Structure elucidation and in vitro rat intestinal fermentation properties of a novel sulfated glucogalactan from Porphyra haitanensis
Peilin Chen,Lu Liu,Zirun Cheng,Yi Zhang,c,Baodong Zheng,c,Xiaoke Hu,Hongliang Zeng,c,*
a Engineering Research Center of Fujian-Taiwan Special Marine Food Processing and Nutrition,Ministry of Education,Fuzhou 350002,China;
b College of Food Science,Fujian Agriculture and Forestry University,Fuzhou 350002,China;
c Fujian Provincial Key Laboratory of Quality Science and Processing Technology in Special Starch,Fujian Agriculture and Forestry University,Fuzhou 350002,China;
d Key Laboratory of Coastal Biology and Bioresource Utilization,Yantai Institute of Coastal Zone Research,Chinese Academy of Sciences,Yantai 264003,China
Keywords:Porphyra haitanensis Structure characterization Fecal microf lora Short-chain fatty acids Metabolic function prediction Polysaccharide
ABSTRACT This study was to investigate the structure and rat fecal microbial fermentation properties of a polysaccharide fraction (PHP2) isolated from the red marine alga Porphyra haitanensis.PHP2 was characterized as a sulfated glucogalactan,with a hypothetical backbone structure of →4)Gα(1→6)G4Sβ(1→4)Glc(1→ and a side chain of Man(1→6)Glc.PHP2 had an irregular spherical chain conformation.The 16S rRNA sequence analysis revealed that PHP2 modulated the rat fecal micro-f lora composition,with a similar effect to inulin,changing the dominant genus (Lactobacillus and Escherichia-Shigella) and promoting the growth of organisms that degrade sulfur-containing polysaccharides,such as Desulfovibrio,Ruminococcaceae_UCG-005,and Ruminococcus_2.PHP2 can promote production of acetic,propionic and butyric acid by rat fecal micro-f lora.Prediction of metabolic function suggested that PHP2 could modulate cholesterol metabolism.The sulfated glucogalactan fermentation behavior may be associated with its monosaccharide composition,chain branching and chain conformation.PHP2 appeared to have considerable potential as functional food,and was associated with sulfur-containing polysaccharides in general.
1.Introduction
Sulfur-containing or sulfated polysaccharides,which are also known as sulfated ester polysaccharides,are structural components widely found in marine algae [1,2]and are widely used in the food industry as gelling (agar) and thickening (carrageenan) agents.They also possess useful biological activities,such as hypolipidemic,antioxidant and pre-biotic activities [2,3].Porphyrahaitanensis,an economically important red macroalga (Order: Bangiales;Division:Rhodophyta),is extensively cultivated along the coasts of China,especially in Fujian and Zhejiang provinces,China [4].The main sulfated polysaccharide component ofP.haitanensisis mainly composed of galactose and 3,6-anhydrogalactose [5].In recent years,P.haitanensispolysaccharides have attracted increasing attention for their biological activities,including hypolipidemic,antioxidant and anticoagulant activities [6],and there is great potential for the application of polysaccharides in functional foods.
The gut micro-floral community has an important effect on human health and has many functions,such as helping to maintain the epithelial barrier,regulating and maturing the immune system and producing various health-beneficial metabolites [7].Non-digested polysaccharides are fermented and utilized by the intestinal flora in the colon,regulating the composition of the intestinal micro-flora and increasing the production of short chain fatty acids (SCFAs) [8,9].SCFAs are fatty acids with between one and six carbon atoms and acetic,propionic,and butyric acids are the predominant SCFAs in the gut lumen [10].The distribution between the different SCFAs produced from a particular polysaccharide species is associated with its monosaccharide composition,glycosidic linkages,chain branching and conformation,and surface topography [11-13].Therefore,determining the structure and chain conformation of a polysaccharide is helpful for understanding its structure-activity relationship.Polysaccharide from the brown seaweedEckloniaradiatahas prebiotic effects and enhances SCFA production [14].Polysaccharide from the red seaweedKappaphycusalvareziialso has prebiotic effects and promotes the production of SCFAs [15].Although crudeP.haitanensispolysaccharides have been shown to modulate the micro-floral composition and promote SCFA production [3],the composition ofP.haitanensispolysaccharides is well defined.To date,regulation of the gut micro-flora by purifiedP.haitanensispolysaccharides and the relationship between their structure and regulation of the gut micro-floral composition has not been reported.The hypothesis of this study was to ask whether the polysaccharide fraction(PHP2) isolated fromP.haitanensismay be an active compound in regulating micro-flora.
Therefore,the aim of this study was to investigate the structure and fermentation properties of aP.haitanensisPHP2.The structure was characterized by FT-IR,methylation analysis,NMR,and atomic force microscopy (AFM).16S rRNA gene sequencing amplification and gas chromatography (GC) were used to evaluate the changes in micro-floral composition and SCFA production,respectively.
2.Materials and methods
2.1 Materials and chemicals
P.haitanensiswas purchased from Pingtan county,Fujian province,China.Preculture medium: yeast powder (5 g/L),sodium chloride (10 g/L),glucose (5 g/L),tryptone (10 g/L) and maltose(6 g/L);carbon-free medium: soybean peptone (2 g/L),yeast powder(2 g/L),2 g/L NaHCO3(2 g/L),NaCl (0.1 g/L),KH2PO4(0.04 g/L),MgSO4·7H2O (0.01 g/L),L-cysteine hydrochloride (0.5 g/L),CaCl2·6H2O (0.01 g/L),heme chloride (0.05 g/L),vitamin K1(10 μL/L),bile salt (0.5 g/L),and resazurin (4 mL/L,0.025%).
Acetic anhydride,ethanol,sodium borohydride,dichloromethane and toluene were from China Pharmaceutical Group Chemical Reagents Co.,Ltd.(Shanghai,China).Rhamnose,xylose,arabinose,glucose,mannose,and galactose standards were from Sigma-Aldrich,Inc.(St.Louis,MO,USA).Heme chloride,trifluoroacetic acid (TFA),methanol,hydroxylamine hydrochloride,pyridine,KBr,D2O,dimethyl sulfoxide,sodium dimethylsilopentane sulfonate,anhydrous sodium sulfate,acetate acid,propionic acid,and butyric acid were from Shanghai Macklin Biochemical Technology Co.,Ltd.(Shanghai,China).
2.2 Preparation of PHP2
DryP.haitanensiswas ground into powder and sieved through an 80-mesh screen.P.haitanensiscrude polysaccharides were extracted as described previously [16],with minor modifications.Crude polysaccharides were extracted using deionized water at a ratio of 1:20 (m/V),at 80 °C for 2 h.After ethanol precipitation and purification with DEAE-Sepharose FF,Sephacryl S-400 HR,and ultrafiltration,PHP2 was obtained.
2.3 Structural characterization of PHP2
2.3.1 Chemical composition
The total sugar and protein contents were determined using the phenol-sulfuric acid method [17]and a BCA protein quantification kit (Yeasen Biotech Co.,Ltd.,Shanghai,China),respectively.The monosaccharide composition was determined as described previously [18],with minor modifications.PHP2 was hydrolyzed using TFA (2 mL,2 mol/L) for 1.5 h at 95 °C.Then,the hydrolysate was treated with hydroxylamine hydrochloride (10 mg) and pyridine (0.5 mL) at 90 °C for 30 min.After cooling to room temperature,acetic anhydride (0.5 mL)was added and heated at 90 °C for 30 min.After filtering through a 0.45 μm organic filter membrane,the resulting mixture of alditol acetates was analyzed by GC (Agilent,7890A;Agilent,Santa Clara,CA,USA;HP-1701 column,30 m × 0.32 mm,0.25 µm;Agilent,USA).The initial temperature was 170 °C,held for 2 min,increased to 230 °C at 10 °C/min,held at 230 °C for 2 min,then increased to 250 °C,at 2 °C/min and held at 250 °C for 5 min.
2.3.2 FT-IR spectral analysis
PHP2 powder and KBr (~1:100) were ground uniformly in an agate mortar,then pressed into a thin sheet for FT-IR analysis.The FT-IR spectrum was recorded on a Nicolet AVATAR360 (Madison,WI,USA) in the range 400–4 000 cm−1.The scanning time was 24 and resolution 4 cm−1[19].The FT-IR data were analyzed by OPUS Spectroscopy Software (Madison,WI,USA).
2.3.3 Methylation analysis
PHP2 was reduced using automatic glucuronic acid reduction(ARCGI,BR-HYY-001;Yangzhou Borui Saccharide Biotech Co.,Ltd.,China).The reduced sample (2–3 mg) was dissolved in dimethyl sulfoxide,then methylated using a methylation kit (Yangzhou Borui,China).The methylation products were hydrolyzed with TFA(2 mol/L,1 mL) at 100 °C for 90 min.After removing TFA under a stream of nitrogen at 70 °C,the hydrolysate was reduced with sodium borohydride (60 mg) for 8 h,then neutralized with acetic acid and acetylated with acetic anhydride (1 mL) at 100 °C for 1 h [20].The acetylated product was extracted with dichloromethane.After removing residual water with anhydrous sodium sulfate,the solution of partially methylated alditol acetates was analyzed by GC-MS (GCMSQP 2010,Shimadzu,Kyoto,Japan).The column was an RXI-5 SIL MS column (30 m × 0.25 mm,0.25 μm,Shimadzu,Japan),with a temperature program of: held at 120 °C for 5 min,then increased to 250 °C,at 3 °C/min.The inlet and detector temperatures were both 250 °C,with helium as carrier gas,flow rate 1 mL/min.The GC-MS data were analyzed with GC-MS Lab Solution (Version 4.30,GCMSQP201;Shimadzu,Kyoto,Japan).
2.3.4 NMR analysis
PHP2 solution (4%) was prepared in D2O,then centrifuged at 1 500 ×g(Sorvall™ Legend™ Micro 17R;Thermo Fisher Scientific(China) Co.,Ltd.,China).The supernatant was transferred to a 5 mm NMR tube.1 D,1H-and13C-NMR and 2 D,correlation spectroscopy(COSY),total correlation spectroscopy (TOCSY),nuclear overhauser effect spectroscopy (NOESY),heteronuclear single quantum coherence (HSQC),and heteronuclear multiple-bond correlation(HMBC) NMR spectra were recorded on an AVANC III 600 NMR spectrometer (Bruker,Hamburg,Germany).The internal standard was sodium dimethylsilopentane sulfonate and the probe temperature was 35 °C [21].The NMR data were analyzed by Topspin 4.0.7 software(Bruker,Germany).
2.3.5 AFM analysis
PHP2 was dissolved (1 µg/mL) in ultrapure water [22],then pipetted onto a silicon plate and air-dried at room temperature.Data were collected with an AFM microscope (Dimension Icon,Bruker,Germany) and analyzed by NanoScope Analysis 1.8 software (Bruker,Germany).
2.4 In vitro fermentation
Sprague Dawley rat fecal material (~10 g) was obtained from ten 8-week-old specific pathogen free (SPF) rats.Rat fecal suspension(10%) was prepared with phosphate-buffered saline (PBS,0.1 mol/L,pH 7.2),then added (16%,V/V) to preculture medium and incubated at 37 °C for 18 h under an atmosphere of 10% H2,10% CO2,and 80% N2in an aluminum-sealed anaerobic culture bottle (Guangzhou Tingyinhui Trading Co.,Ltd.,Guangzhou,China),to form the public bacterial mother liquor (PB group).
PHP2 and inulin were added to carbon-free medium,to a final concentration of 10 g/L and designated as PHP2 and INU groups,respectively.The pH of the media was adjusted to 6.83 ± 0.04.Then,the mother liquor (5%,V/V) was added to PHP2 and inulin media (10 mL),respectively,followed by incubation in aluminumsealed anaerobic culture bottles,under an atmosphere of 10% H2,10% CO2,and 80% N2,at 37 °C for 24 h in a thermostatic culture oscillator (ZWY-200D,Shanghai Zhicheng Analytical Instrument Manufacturing Co.,Ltd.,Shanghai,China) at 150 r/min.All cultures were performed in triplicate.
2.5 Micro-flora diversity analysis
Microbial DNA from fermented samples was extracted using an E.Z.N.A.®soil DNA kit (Omega Bio-tek,Norcross,GA).The primers for PCR amplification were 338F (5’-ACTCCTACGGGAGGCAGCAG-3’) and 806R(5’-GGACTACHVGGGTWTCTAAT-3’) [3].The 16S rRNA gene sequencing analysis was performed on an Illumina MiSeq platform(Illumina,San Diego,CA,USA) according to standard protocols from Majorbio Bio-Pharm Technology Co.,Ltd.(Shanghai,China).
2.6 Determination of SCFAs
SCFAs were determined as described previously,with minor modifications.Fermentation culture medium was centrifuged at 9 600 ×g(4 °C) for 10 min (Sorvall™ Legend™ Micro 17R,Thermo Fisher Scientific (China) Co.,Ltd.,China).SCFAs were determined by GC (7890A,Agilent,USA) on an HP-INNOWAX column (30 m ×0.32 mm,0.25 µm,Agilent,USA).The initial column temperature was 100 °C,held for 0.5 min,then increased to 200 °C at 4 °C/min.The carrier gas was N2at a flow rate of 20 mL/min,the flame ionization detector (FID) fuel gas was H2at 300 mL/min and the auxiliary gas was air at 30 mL/min [23].The FID and injector temperatures were both 240 °C and data were analyzed with HP Chem Station Plus software (Agilent,USA).
2.7 Statistical analysis
The 16S rRNA sequencing data were analyzed on the Majorbio Cloud Platform (http://www.majorbio.com).One-way ANOVA was used to analyze SCFA data,using SPSS 11 (IBM,Chicago,IL).
3.Results and discussion
3.1 Chemical composition of PHP2
PHP2 had a total sugar content of 93% and protein content of 0.53% (Table 1),and the sulfate content was 5.07% (all in wt.%),as discovered in our previous study [24].PHP2 was mainly composed of galactose (69.27%),mannose (21.32%),and glucose (9.41%) (all molar ratio%) (Fig.1A,Table 1),which was in agreement with a previous study that reported theP.haitanensisfraction (PP5) was composed of mannose,glucose,and galactose [4].
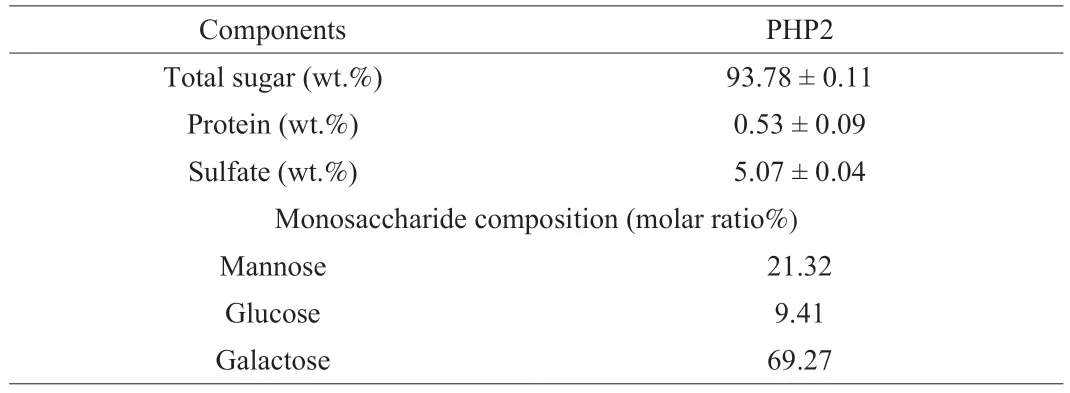
Table 1 Chemical composition of PHP2.

Fig.1 (A) Monosaccharide composition of PHP2,determined by GC.(B) FT-IR spectrum of PHP2;(C) GC-MS total ion chromatogram of PHP2 after methylation analysis to determine linkages between residues.
3.2 FT-IR characteristics
The FT-IR spectrum of PHP2 was recorded (Fig.1B).The intense wide peak at 3 436 cm−1and the weak peak at 2 928 cm−1correspond to the hydroxyl group stretching vibration and the C–H bending vibration,respectively [25].The peak at 1 637 cm−1corresponds to H–O–H bending and the peak at 1 417 cm−1to the stretching vibration of the carboxyl C=O [26].The peak at 1 384 cm−1corresponds to the stretching vibration of the sulfate groups [27]and that at 1 254 cm−1to the S=O stretching vibration of the sulfate groups [28].The three peaks between 1 200 and 1 000 cm−1(1 155,1 073 and 1 035 cm−1) correspond to vibrations of the sugar C–OH groups and C-O-C of the glycosidic bond [29],indicating that PHP2 is composed of pyranose rings.The peak at 773 cm−1corresponded to the symmetric stretching vibration of theα-pyranose ring [30].The peak at 990 cm−1corresponded to the C–O stretching vibration [31]and that at 933 cm−1,to the 3,6-anhydro linkage C–O stretching vibration [32].The peaks at 894 and 817 cm−1corresponded to theβ-configuration,and theα-configuration and C–O–S stretching vibration,respectively [33].The peaks at 617 cm−1and 580 cm−1corresponded to the O=S=O bending vibration [27].
3.3 Methylation characteristics
To further investigate the linkage pattern of PHP2,methylation analysis was carried out.The GC-MS total ion chromatogram(Fig.1C) has peaks that were identified as 2,3,4,6-Me4-Manp,2,3,6-Me3-Galp,2,3,4-Me3-Galpand 2,3-Me2-Glcp(Table 2),indicating that PHP2 contained Manp-(1→,→4)-Galp-(1→,→6)-Galp-(1→ and →4,6)-Glcp-(1→ residues,in agreement with the monosaccharide composition.The presence of 2,3,4,6-Me4-Manpsuggested that PHP2 had a side chain of a single Man residue.This result is not consistent with a previous report [16],but the reported monosaccharide composition was also different,in that residues other than galactosyl were present in larger amounts than found here.
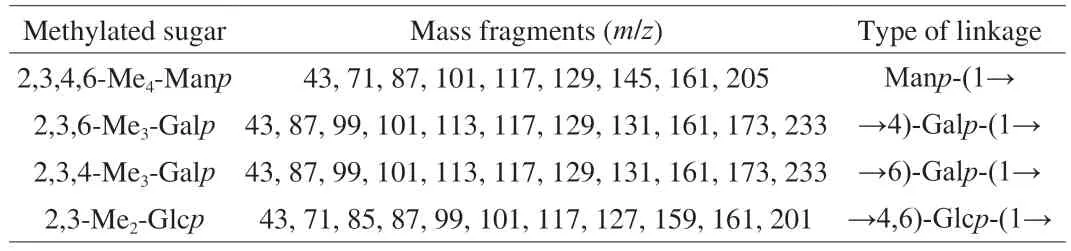
Table 2 Methylation linkage-analysis of PHP2.
3.4 NMR characteristics
Four hetero protons were observed,atδ5.20,5.07,4.77,and 4.64(Fig.2A) and four anomeric carbons,atδ103.08,101.80,101.02,and 97.99 (Fig.2B).The chemical shift atδ5.20 corresponded to the H1 of mannose (Man) [34],that atδ5.07 corresponded to galactose(Gα) [26,33],that atδ4.77 corresponded to glucose (Glc) [35]and that atδ4.64 corresponded toD-galactose-4-sulfate (G4Sβ) [21].

Fig.2 1 D NMR spectra of PHP2,(A) 1H-NMR spectrum;(B) 13C-NMR spectrum.
The correlations between anomeric carbons and hetero protons were determined by HSQC (Fig.3A).The anomeric chemical shifts atδ103.08/4.64,101.80/4.77,101.02/5.20,and 97.99/5.07 in the HSQC spectrum (Fig.3A) corresponded to the H1/C1 of the G4Sβ,Glc,Man,and G residues,respectively.The other proton signals of the G4Sβresidue were confirmed by COSY (Fig.3B),TOCSY (Fig.3C)and NOESY spectra (Fig.3D);the chemical shifts of H2−H5 wereδ3.66,4.38,4.22,3.89,and 3.91,respectively (Table 3).The carbon assignments of the G4Sβresidue were obtained from the HSQC spectrum (Fig.3A);the corresponding carbon signals wereδ80.71,79.54,78.46,67.33,and 61.04 for C2−C5,respectively.Based on the adjacent correlations in the COSY (Fig.3B),TOCSY (Fig.3C)and NOESY spectra (Fig.3D),as well as the corresponding carbon signals in the HSQC spectrum,the H2/C2,H3/C3,H4/C4,H5/C5,and H6/C6 of the Glc residue wereδ3.52/72.05,3.73/70.33,3.65/75.69,3.88/67.36,and 3.80/69.14,respectively.The complete proton chemical shifts of the Man and Gαresidues were obtained from the COSY (Fig.3B),TOCSY (Fig.3C) and NOESY spectra (Fig.3D);the corresponding carbon signals were identified from the HSQC spectrum (Fig.3A).The NMR assignments are summarized in Table 3.
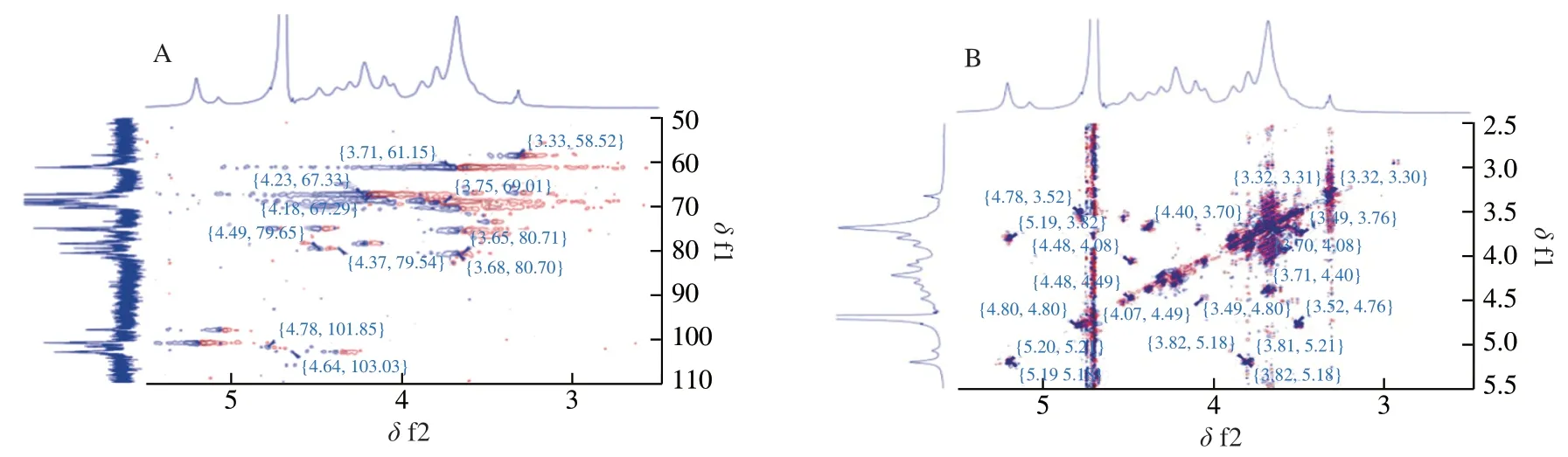
Fig.3 2 D NMR spectra of PHP2;(A) 1H/1H COSY;(B) 1H/1H NOESY;(C) 1H/1H TOCSY;(D) 1H/13C HSQC;(E) 1H/13C HMBC;(F) Proposed chemical structural unit of PHP2.
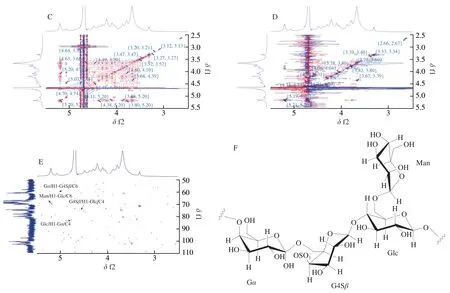
Fig.3 (Continued)
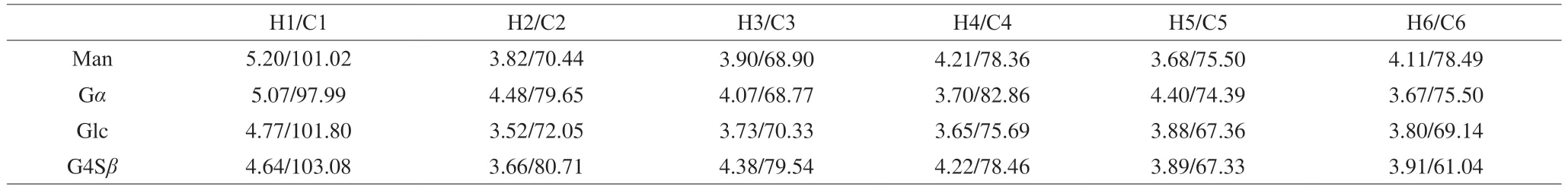
Table 3 1H and 13C NMR chemical shifts (δ) of PHP2.
The glycosidic linkage sequence between the different PHP2 residues was based on the correlation peaks in the HMBC spectrum(Fig.3E).The correlation peak between Glc (H1) and Gα(C4) atδ4.77/82.86 indicated the presence of Glc(1→4)Gα.Similarly,the cross-peaks atδ4.64/75.69 (H1 of G4Sβ/C4 of Glc),5.07/61.04 (H1 of Gα/C6 of G4Sβ),and 5.20/69.14 (H1 of Man/C6 of Glc) showed that G4Sβwas linked to Glc by 1→4 glycosidic linkages,Gαwas linked to G4Sβby 1→6 glycosidic linkages and Man was linked to Glc by 1→6 glycosidic linkages.Combining the NMR assignments with the FT-IR assignments,monosaccharide composition and methylation analysis,PHP2 was characterized as a novel sulfated glucogalactan and a hypothetical backbone structure was proposed as:→4)Gα(1→6)G4Sβ(1→4)Glc(1→,with a side chain Man(1→6)Glc(Fig.3F).This was different from that reported by Khan et al.[36],who showed that polysaccharides contained galactose and anhydrogalactose.Qiu et al.[37]reported that polysaccharides has a typical porphyran structure and has a backbone of alternating(1→4)-linked 3,6-anhydro-α-L-galactopyranose units or (1→4)-linkedα-L-galactose 6 sulphate units.According to our previous study,the molecular weight of PHP2 was (1.14 × 106(± 3.44%)) g/mol [24].The repeated backbone structure unit of PHP2 was~1 530.
3.5 AFM characteristics
AFM provides direct evidence for the chain conformation of polysaccharides in aqueous solution.The chain features,width,and height of PHP2 were determined from planar images (Fig.4A),PHP2 had an irregular spherical chain conformation and was uniform in size.Calculations by NanoScope analysis software found that thelength and width of the biggest aggregate were 0.142 and 0.083 μm,respectively and the height was approximately 7.4−9.1 nm,which was much more than the width of a single polysaccharide chain(0.1−1.0 nm) [38].This observation may be related to the hydroxyl groups,sulfate groups and branch structure of PHP2 being more accessible for molecular interaction [22,39,40].The 3D rendering(Fig.4B) showed that PHP2 did not have a completely spherical chain conformation,and was vertically aggregated.
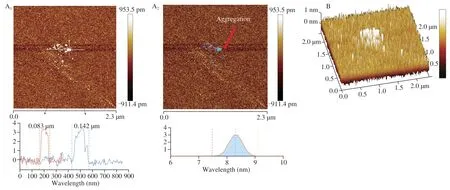
Fig.4 Scanning atomic force microscopy image of PHP2,dried onto a mica surface.(A) 2D image;(B) 3D image.
3.6 Effect of PHP2 on rat fecal microbial composition
3.6.1 Microbial diversity
Theα-diversity is a well-established measure of the richness(Chao index) and diversity (Shannon and Simpson indices) of the gut micro-flora.The Chao index (Fig.5A) indicated PHP2 treatment enhanced micro-floral richness compared with PB (P<0.05).The Shannon index (Fig.5B) after PHP2 treatment was significantly lower than that of PB,whereas conversely,the Simpson index (Fig.5C) of PB was significantly lower than that of PHP2.Gut microbial diversity is positively correlated with a predisposition to diseases [41].However,in this study,PHP2 decreased gut microbial diversity,which is not consistent with another study.Xu et al.[3]reported thatP.haitanensispolysaccharides can effectively increase the diversity of the gut microbial community in humans.This may be that the different molecular weight and sulfate content,which was different from the results of Xu et al.[3].Zeng et al.[42]reported that different diets were the main factor for gut microbiota.To study the similarity,or difference between the microbial composition of different samples,a cluster analysis of the sample micro-flora distance-matrix was carried out and a hierarchical cluster tree was constructed (Fig.5D).This revealed a significant distinction between the micro-flora of the PB and PHP2 treatments,and a clear separation was also observed between the INU and PHP2 treatments.Theβ-diversity was shown using principal components analysis (PCA),which was performed to visualize the differences between the micro-flora in the different cultures (Fig.5E).PC1 and PC2 accounted for 97.85% and 2.14% of the variation,respectively.The different culture samples were well separated,but the replicates of each culture were tightly grouped.Theβ-diversity calculations showed that the micro-floral composition of the PHP2 culture was significantly separated from those of INU and PB.
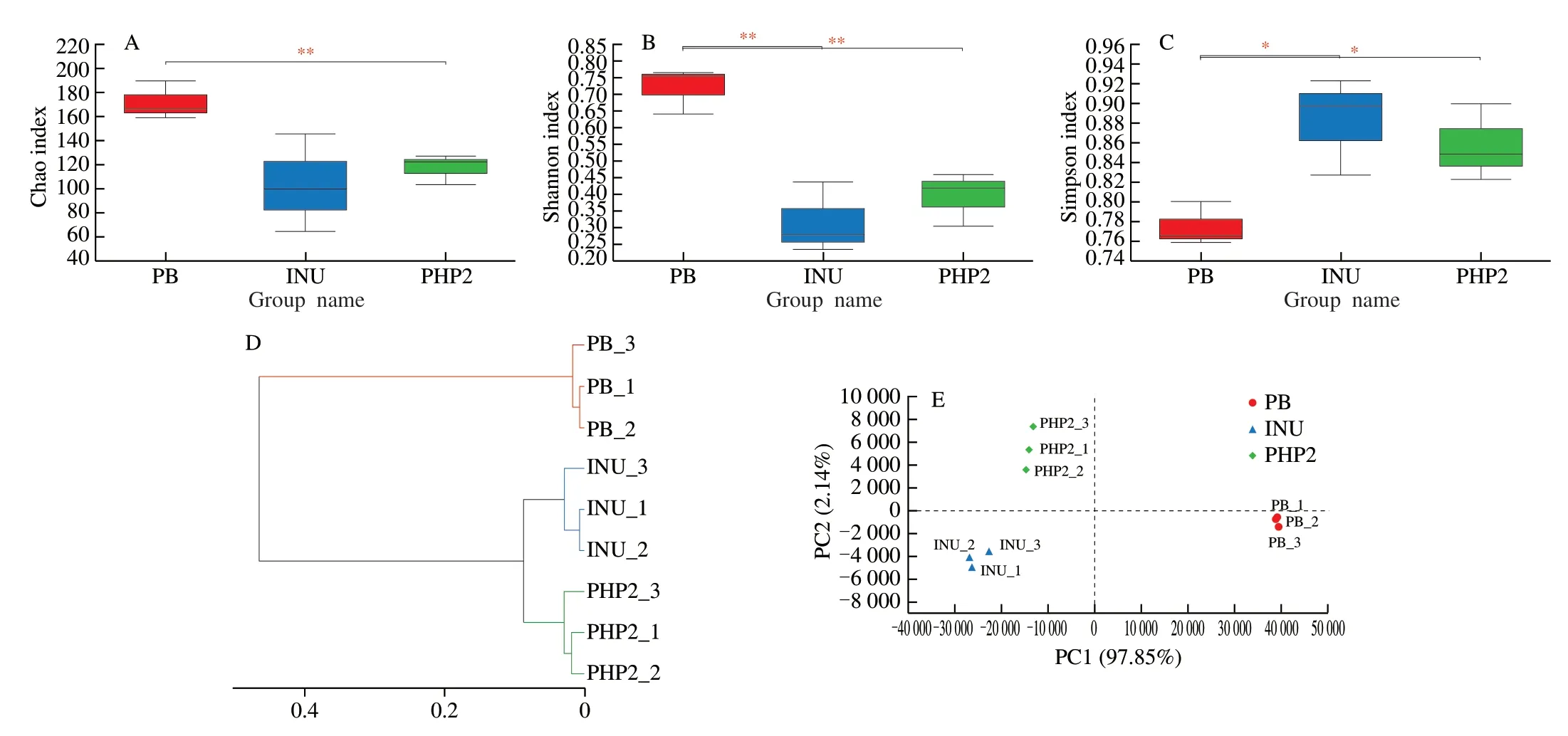
Fig.5 Comparison of microbial composition changes between fermentation with PHP2,inulin (INU) and public bacterial mother liquor (PB).(A) Chao index;(B)Shannon index;(C) Simpson index;(D) Hierarchical clustering tree;(E) Principle components analysis.* P <0.05,** P <0.01.
3.6.2 Key specific phylotypes
To illustrate the modification of the gut micro-flora by PHP2,the microbial distribution at the genus level was determined(Fig.6A).Compared with PB,in which the genusLactobacillusmade up over 95% of the microbial population,fermentation with INU and PHP2 radically changed the distribution,withEscherichia-Shigellamaking up over 90% of the population.The huge relative abundance ofEscherichia-ShigellaandLactobacillusmade other genera almost undetectable in the genus level bacterial taxonomic profiling.Similarly,HelicteresangustifoliaL.polysaccharide treatment increased the abundance ofEscherichia[43]and treatment withP.haitanensispolysaccharides,increased the relative abundance ofEscherichia-Shigella[44].According to our previous study,the molecular weight of PHP2 is (1.14 × 106(± 3.44%)) g/mol [24].The increase inEscherichia-Shigellamay be related to degradation of PHP2 into oligosaccharides by carbohydrate hydrolyzing enzymes.Low molecular weight carbon sources can be used byEscherichia-Shigella,but polysaccharides cannot [45].However,minor differences in microbial composition between the different cultures were visible in a correlation heat map (Fig.6B).Compared with PB,the relative abundances ofEscherichia-Shigella,Ruminococcaceae_UCG-005,Ruminococcus_2,LactococcusandCandidatus_Saccharimonasin PHP2 increased,whereasEnterococcus,Prevotella_9,Dubosiella,Coriobacteriaceae_UCG-002,Parabacteroides,Adlercreutzia,Rikenellaceae_RC9_gut_group,Bacteroides,Helicobacterand norank_o__Gastranaerophilales decreased.Compared with INU,the relative abundances of Ruminococcaceae_UCG-005,Prevotella_9,Ruminococcus_2,Fusicatenibacter,Phascolarctobacterium,Lactococcus,Desulfovibrio,Candidatus_Saccharimonas,Prevotellaceae_Ga6A1_group,and norank_f__Eggerthellaceae increased in PHP2,whereas the relative abundances ofEnterococcus,KosakoniaandAdlercreutziadecreased.Ruminococcaceae_UCG-005 andRuminococcus_2 belong to the phylum Firmicutes,which have a high capacity to degrade polysaccharides,such as resistant starch [46].The decrease inPrevotella_9 abundance may result from a relative lack of coding genes for degradation of PHP2 [44].Helicobacter,which can trigger inflammation,resulting in various diseases [9],decreased,indicating that PHP2 inhibited its growth.The increase inDesulfovibrioabundance with PHP2 may be related to the sulfation of PHP2,becauseDesulfovibriois a sulfate-reducing bacterium [47].Phascolarctobacteriumis a substantial acetic acid and propionic acid-producer [48]and may be responsible for much of the observed propionic acid production.Gut microbial fermentation with tea polysaccharides increased production of acetic,propionic and butyric acids,but propionic acid production was lower than with inulin [49],in agreement with our findings.
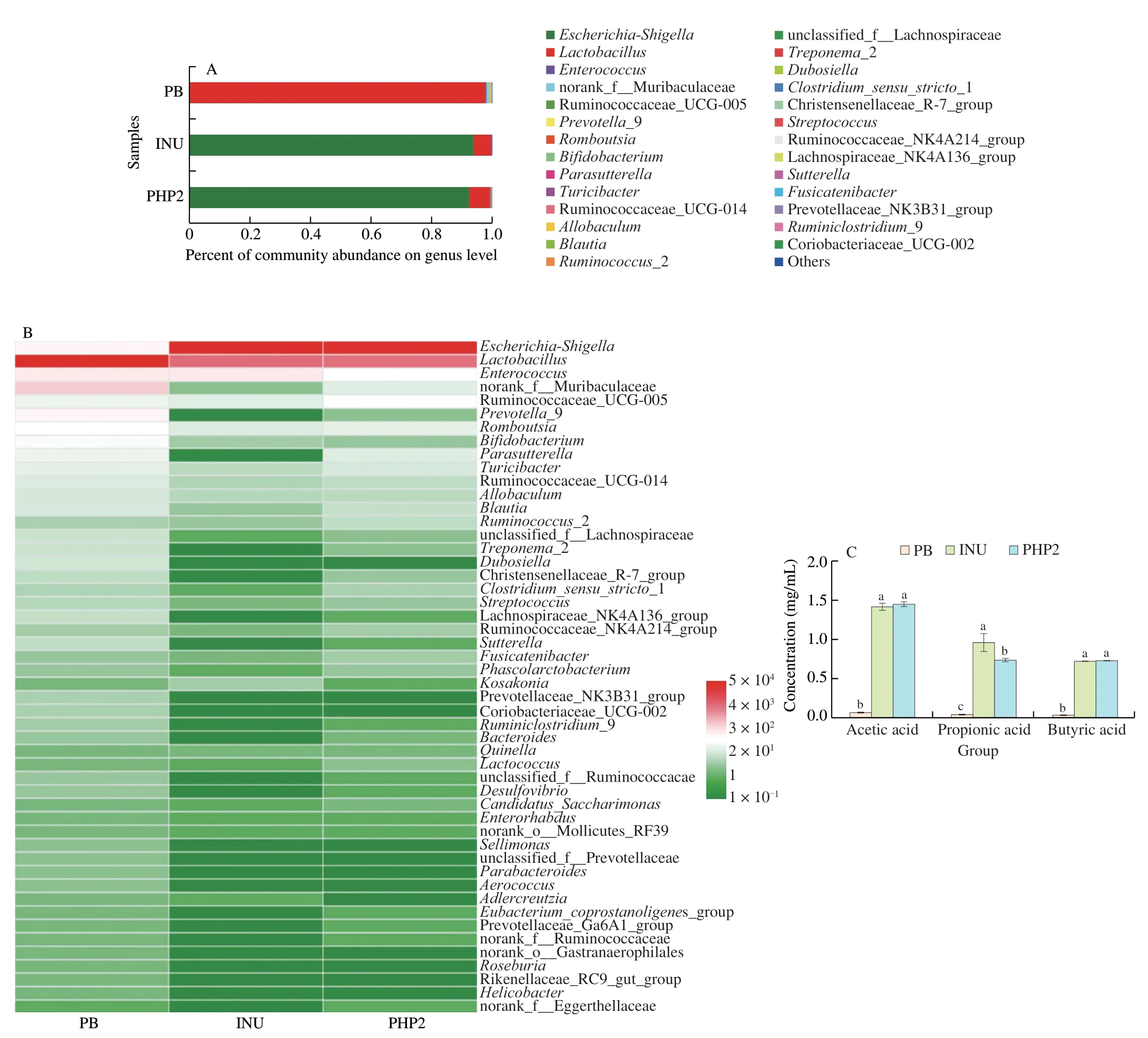
Fig.6 (A) Stacked column plot of microbial genus relative abundance;(B) heatmap analysis of relative abundance of top 50 genera;(C) concentrations of acetic,propionic and butyric acids,Different lower case letters (a,b and c) represent significant differences between different treatments (P <0.05);(D) correlation heatmap between SCFA production and fermentations;(E) correlation heatmap of the most abundant 50 genera.*.P <0.05,**. P <0.01,***.P <0.001.
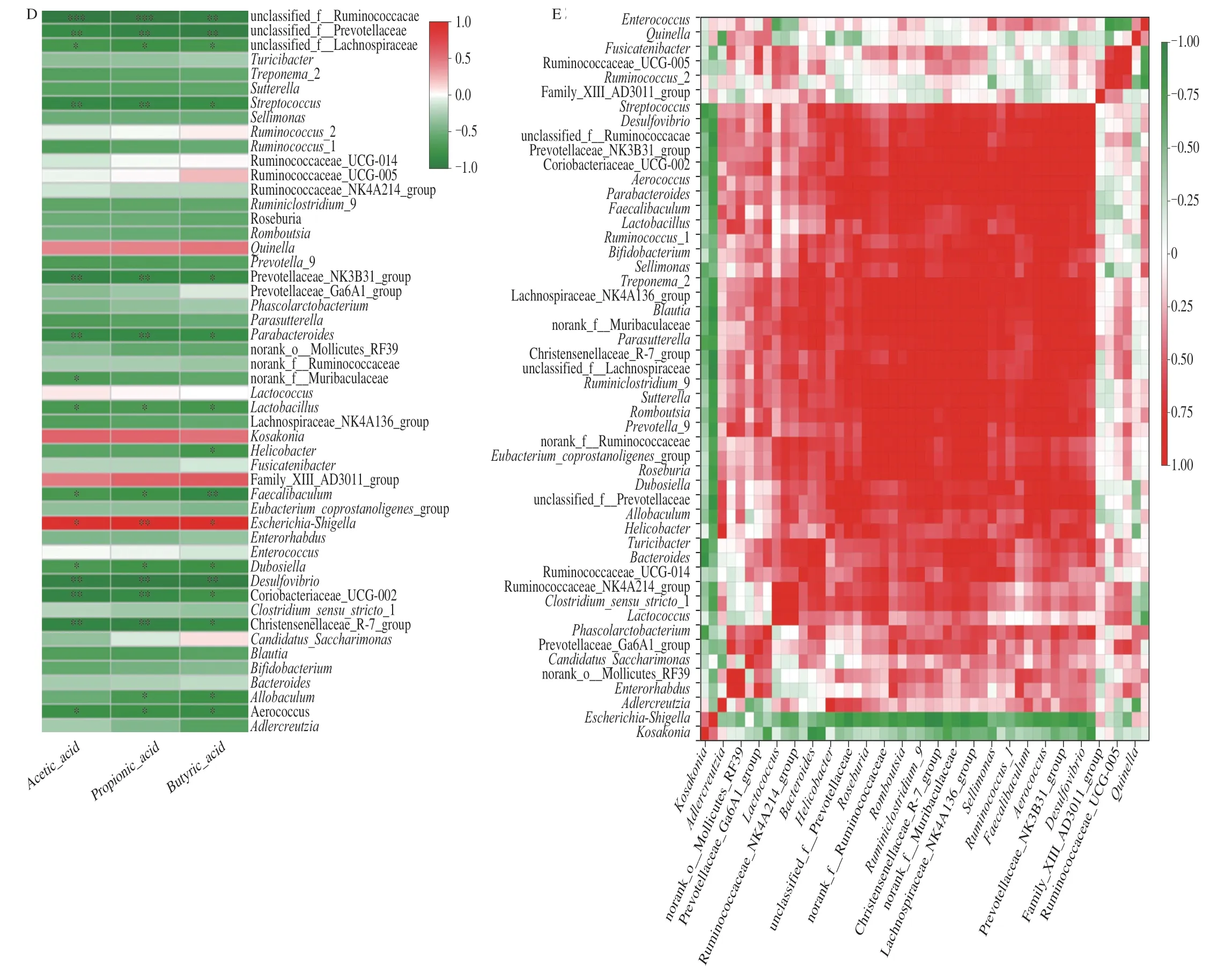
Fig.6 (Continued)
3.6.3 Correlation between specific phylotypes and SCFAs
The major SCFAs produced duringinvitroanaerobic fermentation were acetic,propionic and butyric acids (Fig.6C).After fermentation for 24 h,these SCFAs markedly increased in the INU and PHP2 fermentations (P<0.05),by~10−15-fold compared with PB.The content of propionic acid in INU was significantly higher(P<0.05) than that in PHP2.To evaluate the effect of PHP2 on the relationship between the microbial composition and SCFA production,a correlation analysis was performed (Fig.6D).Streptococcus,Prevotellaceae_NK3B31_group,Parabacteroides,Lactobacillus,Faecalibaculum,Dubosiella,Desulfovibrio,Coriobacteriaceae_UCG-002,Christensenellaceae_R-7_group andAerococcuswere negatively correlated with acetic,propionic and butyric acid levels(P<0.05).Norank_f__Muribaculaceae was negatively correlated with acetic acid (P<0.05).Helicobacterwas negatively correlated with butyric acid (P<0.05),indicating that butyric acid inhibits the growth ofHelicobacter.Allobaculumwas negatively correlated with propionic and butyric acids (P<0.05).Escherichia-Shigellawas positively correlated with acetic,propionic and butyric acids(P<0.05).The micro-floral distribution was not only affected by the carbon source and SCFA levels,but the genera were also partially antagonistic to each other,according to a species correlation heatmap (Fig.6E).Escherichia-Shigellawas negatively correlated withLactobacillus,Prevotella_9,Dubosiella,Coriobacteriaceae_UCG-002,HelicobacterandAdlercreutzia.Lactobacilluswas negatively correlated with Ruminococcaceae_UCG-005,Ruminococcus_2,andLactococcus,but positively correlated withEnterococcus,Prevotella_9,Dubosiella,Coriobacteriaceae_UCG-002,Helicobacter,andAdlercreutzia.SCFA production is influenced by the monosaccharide composition,glycosidic linkages,chain branching and conformation of a polysaccharide [11-13].PHP2 was composed of galactose and glucose,with single-residue mannose side chains.Guar gum,which is composed of galactose and mannose also promoted SCFA production [50].The chain conformation of PHP2 was irregularly spherical and uniform in size.This would provide easier access for microbial polysaccharide-degrading enzymes [51].
3.6.4 Metabolic function prediction
A heat map of the predicted function of microbial metabolic pathways involved in carbohydrate,amino acid and lipid metabolism,included 43 Kyoto Encyclopedia of Genes and Genomes (KEGG)pathways (based on Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUST) 2;Fig.7).The metabolic pathway profiles of INU and PHP2 were very similar,but clearly different from PB.The metabolic flux through pathways related to carbohydrate metabolism,including starch and sucrose metabolism,amino sugar and nucleotide sugar metabolism,glycolysis/gluconeogenesis,fructose and mannose metabolism,galactose metabolism,and inositol phosphate metabolism,all decreased after PHP2 treatment.The observed decrease in microbial fructose,mannose,and galactose metabolism may be related to the mannose and galactose content of PHP2 being depleted by microbiota.Conversely,pathway flux through pyruvate metabolism,butanoate metabolism,glyoxylate and dicarboxylate metabolism,propanoate metabolism,citrate cycle,pentose and glucuronate interconversions,C5-branched dibasic acid metabolism and ascorbate,and aldarate metabolism,increased after PHP2 treatment.Amino acid metabolism,such as lysine biosynthesis,tyrosine metabolism,lysine degradation and tryptophan metabolism,decreased after PHP2 treatment,which was in accordance with another study.Treatment of gut micro-flora withArtemisiasphaerocephalaKrasch seedpolysaccharides decreased tyrosine and tryptophan metabolism,and lysine degradation [23],whereas glycerophospholipid metabolism and fatty acid biosynthesis increased.Glycerolipid metabolism,fatty acid degradation,biosynthesis of unsaturated fatty acids,sphingolipid metabolism,secondary bile acid biosynthesis,synthesis,and degradation of ketone bodies and primary bile acid biosynthesis also decreased with PHP2.The increase in butanoate and propanoate metabolism appears to be related to the increase in propionic and butyric acid production resulting from PHP2 treatment and their use as an energy source by the micro-flora.Propionic acid is a substrate for glycolysis/gluconeogenesis which is related to obesity and glucose intolerance [52].The decrease in glycolysis/gluconeogenesis may be related to gut microbial use of propionic acid as an energy source.Protein can be used by some gut microbes to produce toxic metabolites,whereas a low pH environment inhibits their ability to do this [53].Bacteroidesproduces a bile acid hydrolase which can degrade bile acids and in combination with suppression of secondary bile acid production by SCFAs,can decrease intestinal bile acid levels [54,55].A decrease in bile acid levels may be beneficial to health,by promoting conversion of cholesterol to bile acid in the liver [56,57],so PHP2 may be able to modulate cholesterol metabolism.
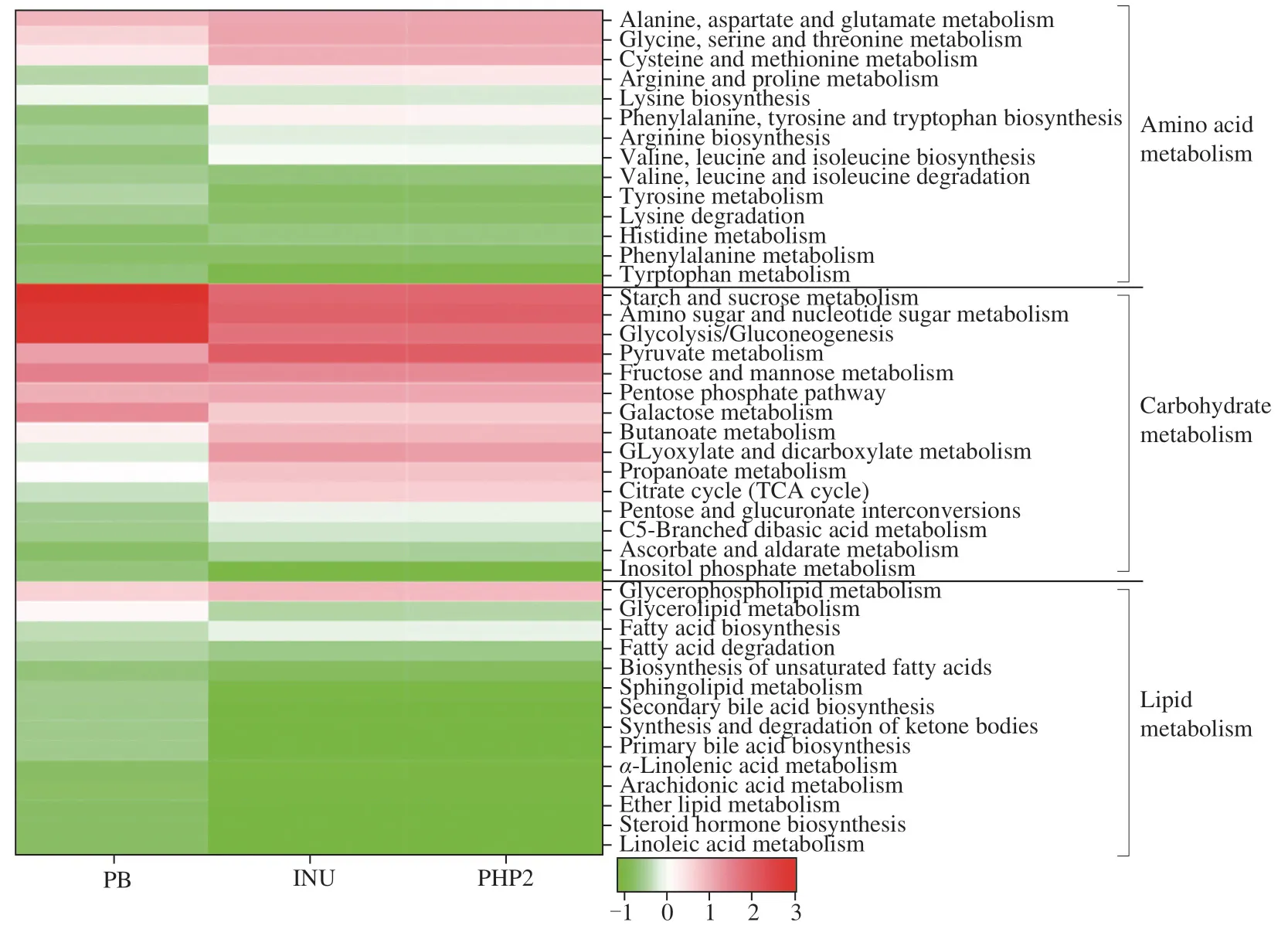
Fig.7 Heatmap showing effect of PHP2,INU and PB on regulation of KEGG pathways related to amino acid metabolism,carbohydrate metabolism and lipid metabolism.
A possible mechanism of PHP2 degradation by rat fecal microflora is initial degradation byDesulfovibrio(sulfate removal),Ruminococcaceae_UCG-005 andRuminococcus_2 (polysaccharide chain degradation),followed by metabolism of the mono-and oligo-saccharides by other genera,such asPhascolarctobacterium,Escherichia-ShigellaandPrevotella_9.
4.Conclusion
A novel sulfated glucogalactan (PHP2) was extracted and purified from the red marine alga,P.haitanensis.PHP2 was mainly composed of galactose,mannose,and glucose.PHP2 had hydroxyl groups,sulfate groups and branch structure.A hypothetical structure for PHP2 was proposed,consisting of a backbone structure: →4)Gα(1→6)G4Sβ(1→4)Glc(1→,with a side chain of Man(1→6)Glc.PHP2 had an irregular spherical chain conformation and was vertically aggregated.PHP2 treatment strongly promoted the production of SCFAs of the rat fecal micro-flora and affected the microbial metabolic pathway flux profile.Prediction of metabolic function indicated that PHP2 may be able to modulate cholesterol metabolism and provide other effects of benefit to health.A possible mechanism of PHP2 degradation by rat fecal micro-flora is initial degradation byDesulfovibrio(sulfate removal),Ruminococcaceae_UCG-005,andRuminococcus_2 (polysaccharide chain degradation),followed by metabolism of the mono-and oligo-saccharides by other genera,such asPhascolarctobacterium,Escherichia-ShigellaandPrevotella_9.The current study shows that PHP2 appears to have considerable potential as a prebiotic functional food ingredient,in addition to the functional food associated with sulfated polysaccharides in general.
Declarations of interest
None.
Acknowledgements
This work was supported by the Scientific Research Foundation of Graduate School of Fujian Agriculture and Forestry University(1122yb065),the Program for Leading Talent in Fujian Provincial University (660160190).
- 食品科学与人类健康(英文)的其它文章
- Colloidal nanoparticles prepared from zein and casein:interactions,characterizations and emerging food applications
- Biological factors controlling starch digestibility in human digestive system
- Preparation methods,biological activities,and potential applications of marine algae oligosaccharides: a review
- Development of hyaluronic acid-based edible film for alleviating dry mouth
- Mushroom β-glucan and polyphenol formulations as natural immunity boosters and balancers: nature of the application
- Preparation of multicore millimeter-sized spherical alginate capsules to specifically and sustainedly release fish oil

