Melphalan-loaded methoxy poly(ethylene glycol)-poly(D,l-lactide)copolymer nanomicelles in the treatment of multiple myeloma
Yingying Chen, Qiang Zeng, Bingyang Chu, Zhigang Liu, Xue Wei, Mengran Chen,Peipei Yang, Minghai Tang, Ting Niu, Yongqian Jia, Ying Qu, Zhiyong Qian
Department of Hematology and Institute of Hematology, State Key Laboratory of Biotherapy, West China Hospital, Sichuan University, Chengdu 610041,China
Keywords:Melphalan Multiple myeloma MPEG-PDLLA Nanomicelle
ABSTRACT Multiple myeloma (MM) is the second most common hematological tumor characterized by the proliferation of monoclonal plasma cells.Melphalan (MEL) is commonly used in the treatment of MM and is especially essential for patients undergoing autologous stem cell transplantation (ASCT).Although many drugs for MM have been developed in recent years, chemotherapy followed by ASCT remains the optimal option.Melphalan, the backbone of the conditioning regimen, brings severe toxicities at a high dose.Nanodrug delivery systems enable drugs to be highly effective and have low toxicity.In this study, methoxy poly(ethylene glycol)-poly(D,l-lactide) copolymer (MPEG-PDLLA) was chosen to encapsulate melphalan,and the characteristics, effectiveness, and safety of MEL/MPEG-PDLLA in vitro and in vivo were investigated.MEL/MPEG-PDLLA showed slow release and was easily engulfed by MM cells despite a result of the antitumor assay comparable to that of free melphalan in vitro.The in vivo results showed that MEL/MPEG-PDLLA could significantly alleviate tumor burden and prolong survival time without increasing the toxicity to vital organs.In addition, MEL/MPEG-PDLLA could significantly reduce the damage to the intestinal mucosa caused by melphalan.In conclusion, MEL/MPEG-PDLLA shows improved antitumor activity and has the potential to alleviate pains of MM patients undergoing ASCT.
Multiple myeloma (MM) is the second most common hematological tumor characterized by the proliferation of monoclonal plasma cells [1,2].The world health organization (WHO) estimated in 2019 that approximately 160,000 patients suffer from MM globally, with a 5-year overall survival rate of approximately 52% [3].Since the 1960s, melphalan (MEL) has already been used in MM and has achieved good results.Melphalan, a synthetic bifunctional alkylating agent, is an l-isomer of the phenylalanine derivative of nitrogen mustard which is nonspecific cytotoxic either by forming cross-links with deoxyribonucleic acid (DNA) or DNA protein complexes [4].The pharmacokinetics of melphalan appears to be doseand age-independent and the half-life period ranges from 1.3 h to 2.3 h [4,5].The toxicities of melphalan depend on the dose, mainly myelosuppression, gastrointestinal toxicities, hypersensitivity and so on [6].Owing to the short half-life period and toxicities of melphalan, many novel drugs for MM have been developed for different targets in recent years, including anti-CD38 monoclonal antibodies, the new generation of immunomodulators and proteasome inhibitors, and Chimeric Antigen Receptor T-Cell (CAR-T) therapy targeting B cell maturation antigen (BCMA).Despite the improvement in efficacy and prognosis, these new drugs were not added to the conditioning regimen currently.It appeared that melphalan remains an indispensable drug for MM patients, especially for patients undergoing autologous stem cell transplantation (ASCT)[7], a treatment that hematopoietic stem cells from patients in remission are imported back into the patients themselves to restore hematopoiesis of the bone marrow.
High-dose (HD) melphalan followed by ASCT is currently the standard treatment for MM patients [8-10].Although HD melphalan can increase the concentration in tumor tissues and prolong the duration of drug action, the accompanying severe adverse events should also be given more attention, such as mucosal damage, interstitial pneumonia, and arrhythmia [4,11].One of the studies regarding MM patients receiving HD melphalan as a conditioning regimen showed that severe diarrhea and bloody stools were the common complications and compromised the patients’life.Another case report described that five patients experienced severe paroxysmal atrial fibrillation after HD melphalan and one of them died three months later [12].Currently, melphalan reduction alone or combined with other treatments, such as total body irradiation(TBI) or busulfan, is used in conditioning regimens clinically [13-17].However, this brings new problems, such as relapse and other adverse events.Therefore, the enhancement and detoxification of melphalan need to be carried out in other ways.
At present, the nanodrug delivery system is very popular and has solved many drug-related problems [18,19].Nanomaterials are characterized by large surface area, high surface activity, and easy assembly [20].Drugs loaded with nanomaterials can improve the dispersion and water solubility of a liquid environment, reduce drug toxicityviaslow release, and improve biocompatibilityin vivo[21,22].Additionally, nanomaterials can prolong the half-life by reducing drug degradation [23].These characteristics of nanomaterials perfectly fit our ideal antitumor drug delivery system[24-26].Polymeric micelles were shown to increase water solubility, slow drug release, and reduce toxicity.Furthermore, a synthesized copolymer from monomers of D,l-lactide and methoxy poly(ethylene glycol) by a ring opening bulk polymerization, called methoxy poly(ethylene glycol)-poly(D,l-lactide) copolymer (MPEGPDLLA), also demonstrated improved water solubility, great effi-cacy, and non-toxicity when loading drugs [27].MPEG-PDLLA can be self-assembled in aqueous solution.Previous studies demonstrated that MPEG-PDLLA micelles did not cause extra cytotoxicity on tumor or normal cells [28].We have previously successfully synthesized MPEG-PDLLA.Docetaxel (DTX) and paclitaxel (PTX)have been shown to be stably encapsulated in the self-assembled MPEG-PDLLA in our previous studies [29-31].Compared to free drugs, both DTX-MPEG-PDLLA and PTX-MPEG-PDLLA presented a higher drug accumulation and better anti-tumor effect in the treatment of cancers.Despite a long way to clinical use, it was a good choice that MPEG-PDLLA was employed to encapsulate antitumor drugs to improve efficacy and reduce toxicity.In addition, nanomaterials can enhance drug targeting to tumors due to their selective accumulation in tumor tissues through enhanced permeability and retention (EPR) effects, which is unconclusive in hematological malignancies.Given that current studies of MPEG-PDLLA-encapsulated drugs have mainly focused on treating solid tumors, we chose a drug to be encapsulated and explored its role in the hematological tumor.
The use of melphalan in the treatment of MM is unquestionable.Despite the boost of novel drugs for MM, melphalan has always been the cornerstone in MM patients, especially in patients undergoing ASCT.Few studies are currently performed to investigate the nanodrug delivery system of melphalan in MM, and a stable, effective and simple method for melphalan transported by the nanodrug delivery system has not been established so far [32,33].Here, we chose MPEG-PDLLA to encapsulate melphalan and constructed MEL/MPEG-PDLLA micelles, investigated its efficacy and safety in MM treatment and expected the micelles could improve the antitumor activity of melphalan and solve problems of adverse effect, especially for patients with ASCT.
MPEG-PDLLA copolymers were synthesized according to our previous studies [34,35].MPEG-PDLLA was synthesized from the D,l-lactide and MPEG by ring opening polymerization.The MPEGPDLLA copolymers were purified and kept at -20 °C before application.The encapsulated micelles were preparedviathin film hydration method.The MPEG-PDLLA powder was dissolved in methanol.Next, the clarified solution was evaporated by rotation at 30 °C for 2 h.Subsequently, self-assembly of MPEG-PDLLA was performed with 1×phosphate buffer solution (PBS) at 60 °C.Melphalan and MPEG-PDLLA were co-dissolved in methanol at a certain ratio.The next steps were the same as described above, and then MEL/MPEG-PDLLA was prepared.
The particle sizes of MEL/MPEG-PDLLA micelles were tested by dynamic light scattering (DLS).As illustrated in Fig.1A, the average sizes were 26.30±0.44 nm, 30.63±2.78 nm, 35.95±1.62 nm, and 54.62±4.99 nm for MPEG-PDLLA, MEL/MPEG-PDLLA (MEL:MPEGPDLLA=1:24), MEL/MPEG-PDLLA (MEL:MPEG-PDLLA=1:9), and MEL/MPEG-PDLLA (MEL:MPEG-PDLLA=1:4), respectively.As shown in Fig.1B, compared to another two groups, the sizes of MEL/MPEG-PDLLA (MEL:MPEG-PDLLA=1:4) fluctuated over a larger range within 8 h at room temperature.Considering the particle size, stability and the amount of drug package, we finally chose 1:9 as the ratio of melphalan to MPEG-PDLLA in our study.Next, the morphology characteristics of MEL/MPEG-PDLLA micelles were determinedviatransmission electron microscopy (TEM) (Fig.1C).In addition, to investigate the stability of the preparation when stored in the refrigerator or kept at room temperature,the size of micelles was also detected every two hours at 4 °C(refrigerated temperature) and 25 °C (room temperature).The results are shown in Fig.1D, after encapsulating melphalan, the size of the nanoparticles increased, and the sizes of MPEG-PDLLA and MEL/MPEG-PDLLA fluctuated over a small range, which indicated that MPEG-PDLLA was stable before and after melphalan encapsulation.Furthermore, we detected the amount of melphalan encapsulated in MPEG-PDLLA nanomicelles at 4 °C and 25 °C at 2, 4, and 8 h.After 2 h, the amount of melphalan in MPEG-PDLLA nanomicelles at 4 °C and 25 °C did not change too much.However,the MEL/MPEG-PDLLA at 4 °C was remained stable for a longer period than that at 25 °C (P< 0.001; Fig.1E).
Melphalan release behavior was performedviathe dialysis bag method.The concentration of melphalan was measured by highperformance liquid chromatography (HPLC).As shown in Fig.1F,the cumulative release amount of melphalan had a rapid increase in the first twelve hours.The cumulative release amounts of melphalan were 58.74% ± 3.58% at the first hour and 81.19% ± 4.76%at the 72ndhour in the free melphalan group.In the MEL/MPEGPDLLA group, the cumulative release amount was 39.73% ± 1.41%at the first hour and 61.80% ± 5.99% at the 72ndhour (P< 0.01).This significant difference showed that the nanomicelles could delay the release of melphalan and showed a good sustained-release behavior.
The cytotoxicity experiment was performed with a CCK-8 kit in ARD cells (Human MM cell lines).1×104cells were seeded in 96-well plates and free melphalan or MEL/MPEG-PDLLA was added at gradient concentrations.After incubation for 24 and 48 h, 20 μL of CCK-8 reagent was added for 2 h and the optical density (OD) values were detected at a wavelength of 450 nm.As illustrated in Fig.2A, the inhibition of ARD cells by free melphalan and MEL/MPEGPDLLA were both dose dependent, especially when the concentration surpassed 10 μg/mL.The cell viability in the MEL/MPEG-PDLLA at different concentrations was comparable with that in the free melphalan after either 24 or 48 h.
In addition, the cell apoptosis experiment was performed using flow cytometry.ARD cells were seeded in 12-well plates at a density of 2×105cells per well.Free melphalan or MEL/MPEGPDLLA was added at a dose of 10 μg/mL.After incubation for 24 h, cells were collected and stained with propidium iodide (PI)and Annexin V.As illustrated in Fig.2B, the apoptosis rates of ARD cells were 26.18% ± 2.64% and 25.11% ± 3.12% in the free melphalan and MEL/MPEG-PDLLA groups after 24 h, respectively.MEL/MPEG-PDLLA was shown to be not superior to free melphalan (P=0.73).To some extent, the results indicated that MPEGPDLLA was almost harmless to cells.In a further breakdown of Fig.2B, we found that more cells experienced early apoptosis in the MEL/MPEG-PDLLA group than those in the free melphalan group (MEL/MPEG-PDLLAvs.free melphalan, 16.87% ± 3.40%vs.11.73%±3.01%), which seemed to imply a tender tumor-killing effect and toxicity of MEL/MPEG-PDLLA.This may explain why MEL/MPEG-PDLLA was ingested more but the efficacy is similar to free melphalan.The melphalan release behavior showed that MEL/MPEG-PDLLA was released more slowly than free melphalan.When many MEL/MPEG-PDLLA nanoparticles were absorbed into cells, they did not work at once.Instead, the efficacy of MEL/MPEGPDLLA is not strong initially, and over time, it achieved a similar efficacy to that of free melphalan.
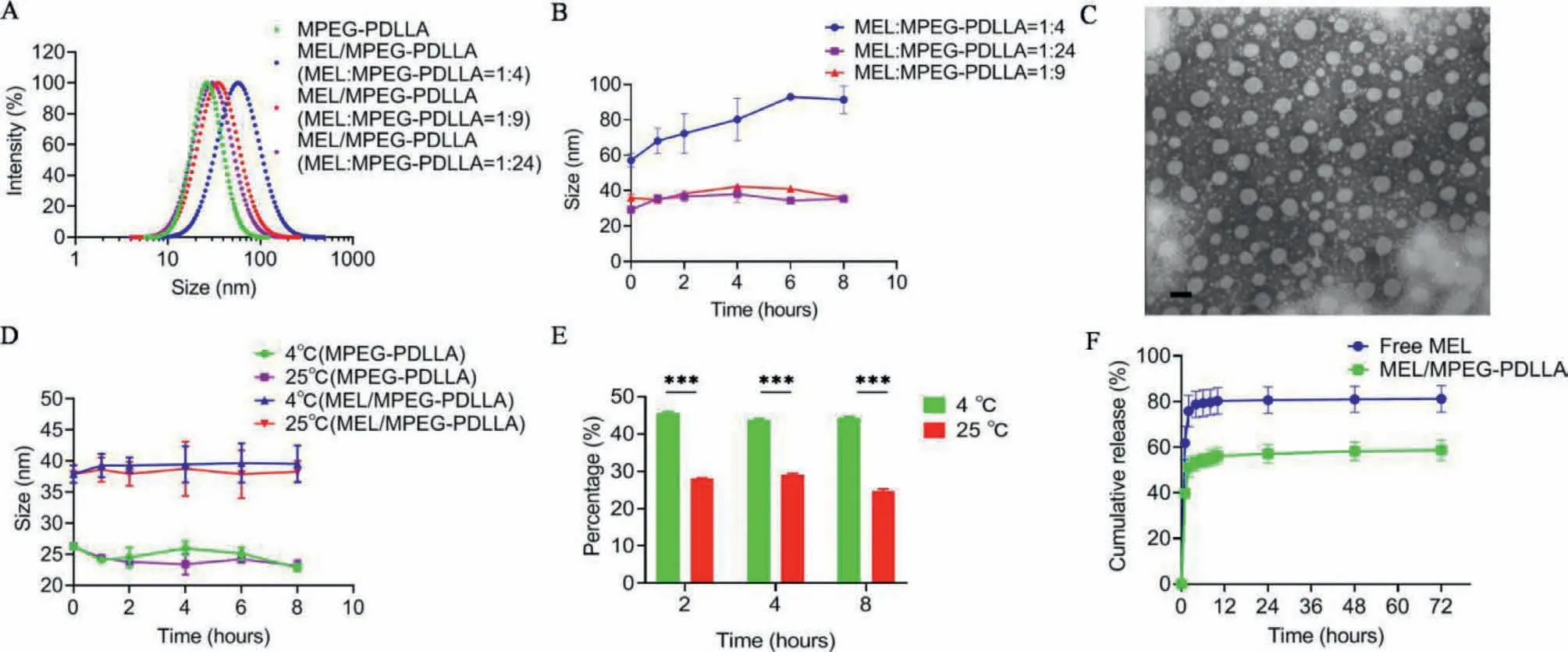
Fig.1.Characteristics of MEL/MPEG-PDLLA nanomicelles.(A) Size distribution of MPEG-PDLLA and melphalan at different ratios; (B) Stability of MEL/MPEG-PDLLA with different ratios of melphalan: MPEG-PDLLA at different times; (C) Morphology of MEL/MPEG-PDLLA nanomicelles; Scale bar: 100 nm; (D) Size of MPEG-PDLLA and MEL/MPEGPDLLA nanomicelles at 4 °C and 25 °C; (E) Melphalan retained inside MPEG-PDLLA nanomocelles at 4 °C and 25 °C at different times; (F) Release behavior of MEL/MPEGPDLLA and free melphalan in vitro (∗∗∗P < 0.001).
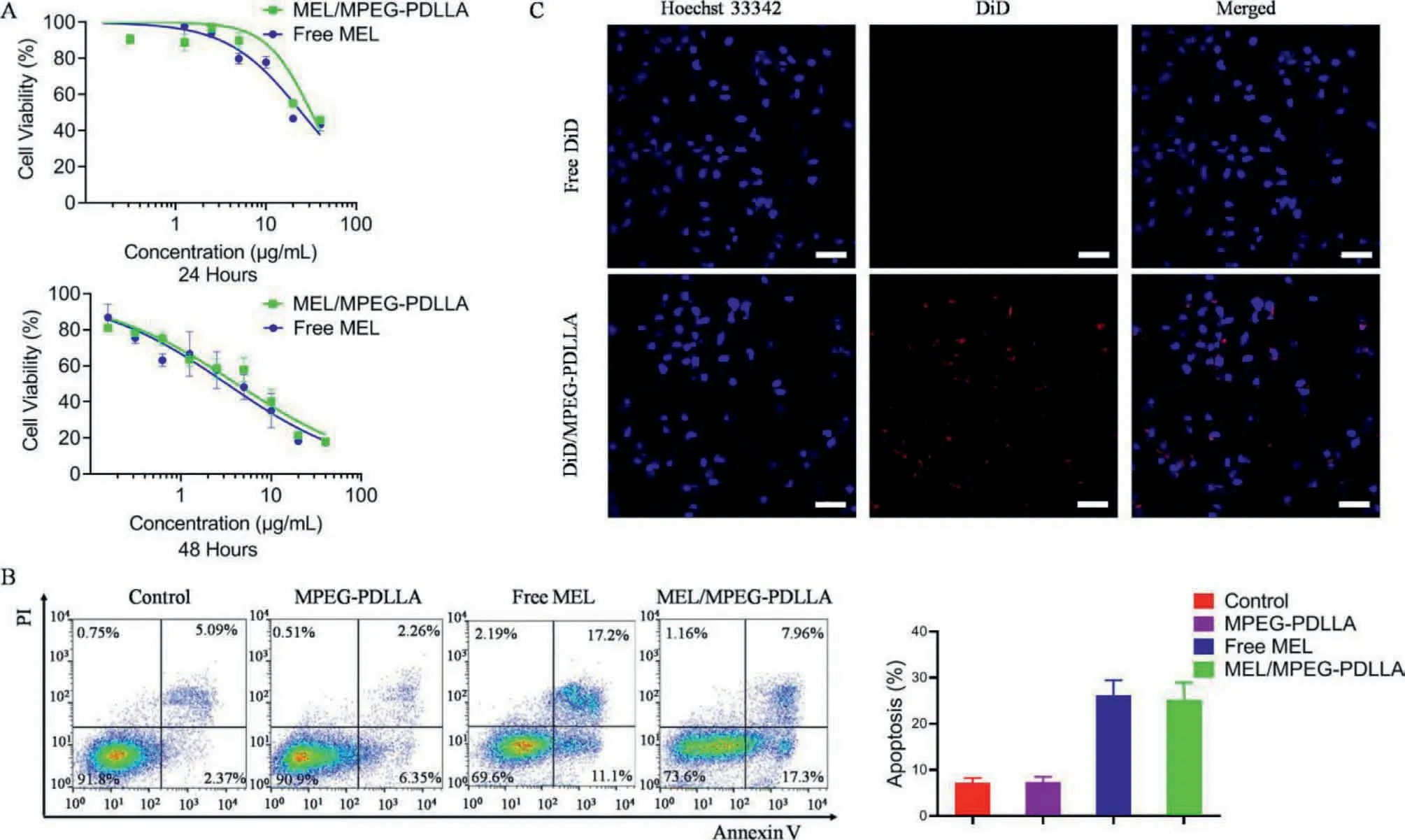
Fig.2.(A) Cytotoxicity in vitro of MEL/MPEG-PDLLA and free melphalan; (B) Apoptosis of ARD cells after addition of MPEG-PDLLA, MEL/MPEG-PDLLA, and free melphalan at 10 μg/mL after 24 h; (C) Confocal images of intracellular DiD/MPEG-PDLLA; Scale bar: 50 μm.
DiD and DiO were fluorescent label and used to imitate melphalan as a probe for exploring the transmembrane ability of the nanomicelles.Using the same method described above, DiD or DiO was encapsulated in nanomicelles as DiD/MPEG-PDLLA or DiO/MPEG-PDLLA.ARD cells were seeded in a confocal dish and incubated with free DiD and DiD/MPEG-PDLLA for 5 h, then washed with PBS, fixed with 4% paraformaldehyde, stained with Hoechst 33342 and washed again.Finally, confocal images were captured using a confocal microscope.The result showed that DiD/MPEGPDLLA was more easily accessible to ARD cells (Fig.2C).In addition, ARD cells were incubated with free DiO and DiO/MPEGPDLLA in a 6-well plate for 5 h.Then cells were collected and detected using flow cytometry.The results showed that the fluorescence intensity in the cytoplasm was higher and more abundant in the DiO/MPEG-PDLLA group than those in the control and free DiO groups, which implied that DiO/MPEG-PDLLA was more readily absorbed by ARD cells than free DiO (Fig.S1 in Supporting information).This finding suggested that MPEG-PDLLA could promote melphalan absorption and internalization by ARD cells and was suitable for melphalan transported into cells.

Fig.3.(A) Schematic illustration of the experiment in vivo; (B) IVIS images of mice treated with different treatments; (C) Survival curve of mice with different treatments; (D)Flow cytometry of CD138 positive cells in the bone marrow.(E) HE staining of femurs after different treatments; Scale bar: 100 μm; (F) Plasma concentration of MEL/MPEGPDLLA and free MEL in rats within 24 h (∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001).
Considering the complexity of thein vivoenvironment, we conductedin vivoexperiments in mice to assess the antitumor effect of MEL/MPEG-PDLLA and free melphalan.As showed in Fig.3A, a model of B-NDG mice bearing MM was established as follows: 1×106ARD-luciferase cells were injected into mice intravenously, and the mice were imagedviaanin vivoimage system (IVIS) to monitor the tumor burden.When mice were inoculated with MM successfully, they were randomly divided into four groups and treated with PBS, MPEG-PDLLA, free melphalan,or MEL/MPEG-PDLLA every four days three times at a melphalan dose of 5 mg/kg.As shown in Fig.3B, the bioluminescence intensity of mice treated with PBS and MPEG-PDLLA increased rapidly, while the bioluminescence intensity of those mice treated with free melphalan and MEL/MPEG-PDLLA increased very slowly,especially the mice treated with MEL/MPEG-PDLLA.This finding showed that MPEG-PDLLA itself almost has no antitumor effect,and MEL/MPEG-PDLLA significantly inhibited MM progression better than free melphalan, which indicated that MEL/MPEG-PDLLA had the advantage of tumor-killing effectin vivo.In addition, a quantitative analysis of the bioluminescence intensity of these four groups was performed to demonstrate these results.
In addition, we found that all mice from the PBS and MPEGPDLLA groups were dead until days 34 and 36, respectively, which suggested that there was no difference between the two groups(Fig.3C).Additionally, the survival of mice from the free melphalan and MEL/MPEG-PDLLA groups was 40 and 46 days, longer than that of the other two groups (P< 0.01).Between the free melphalan and MEL/MPEG-PDLLA groups, the latter presented a significantly longer survival of mice (P< 0.05).

Fig.4.(A) HE staining of vital organs; Scale bar: 50 μm; (B) The weight change of mice with different treatments; (C) The mRNA expression of Occludin after different treatments; (D) The fluorescence intensity of the colon of mice receiving different treatments; Scale bar: 100 μm (∗∗P < 0.01; ∗∗∗P < 0.001).
To further confirm the efficacy of MEL/MPEG-PDLLA, we continued to detect CD138 positive cells from bone marrow using flow cytometry.As shown in Fig.3D, the CD138 positive cells from the mice treated with MEL/MPEG-PDLLA were fewer than those of mice treated with free melphalan.However, there was no significant difference between them.Moreover, HE staining was performed on the femur.The results showed that the MM cells almost filled the bone marrow cavity in the control and MPEGPDLLA groups while fewer MM cells infiltrated the bone marrow in the free melphalan and MEL/MPEG-PDLLA groups.Especially in the MEL/MPEG-PDLLA group, only scattered MM cells were observed (Fig.3E).Although extramedullary infiltration of myeloma cells was observed in the late stage, MEL/MPEG-PDLLA helped MM mice significantly prolong the survival time.The results suggested that MEL/MPEG-PDLLA enhanced the antitumor effect compared to free melphalan to some extent.
To evaluate the pharmacodynamics of the preparation, a pharmacokinetic study was performed in female SD rats.All rats were randomly divided into two groups.Each group was administrated intravenously with free melphalan or MEL/MPEG-PDLLA at a melphalan dose of 10 mg/kg.The blood concentration of melphalan was illustrated in Fig.3F.The mean peak level of MEL/MPEG-PDLLA was 14,973.5±1729.1 μg/L while the mean peak level of free MEL was 6719.3±1537.0 μg/L (P=0.003).The plasma concentrations of MEL/MPEG-PDLLA and free MEL decreased rapidly.Thein vivoclearance of MEL/MPEG-PDLLA was significantly lower than that of free melphalan (0.26±0.02 L h-1kg-1vs.0.53±0.09 L h-1kg-1,P< 0.01).Thus, the plasma concentration of MEL/MPEG-PDLLA was significantly higher than that of free melphalan at the same dose.The AUCs for MEL/MPEG-PDLLA and free melphalan were 28,849.38±835.15 μg h/L and 14,387.59±2480.43 μg h/L, respectively (P< 0.001).The half-life was 0.65±0.05 hvs.0.72±0.07 h in the MEL/MPEG-PDLLA and free melphalan (Fig.S2 in Supporting information).The improved AUC and minimized CL may be attributed to the controlled release capacity of nanomicelles.All animal experiments were approved by the Institutional Animal Care and Treatment Committee of Sichuan University (Chengdu, China)
In addition, hearts, livers, lungs, kidneys, and colons were dissected for hematoxylin and eosin (HE) staining to detect toxicityin vivo.As shown in Fig.4A, no pathological changes caused by the nanomicelles were observed in the heart, liver, lung, kidney,or colon among the four groups.The weights of the mice did not change much throughout the observation period, and no significant difference was observed among the four groups (Fig.4B).Interestingly, in the colon, more pronounced mucosal detachment was observed in the free melphalan group than in the MEL/MPEG-PDLLA group.Subsequently, Ribonucleic acid (RNA) was extracted from colon tissues, and quantitative real-time polymerase chain reaction(qPCR) was performed to assess the mRNA expression ofOccludin,the major component of tight junctions, which has a protective effect on the intestinal mucosa.The results showed that the mRNA expression was significantly higher in the MEL/MPEG-PDLLA group than in the free melphalan group (Fig.4C).Furthermore, the immunofluorescence results showed that the fluorescence intensity of the MEL/MPEG-PDLLA group was higher than that of the free melphalan group (Fig.4D).These findings indicated that compared to free melphalan, MEL/MPEG-PDLLA presented less toxicity to the colon.
In conclusion, the melphalan was encapsulated in MPEG-PDLLA and successfully made into MEL/MPEG-PDLLAviathin film hydration method.The MEL/MPEG-PDLLA nanomicelles showed high encapsulation efficiency and good sustained-release capability, and was taken up easily by MM cells.MEL/MPEG-PDLLA nanomicelles presented a better anti-tumor effect and less toxicity than free melphalan in the mouse model of MM.Especially for patients who had to receive HD melphalan or underwent ASCT, MEL/MPEGPDLLA nanomicelles were expected to reduce the shedding of the intestinal mucosa and relieve the pain from severe diarrhea and bloody stools.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos.U21A20417, 31930067, 32271450, 31700868),PostDoc Research Project, West China Hospital, Sichuan University(No.2020HXBH165), and 1·3·5 Project for Disciplines of Excellence,West China Hospital, Sichuan University (No.ZYGD18002).
 Chinese Chemical Letters2023年9期
Chinese Chemical Letters2023年9期
- Chinese Chemical Letters的其它文章
- Nanoplatform-based cellular reactive oxygen species regulation for enhanced oncotherapy and tumor resistance alleviation
- Recent advances in the synthesis and applications of furocoumarin derivatives
- High-performance and multifunctional organic field-effect transistors
- MOFs-based functional materials for aqueous micro/nanoplastics elimination
- NHC-gold(I)-alkyne complexes induced hepatocellular carcinoma cell death through bioorthogonal activation by palladium complex in living system
- New insight into the synergy of nitrogen-related sites on biochar surface for sulfamethoxazole adsorption from water
