016/j.fshw.2022.07.061.
- 食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil

