Development of biochar electrode materials for capacitive deionization: preparation, performance, regeneration and other challenges
ZENG Zhi-hong, YAN Li-li*, LI Guang-hui, RAO Pin-hua, SUN Yi-ran, ZHAO Zhen-yi
(Shanghai University of Engineering Science, Shanghai 201620, China)
Abstract: Capacitive deionization (CDI) is a potential cost-efficient desalination technology. Its performance is intrinsically limited by the structure and properties of the electrode materials. Biomass materials have become a research hotspot for CDI electrode materials because of their abundance, low cost, and unique structure. The preparation, desalination performance, and regeneration status of biochar electrodes are summarized and clarified. Their preparation and use in CDI in recent years are presented and compared, and the effects of biochar electrode materials and CDI operating parameters on the desalination performance are emphasized.It is found that the salt adsorption capacity is positively correlated with the percent mesoporous material they contain. The selective adsorption of ions mainly depends on ion properties like ionic radius and charge as well as voltage, charging time and feed water characteristics. The current status and methods of electrode regeneration are discussed and future developments are suggested.
Key words: Biomass;Electrode material;Capacitive deionization;Regeneration
1 Introduction
Water pollution and water resource availability have become major challenges for global sustainable development. The development of methods to obtain clean water is an urgent requirement owing to population growth, industrialization, and climate change.Therefore, research and development on water treatment and seawater desalination technologies are key to solving the problem of water resources, which can effectively advance socioeconomic and green development[1].
To date, many desalination techniques, including reverse osmosis[2-3], electrodialysis[4-5], multistage flash[6-7], and multi-effect distillation[8-9], have been developed. However, their application are limited by factors such as high energy consumption and production of secondary chemicals or waste. Because of the relatively high applied voltage, electrodialysis is prone to redox reactions during operation, which accelerates electrode corrosion. In addition, multistage flash and multi-effect distillation are costly, require more energy, and experience equipment consumption abrasion[10-12]. Therefore, it is crucial to develop a relatively low-energy consumption and high-efficiency technology[13]. Capacitive deionization (CDI) is an alternative desalination and purification technology based on the electrical double layers (EDLs) capacitor theory, which mainly includes 2 stages: adsorption and desorption. During the adsorption process, ions in the solution accumulate on the surface and inside of the electrode form EDLs, achieving ion removal.When the electrode is saturated, ions are released from the EDLs through a short or reverse circuit to complete electrode regeneration[14].
In recent years, several novel CDI techniques have been developed (Fig. 1). To prevent the intrinsic“co-ion effect” inside electrodes, a novel and highly efficient membrane CDI (MCDI) system with an ionexchange membrane (IEM) is gaining interest[15]. On one hand, co-ions can be trapped in intraparticle pores, increasing the accumulation of counter-ions in macropores and leading to a higher salt adsorption capacity (SAC). On the other hand, IEMs can prevent the desorbed ions from being re-adsorbed to the counter electrode and can effectively alleviate Faradaic reactions, enhancing electrode regeneration efficiency and lifetime[16-17]. Despite the abovementioned benefits of IEMs, the presence of membranes increases the internal resistance of the system to electrosorption, and contamination issues also need to be addressed[18].
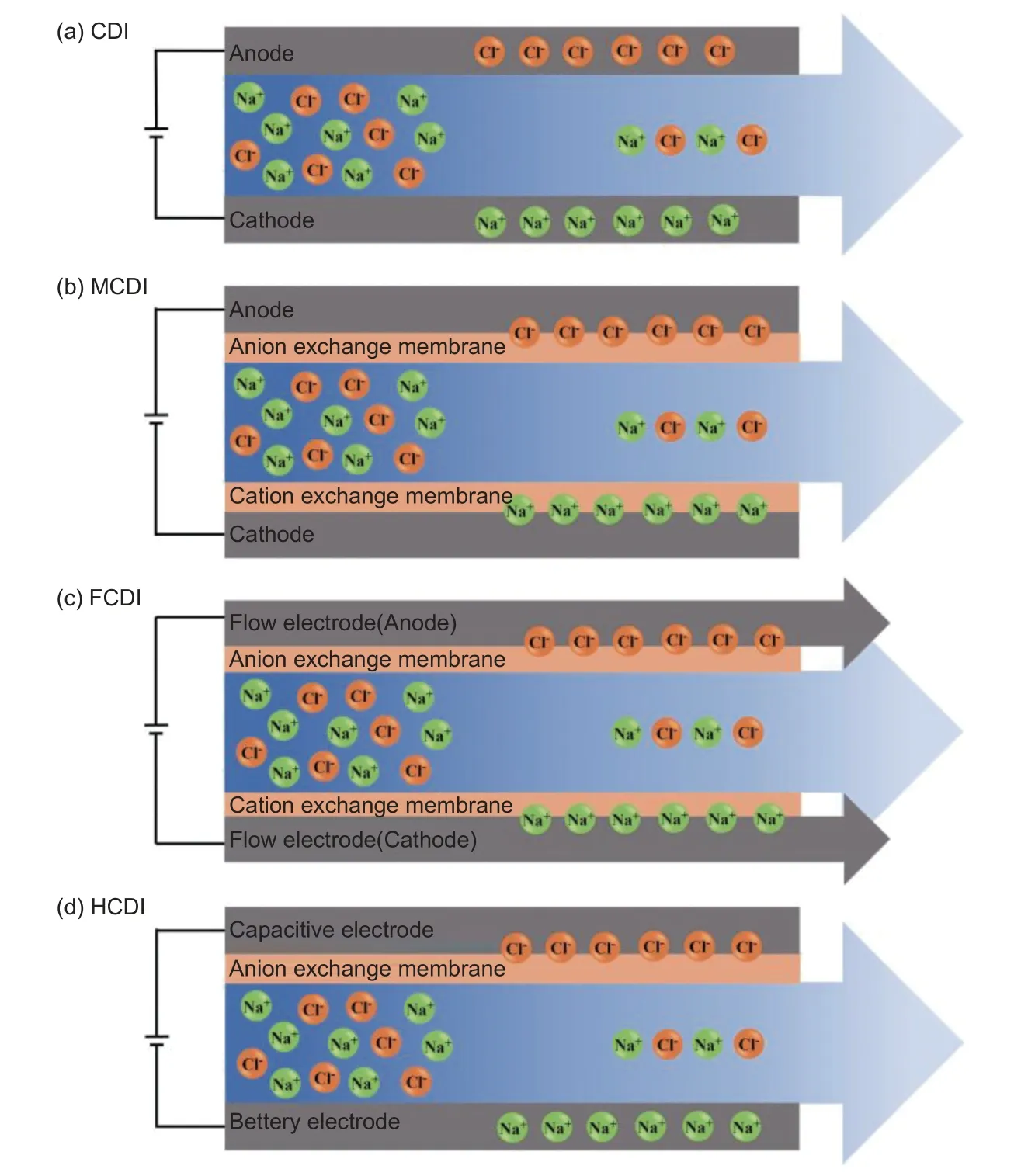
Fig. 1 Architecture diagrams and removal mechanisms of (a) capacitive deionization (CDI), (b) membrane capacitive deionization (MCDI), (c) flow electrode capacitive deionization (FCDI), and (d) hybrid capacitive deionization (HCDI)[21]. (Reprinted with permission)
Flow electrode CDI (FCDI), a new technique based on ion-exchange membranes and flowing electrodes, is developed to address the limitations of MCDI. Unlike conventional fixed electrodes, FCDI uses slurry-type electrodes, which typically contain small particles of activated carbon with a high specific surface area, leading to a higher electrosorption capacity. In addition, flowable carbon electrodes adsorb charged species from the feed stream and continuously circulate and regenerate by desorption outside the electrode chamber, leading to continuous and energy-efficient operation[19-21]. Nevertheless, the activated carbon particles show poor electronic conductivity in the suspended state, and the high carbon content restricts the further development of activated carbon-based flow electrodes[22]. Hence, metal-organic frameworks (MOFs)[23]and intercalation materials[24]such as CuHCF have been developed as flow electrodes with high electronic conductivity and salt removal efficiency.
Furthermore, some unavoidable problems, including carbon oxidation and incomplete desorption,resulting a low SAC and degradation of the cycling properties during long-term desalination. Thus, a system with 2 Faradaic electrodes and a hybrid CDI(HCDI) system have been developed. HCDI combines 2 different electrode materials, one traditional CDI electrode like carbon electrode works based on EDL mechanism, and another electrode such as metal or metal oxide is fabricated by battery material which works through the faradaic reactions including the ion intercalation or electrochemical redox reactions[25-26].Li et al.[27]developed HCDI using a redox-active polyimide as the electrode material. It exhibited a high sodium uptake capacity of 54.2 mg g-1and superior electrochemical stability with a 31.9% retention after 100 charge/discharge cycles. Moreover, Chen et al.[28]found excellent cycling stability with only 4% desalination capacity degradation for over 100 cycles by combining MoS2nanoflakes with MXene as electrode material. Although HCDI shows excellent performance, the imbalanced ion storage capacity and instability of the Faraday material structure require more indepth investigation[29].
Notably, electrode materials play a critical role in CDI. Carbon materials, including biochar, carbon nanotubes, carbon nanofibers, carbon aerogels, graphene,and their modified materials, are common electrode materials[30]. Although carbon nanotubes, carbon nanofibers, carbon aerogels, and graphene have excellent electrosorption performance, their synthesis procedures are more complex, expensive, and limited by non-uniform particle sizes and external porous structures[31]. Biochar is an economical, environmentally friendly carbon material obtained through the pyrolysis of biomass in an oxygen-limited environment,which can stretch back to 2 000 years ago of its application in agriculture[32-33]. The ‘dark earth of the Indians’ (Amazonian Dark Earths) are composed of variable quantities of highly stable organic black carbon waste, providing evidence of the extensive of biochar[33-34]. The high agronomic fertility of these sites has attracted interest and led to in-depth research on biochar whose chemical structure contains highly distorted aromatic rings irregularly stacked with branched functional groups on the surface and rich pore structure[32,35].
Commonly, the main components of biochar include carbon, hydrogen, sulfur, oxygen, nitrogen and the trace elements such as potassium, calcium, and magnesium[36]. Due to biochar’s physical and chemical properties, it has been widely used in many fields.Previous researches have proven that biochar can improve soil quality and crop yield, reduce greenhouse gas emissions from soil, and has a strong affinity to inorganic ions such as heavy metal ions, phosphate,and nitrate[37-39]. Notably, with the large specific surface area and well-regulated structure, biochar can also be prepared as an electrode. This strategy not only solves the environmental problem due to random biomass accumulation but also improves the economic value and application potential of biomass resources[40-41]. The unique advantages of biochar make it a promising resource for CDI, which has applications in the removal of heavy metals from wastewater[35,42-43], water softening[44], brackish water treatment[45], and seawater desalination[46-48].
However, the effects of biochar electrodes on CDI performance and stability remain to be summarized and elucidated. In this review, the preparation methods and properties of biochar have been summaried. The specific objectives of this review were (1) to expound on the influence of the biochar electrodes and CDI operating parameters, (2) to evaluate the selective adsorption of ions and illustrate the selection mechanism and influencing factors, (3) to discuss the current state of electrode regeneration, (4) to show the current direction of research on biochar electrodes and provide a reference for their practical application in CDI.
2 Preparation of biochar
With the increasing demand for carbon, biomassderived carbon materials have attracted wide attention owing to their low cost, broad raw material resources, and environmental friendliness. Biomass materials, such as rice husks[41], sugarcane bagasse[44],coconut shells[49], peanut shells[50], and date seeds[51]have been used to prepare biochar electrodes with excellent performance. The performance depends heavily on the preparation method. Common preparation methods include pyrolysis, hydrothermal carbonization, and template method.
2.1 Pyrolysis
Pyrolysis is the process of thermal decomposition of biomass below 1 000 °C in an inert atmosphere, during this process, biomolecules such as cellulose and lignin are gradually broken down into smaller molecules with the temperature rises, and biomass converts into biochar[52]. Pyrolysis temperature and time play crucial roles in the performance of biochar. Wang et al.[53]prepared the cattle bone biochar with a 2-step carbonization method, which was pre-carbonized at 450 °C for 3 h under N2atmosphere, then the pre-carbonized product and KHCO3were mixed and heated to a target temperature kept for 2 h (Fig. 2a). It’s found that the degree of the graphitic structure was constantly enhanced when the pyrolysis temperature increased from 600 to 900 °C,which could improve the electrical conductivity of biochar. Similarly, Du et al.[54]reported that the graphitic structure of red oak-derived biochar was more orderly, which favored the mass transfer process at a pyrolysis temperature of 1 000 °C and a time of 3 h.
Furthermore, biochar can be pre-oxidized by oxidants to improve its performance. Yan et al.[55]oxidized basswood in O2atmosphere and then transferred it to N2atmosphere at a temperature and time of 1 000°C for 2 h. The results showed that the pseudocapacitance and hydrophilicity improved after pre-oxidation(Fig. 2b). Jin et al.[56]increased the biochar yield and N content by pre-oxidation, enhancing the pore volume of biochar and its adsorption capacity by up to 437.8 mg g-1for toluene. Moreover, Li et al.[57]pretreated the biochar with 25% HNO3and doped its surface with more N/O active sites, in which the adsorption capacity for Hg (II) reached 153 mg g-1.
Typically, activation is an effective strategy for improving the properties of biochar. Common activation methods include thermal, physical and chemical activation, and their combinations[58]. Compared with physical activation using N2or CO2atmosphere,chemical activation usually requires an activating agent such as KOH, KHCO3, HNO3, H3PO4or ZnCl2.Commonly, the added activator reacts with biochar by removing atoms from the carbon framework which can increase the defects as the active and adsorption sites, enrich the pore structure, and introduce oxygencontaining groups to increase the wettability of the materials, leading an excellent desalination performance[59]. For example, Hai et al.[51]found that KOH-activated biochar performed better than ZnCl2and H3PO4. The specific surface area and specific capacitance of KOH-activated biochar were 1 020.85 m2g-1and 400 F g-1, respectively. Lado et al.[44]used sugarcane bagasse as a carbon source. They observed that physicochemical activation significantly increased the porosity of sugarcane bagasse biochar; its specific surface area ranged from 240 to 1 633 m2g-1, and its hydrophilicity improved after KOH activation.
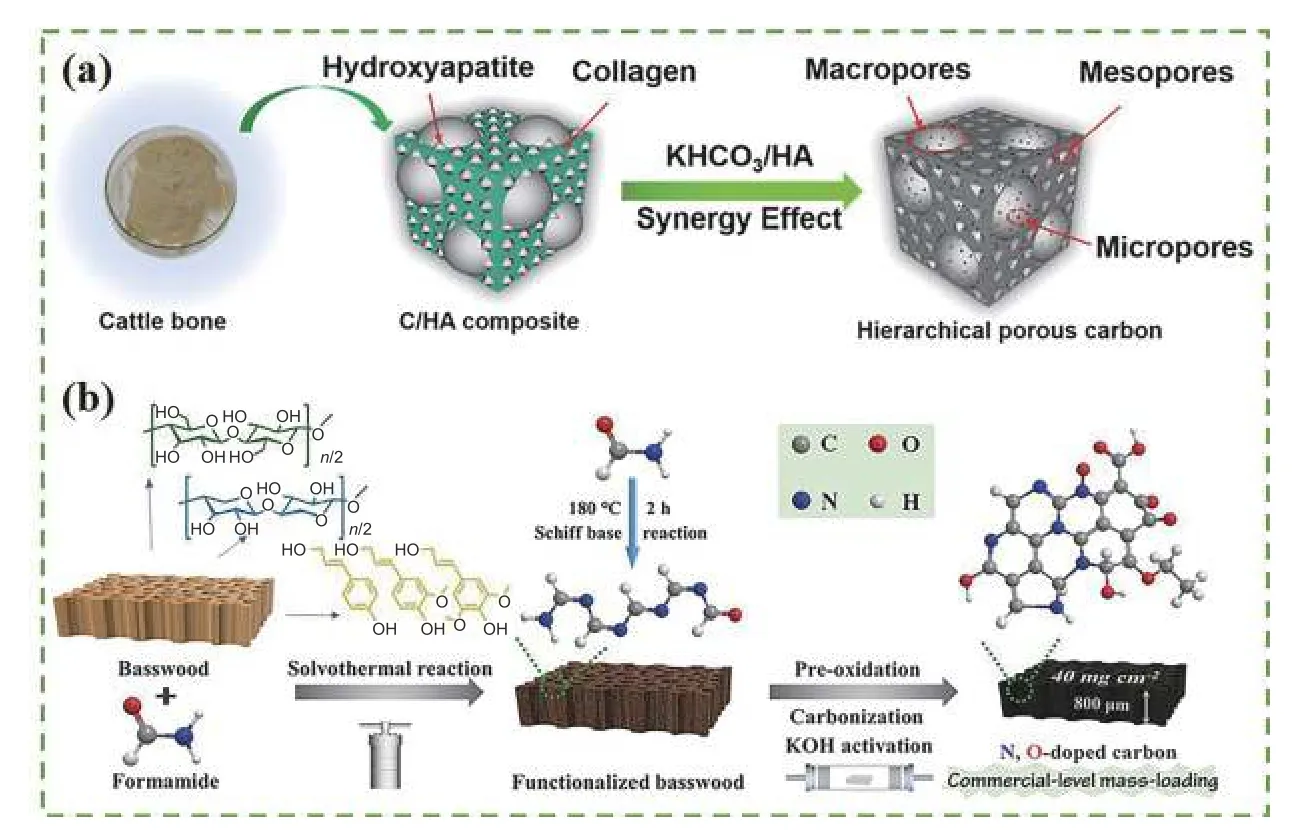
Fig. 2 Illustration of the synthetic procedure using pyrolysis method of (a) cattle bone porous carbon[53], (b) wood-derived biochar[55].(Reprinted with permission)
The weight ratio of biochar to the activator is also an essential factor that determines the performance of the biochar. Shi et al.[60]prepared micro/mesoporous carbon spheres using sucrose as a precursor and NaOH/KOH as an activating agent. When the Na-OH/KOH ratio was 2∶1 and the mixed activating agent/carbon sphere ratio was 3∶1, the specific capacitance of the biochar electrode reached 235 F g-1.During the activation process, NaOH increased the volume of the mesopores, while KOH increased the volume of the micropores. Micropores were beneficial in increasing the specific surface area. However,they negatively affected mass transfer. Therefore, the optimization of biochar performance is closely related to the pore structure of the materials and the pyrolysis method.
2.2 Hydrothermal carbonization
The hydrothermal carbonization method converts biomass in an aqueous solution into biochar at a pressure (2-10 MPa) which comes from self-generated pressures in aqueous media of the reactor and a low reaction temperature (180-280 °C). The biomass raw materials are hydrolyzed into monomers through dehydration and polymerization, and then through aromatization reaction obtain the product[61-62]. Adorna et al.[49]dispersed coconut shells in an HNO3solution to produce coconut shell biochar (AB). Compared with AB, AB-MnO2composite prepared by direct precipitation was more mesoporous, with aVmeso/Vtotalup to 66.1% and better hydrophilicity,which was conducive to electric adsorption desalting.Chen et al.[63]prepared porous carbonviamixing the citric acid and bamboo powder in a stainless-steel autoclave and heated to 180 °C for 6 h. They found that the addition of citric acid improved the degree of carbonization, oxygen content, and the specific surface area through physical characterization. When the concentration of citric acid was 0.69 mol L-1, the biochar had a specific surface area of 3 132 m2g-1and a specific capacitance of 435.5 F g-1(Fig. 3a). With a higher temperature of 200 °C, a hydrothermal time of 24 h, compared with 12 h, resulted in a larger specific surface area of 1 138 m2g-1and a total volume of 0.70 cm3g-1[64]. Therefore, hydrothermal temperature, time,and solvent significantly affect the biochar properties.
Notably, in addition to temperature and time, the activator also influences the structure and performance of biochar. Hu et al.[65]obtained N self-doped porous carbon obtained by hydrothermal carbonization with the 30 g of penicillin fermentation residue and 150 mL of deionized water added into the reactor.Argon was blown into the reactor for 10 min to drive off the residual air inside the vessel. Then it was heated to the reaction temperature and maintained for 2 h (Fig. 3b). Furthermore, the hydrothermal carbon was activated with ZnCl2or KOH as an activator.Compared with KOH, the porous carbon prepared with ZnCl2as an activator had a higher N content of 4.75%. Benefiting from the high N groups, the electrode showed a specific capacitance of 209.2 F g-1at 1.0 A g-1in the 3-electrode system, which displayed excellent electrochemical performance.
2.3 Template method
The basic principle of template method is firstly to fill the pores of the template material with biomass as the carbon source, while the template can build cross-linked porous structures in the carbonization process and the template was removed finally[58].Template method includes the hard template method,which uses mesoporous silicon, zeolite, and MOFs as a template, and the soft template method, which uses surfactant, polymer and biopolymer as a common template[66]. The template method (Fig. 4a) not only provides some active sites and can enrich the pore structure but also improves the hydrophilicity, conductivity, and cyclic stability of the biochar[67]. Wang et al. designed an N-doped porous carbon (NPC) by combining polyacrylonitrile with nanometer-sized SiO2and ZnCl2[68]. The adsorption isotherm was a combination of type IV and type I isotherms, indicating numerous micropore and mesopore structures.Moreover, Shi et al.[69]proposed an innovative strategy to prepare hierarchical porous biocharviaone-step co-pyrolysis of waste rice straw and polyvinyl chloride with CaCO3as a template (Fig. 4b).The mixture was ball milled for 1 h in cylindrical tablets with 20 MPa to eliminate gaps between mixtures.Afterwards, the mixture was pre-carbonized at 400 °C for 1 h in N2atmosphere, and then carbonized at 800°C for 4 h. During the pore formation process, the construction of abundant mesoporous spaces was promoted with the assistance of template-casting role of CaCO3. Moreover, the CO2released by CaCO3decomposition can further create a large number of pinholes inside the material, leading to the total pore volume reaching 0.639 7 m3g-1.
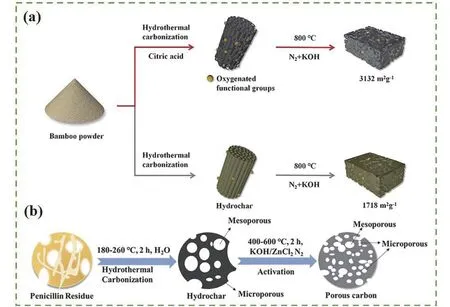
Fig. 3 Illustration of the synthetic procedure using hydrothermal carbonization of (a) bamboo-based porous carbon[63], (b) penicillin fermentation residue derived porous biochar[65]. (Reprinted with permission)
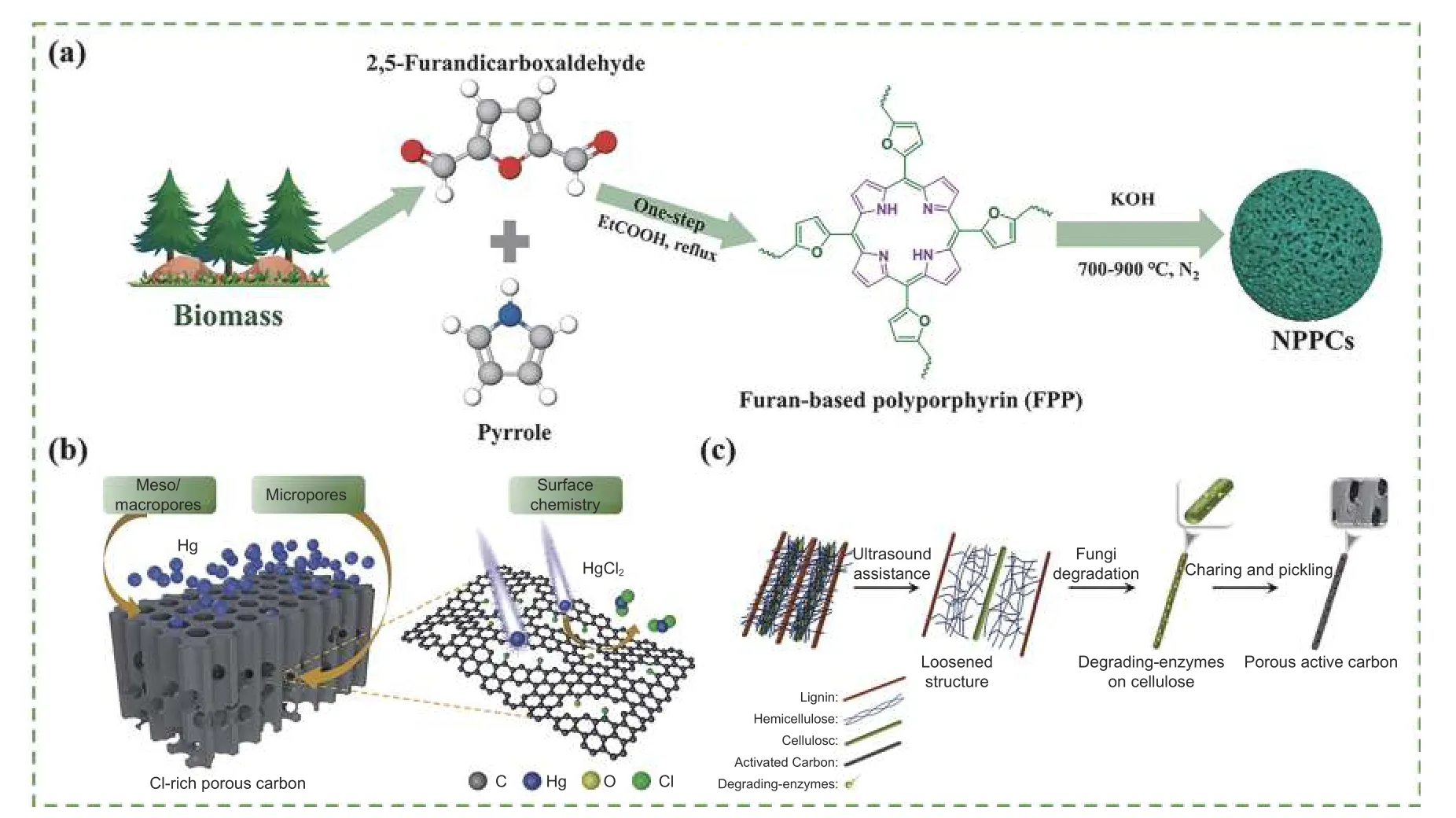
Fig. 4 Illustration of the synthetic procedure using template method of (a) nitrogen-doped polyporphyrin porous carbon[67], (b) Cl-rich porous carbon[69],(c) preparation of the biomass-based hierarchical porous carbon by microwave synergistic[73]. (Reprinted with permission)
In addition to external N-source doping, N-rich biomass can also be used as a carbon and N source to prepare porous carbon. For example, Zhao et al.[70]created an NPC derived from soybean shells with an N content of 1.66%. NPC was further functionalized with sulfonic acid groups to obtain S-NPCs. The desalination rate of S-NPC was 0.89 mg g-1min-1,which was larger than that of NPC (0.56 mg g-1min-1).This was attributed to the functionalization of the sulfonic acid group, which improved the adsorption efficiency of salt ions.
2.4 Other methods
Novel preparation methods have been developed to improve the performance of biochar electrodes.Tang et al.[71]synthesized hierarchical porous biochar by coupling microwave and hydrothermal carbonization with sugarcane bagasse as a precursor. In the carbonization process, 6 g of sugarcane bagasse and 60 mL of deionized water were added into a Teflonsealed reactor with the assistance of a microwave system under 2.45 GHz irradiation at 180 °C for 0.5-1.5 h. Microwave-assisted hydrothermal carbonization for 1.5 h increased the carbon content of samples to 49.8%, which was close to the carbon content obtained after conventional hydrothermal carbonization for 3 h, thus microwave-assisted carbonization reduced the reaction time. Huang et al.[72]used microwave pyrolysis to prepare a N/P double-doped graded porous biochar with an excellent specific surface area and specific capacitance of 1 367.6 m2g-1and 531 F g-1, respectively. The precise inside-out heating characteristics of microwaves prompted suitable mesopore-micropore distribution ratios in carbon, providing abundant active sites for charge accumulation and ion diffusion while improving the electrical conductivity, wettability, and stability with the doping of N/P atoms. Wu et al.[73]prepared lignocellulose biochar using ultrasound-fungi pretreatment combined with a carbonization route (Fig. 4c). Their synergistic effect improved the rigid structure and increased the porosity of the material, providing a better place for ion transport. The ultrasound-fungi assistance resulted in a high specific surface area and adsorption capacity of 1 234 m2g-1and 29.39 mg g-1, respectively. Genovese et al.[74]prepared a low-cost biochar using a novel synthesis strategy involving biomass pre-treatment, N pyrolysis, and high-temperature thermal-chemical flash exfoliation. The biochar electrodes showed a high specific capacitance of 221 F g-1while maintaining a 97% capacitance after 5 000 successive potential cycles, demonstrating their longterm stability. Therefore, the preparation of biochar coupled with other technologies has a promising outlook.
In conclusion, pyrolysis is a simple process with a high biochar yield. However, it requires high temperatures and energy consumption, and the porous structure of biochar mainly depends on the activation process. Hydrothermal carbonization is attractive because it uses low temperatures and is rich in functional groups but has low porosity and conductivity[75].Template method can effectively regulate the pore size structure of the prepared biochar and its distribution, leading a uniform structure. The wettability and mass transfer ability of biochar can also be enhanced by heteroatomic doping. This method is much more complicated and requires physical or chemical methods to remove the template, which causes environmental pollution. Therefore, an appropriate method can be chosen to obtain the best performance of the biochar material, according to the actual situation and application requirements. In addition, novel, low-pollution, and energy-consuming methods for preparing biochar need to be further studied.
3 CDI performance using biochar electrode materials
3.1 Effect of biomass used in biochar electrodes on CDI performance
Biochar, as the electrode material for CDI, plays a decisive role in the CDI system. Its structure and properties directly determine the removal of ions through electrosorption. Table 1 summarizes the properties of the pore volume, specific surface area, specific capacitance, and SAC of the biochar electrodes obtained from different biomass. Cattle bone carbon showed a good mesoporous proportion of 72.83% owing to the presence of hydroxyapatite. Its abundant pore structure promoted the diffusion and transfer of ions[53]. Owing to the different biomass used for preparing biochar, the specific surface area of biochar ranges from 303.59 to 3 557 m2g-1, but the specific capacitance of individual biochar could reach 474 F g-1, resulting in different desalination effects. For example, pomelo peel biochar has a large specific surface area of 2 726 m2g-1and a specific capacitance of 207 F g-1, respectively. Its SAC is 20.78 mg g-1,which can be maintained after 10 cycles. In contrast,the specific surface area of sugarcane bagasse biochar is 1 019 m2g-1, but SAC can reach up to 28.9 mg g-1.Thus, the mechanisms affecting desalination performance are diverse and complex. Fig. 5a shows a strong positive correlation between desalination and mesopore ratio. The existence of mesopore can reduce the overlapping effect of the electric bilayer, providing a channel into the smaller pores for ions and increasing the mass transfer rate, whereas the existence of micropore provides a larger specific surface for biochar[52-53,76]. The specific surface area and total pore volume of the biochar also have a strong correlation(Fig. 5b). A higher total pore volume indicates a more porous structure and larger specific surface area, thus providing more adsorption sites for ions. Fig. 5c and 5d show plots of SAC and regeneration performance versus the specific surface area or specific capacitance, respectively. The specific surface area of two-thirds of the biochar ranged from 1 000 to 2 800 m2g-1and the specific capacitance, SAC, and regeneration time were maintained at 100-250 F g-1, 15-30 mg g-1, and 5-10 cycles, respectively.

Table 1 Properties of biochar electrodes prepared from different biomass
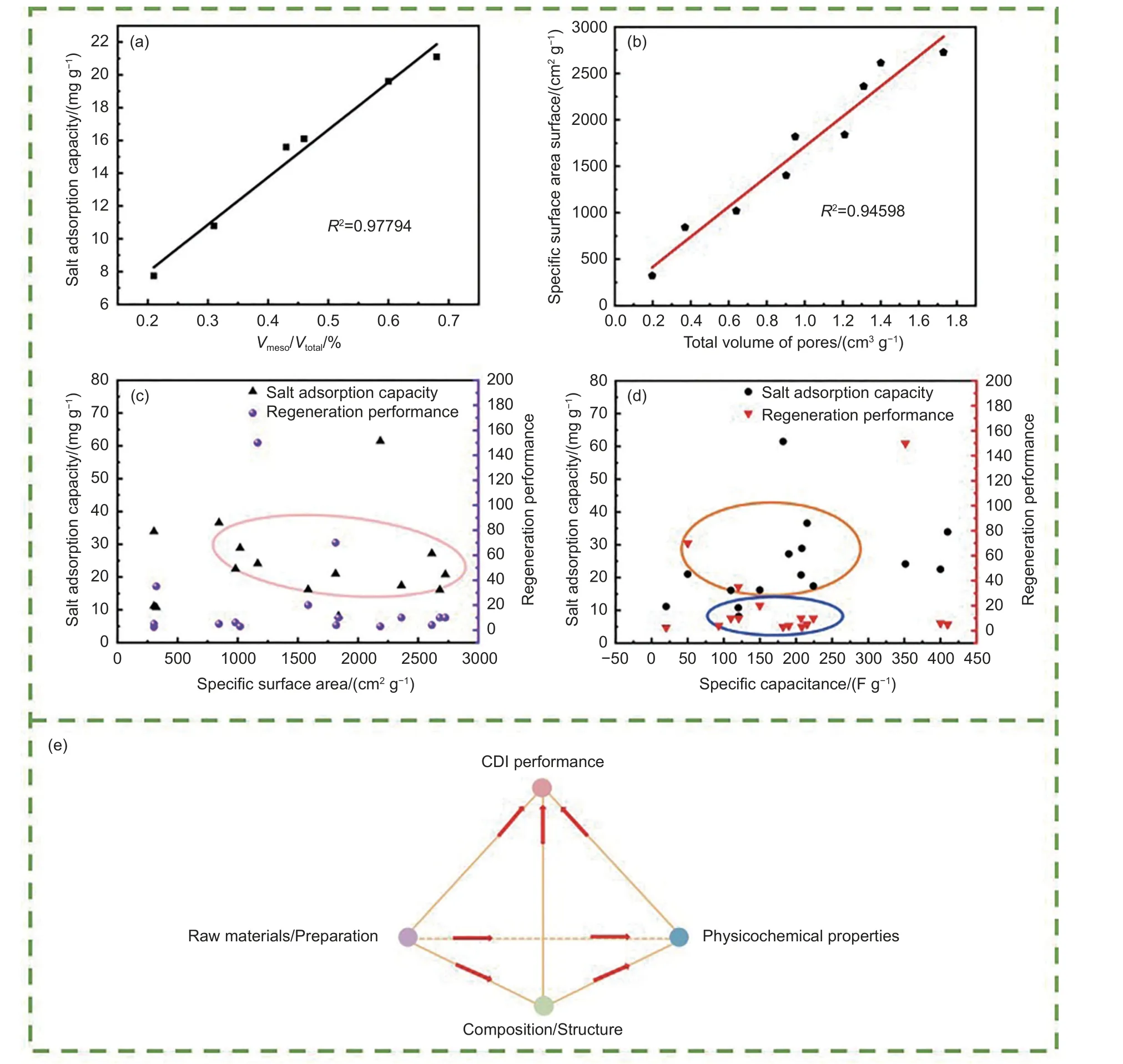
Fig. 5 Relationship between (a) SAC and the proportion of mesopore, (b) the specific surface area of biochar and total volume of pores,(c) SAC and the regeneration performance and specific surface area, (d) SAC and the regeneration performance and specific capacitance,(e) biochar-based electrode and CDI performance
Moreover, the functional groups on the surface will also influence the desalination performance. Adorna et al.[49]found that the specific surface area of the AB-MnO2electrodes was only 304 m2g-1, but their SAC reached 33.9 mg g-1, which was approximately 4 times that of the original biochar electrodes. This was attributed to the presence ofα-MnO2. On one hand, it increased the hydrophilicity of the biochar, accelerating the ion mass transfer rate, and on the other hand, it enhanced the specific capacitance of the biochar, improving the CDI performance. Moreover, heteroatomdoped porous carbons exhibited a higher specific surface area, good electrical conductivity, suitable pore size distribution, and excellent electrosorption performance. O-doping can produce porous carbon with a graded structure and improve its wettability, which is conducive to rapid migration[77-78]. Typically, the incorporation of N and S into pristine biochar considerably improves the hydrophilic energy owing to the increasing in hydrophilic groups. Zhao et al.[70]reported that pyridine N could improve the electrical activity of the material, graphite N could increase the graphitization of the biochar and improve its electrical conductivity, and pyrrole N could participate in the reversible Faraday reaction and provide additional pseudocapacitors according to the electron properties. Moreover,the sulfonic acid groups effectively inhibited the coion effect and provided additional absorptivity for the Na+. The specific capacitance of the modified electrode was 215.3 F g-1, and the SAC was as high as 43.3 mg g-1. Thus, the SAC of the biochar electrode is positively correlated with the mesoporous ratio of carbon but presents irregularity with the specific capacitance.
In conclusion, many factors influence the CDI performance of biochar electrode. Fig. 5e shows the relationship between biochar-based electrode and CDI performance. In order to efficiently improve desalination performance, it is important to regulate the composition and structure to increase the active sites, mass transfer rate, and hydrophilicity of the electrode materialsviaraw material selection and preparation process. Moreover, electrochemical properties such as electrical conductivity and charge storage capacity also need to be improved.
3.2 Effect of biochar electrode preparation on CDI performance
Electrode preparation is a complex process. In addition to basic biochar materials, binders and conductive agents are usually added to enhance the conductivity and bulkiness of electrodes, which are then mixed and coated onto a substrate. It is very important to select a suitable substrate with good conductivity and corrosion resistance, such as graphite paper, titanium mesh, stainless-steel mesh, and carbon cloth[93]. The coated electrode was then dried in an oven to obtain the biochar electrode.
Furthermore, the preparation method, electrode thickness, binder, conductive agent, and mixing ratio had a significant impact on the electrochemical performance. For example, Wang et al.[94]utilized rice straw to prepare electrodesviathe thermosetting and roller pressing methods. The results showed that the removal efficiencies of 2 different electrodes within 240 min were 46.21% and 45.08%, respectively, at a current density of 3.12 mA cm-1(Fig. 6a). On the other hand, the biochar electrode prepared by the thermosetting method could be formed independently at a low cost, while electrodes with good conductivity and mechanical strength could be produced using the roller-pressing method. Furthermore, the raw material ratio also affected the electrode. When the ratio was 10∶5∶2, the electrode molding was good and the electrode had good hardness and strength. Furthermore, when the mass was relatively small, electrode stability decreased, while too high mass ratio resulted in increased internal resistance and decreased conductivity[95]. Chang et al.[96]observed that the absolute current increased with the thickness of the biochar electrode owing to the larger activated area in thicker biochar. When the thickness was increased to 3.5 mm,the biochar electrode exhibited the highest power density of 72 mW m-2(Fig. 6b). In general, the thin electrode could facilitate the electrolyte penetration in the electrode, resulting a faster ion diffusion and better desalination capacity while the ions could not rapidly penetrate the entire electrode structure and the electronic resistance was increased in an overly thick CDI electrode, thus affecting the desalination performance[97-98]. Lang et al.[99]investigated a biochar electrode using graphene as the conductive agent. The electrode possessed many excellent properties, especially a high desalination capacity of 16.88 mg g-1.The conductive agent prepared with biochar also showed great potential. Hao et al.[100]and Kane et al.[101]prepared biomass-based carbon as conductive electrodes. Compared with conventional conductive agents, biochar showed the same electrochemical pseudocapacitance behavior and high electrical conductivity, which indicated fast electron and ion transport. The cell efficiency and rate capability were also slightly improved. However, further research is required for this novel design scheme.
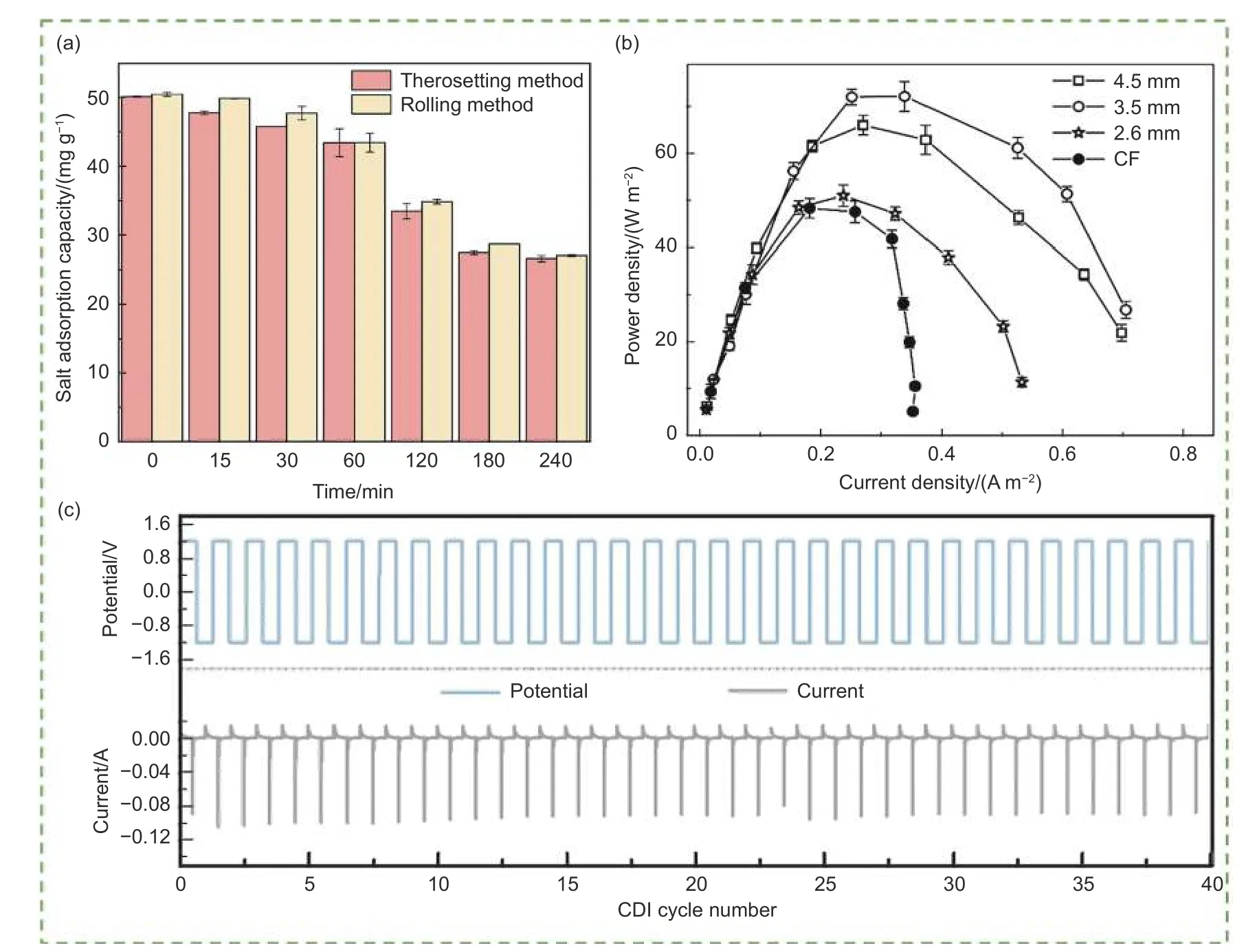
Fig. 6 (a) The removal effect of biochar electrode made via 2 methods on cadmium[94], (b) the curves of power density and electrode potentials polarization[96],(c) the potential and current of the MCDI during a 40 cycles desalination operation[104]. (Reprinted with permission)
Different binders affect electrode performance differently. Notably, conventional binders are harmful to the environment. Therefore, natural, environment-friendly, and novel binders are of high interest.Kiminaitė et al.[102]used flaxseed mucilage as a binder. With the assistance of flaxseed mucilage, the fabricated electrodes exhibited higher mechanical strength, conductivity, and thermal stability. Mian et al.[103]prepared a cellulose nanofiber (CNF) binder.Compared with the polyvinylidene fluoride polymer binders, the CNF binders retained biochar microporosity and improved wettability, leading to a significant reduction in the internal resistance of the electrode from 1.5 to 0.76 Ω. The effect of different ratios of biochar, binder, and conductive agent on the performance of the electrode was also explored. The results showed that the lowest internal resistance of 0.76 Ω was obtained for an 85∶5∶10 ratio. Furthermore,compared with the other ratios, this ratio exhibited a higher capacitance of 268.4 F g-1. Although some novel binders have drawn considerable attention, the existence of binders can also lead to the blockage of pore structures, which causes a reduction in cycle performance. Therefore, many researchers have reported binder-free electrodes with excellent properties. Chen et al.[104]prepared a porous carbon electrode from fungal hyphae in the absence of a binder. The results showed that the binder-free electrode had a lower charge transfer resistance of 1.6 Ω, which would enable accelerated ion transport. More importantly,Fig. 6c showed that current and voltage remain stable after 40 cycles. Yang et al.[105]also found a binder-free electrode with no performance attenuation after 10 000 cycles in supercapacitors, demonstrating the potential of the electrodes for practical applications in enhancing sustainability.
Moreover, cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), and galvanostatic charge-discharge (GCD) cycles are often used to characterize the electrochemical properties of biochar. The CV curve shows the relationship between the current and voltage on the electrode surface, which can be used to evaluate the microscopic reaction process, stability, and reversibility of the electrode surface[106-107]. This curve is usually a regular shape-like rectangle, indicating ideal electric double-layer behavior. The EIS curve can be used to assess the storage of the electrical energy and properties of the electrode materials, and its equivalent circuit can be inferred from the Nyquist plots[51-52,108].The GCD curve shows the current or voltage changes with time under a constant voltage or current, through which the energy consumption and electrode capacity can be obtained. This curve is also a symmetric triangle, indicating low internal resistance and reversible properties of the electrodes[91].
Notably, the electrochemical performance is usually dependent on the electrodes, which strongly rely on many factors, such as the preparation methods,electrode thickness, binders, conductive agents, and mixing ratio. Therefore, when preparing the electrodes, all of these factors must be considered in detail, for which play a critical role in CDI performance.
3.3 Influence of operation parameters on CDI performance
Apart from the impact of carbon electrodes on CDI performance, various parameters of the CDI system, such as voltage, electrode spacing, electrode pair number, flow rate, and initial feed concentration, also have a particular impact on CDI performance.
The voltage is an essential parameter for electrosorption. Tang et al.[71]found that when the voltage increased to 1.2 V, the SAC increased approximately 4 times owing to the stronger electrostatic interaction at a higher voltage[109](Fig. 7a). Wang et al.[68]observed that the current density increased as the voltage increased. However, the formation of a differential concentration polarization layer on the working electrode reduced the charging efficiency of the CDI system. Furthermore, the electrode wear ratio increased due to the redox reaction and the properties of the solution changed, leading the energy consumption of the CDI system[110]. Moreover, excessive voltage caused the electrolytic reaction, which affected adsorption capacity[111].
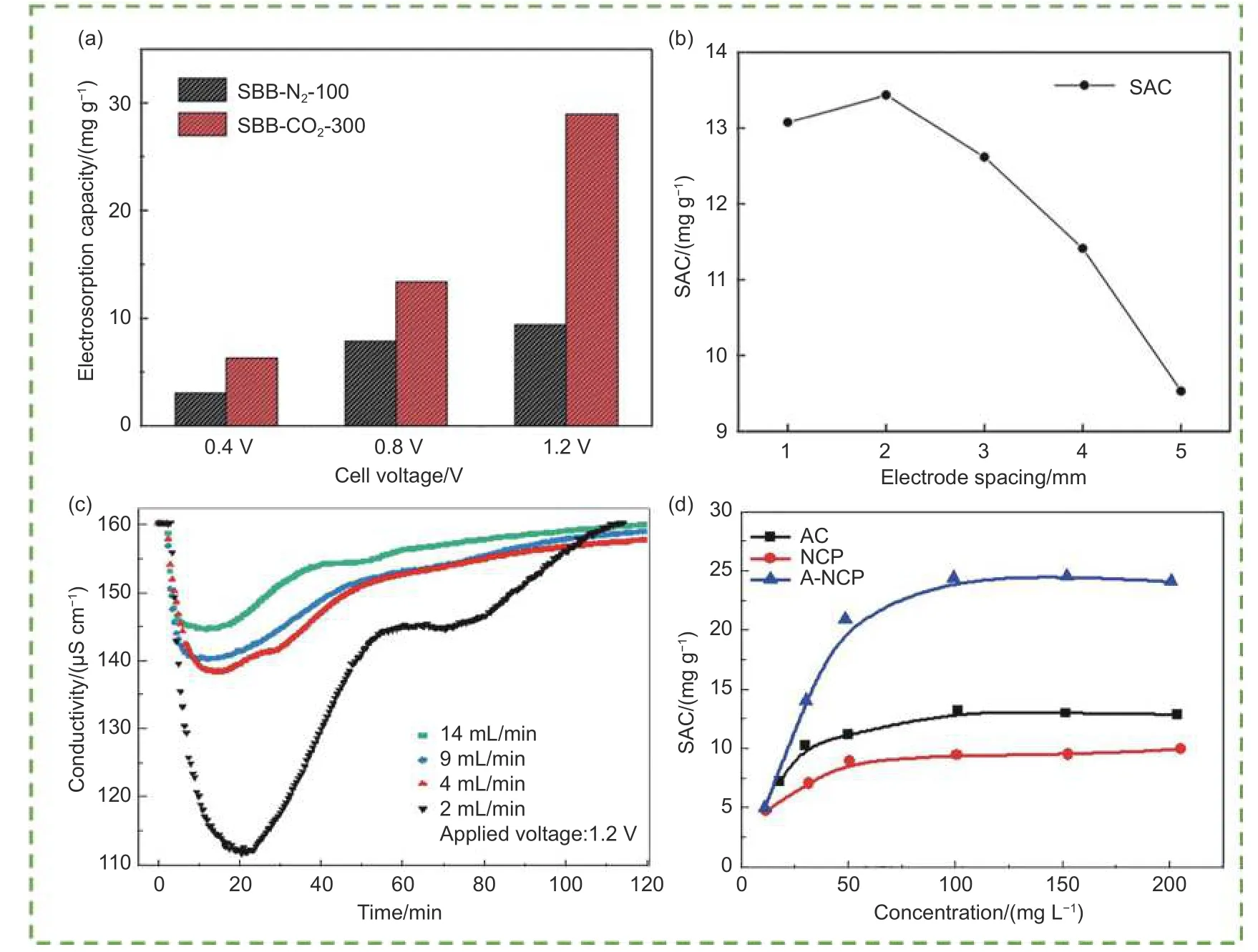
Fig. 7 Influence of the following operating parameters in the CDI system: (a) electrosorption capacity at different applied voltages[71], (b) effect of electrode spacing[54], (c) conductivity with different flow rate[115], (d) SAC at equilibrium state with different initial concentrations[31]. (Reprinted with permission)
Electrode spacing and electrode pairs also influence the CDI performance by affecting the ion diffusion distance and adsorption sites. Du et al.[54]observed that the adsorption capacity increased from 9.53 mg g-1to 13.44 mg g-1as the electrode spacing decreased from 5 to 2 mm, and the adsorption capacity was much lower when the spacing was 1 mm(Fig. 7b). This was because a small spacing reduced the distance between the ions and the double layer on the electrode surface. Thus, the ions could quickly enter the double electric layer and be adsorbed.However, when the spacing was too small, a large resistance would be produced, which was not conducive to electrosorption[112]. Zhang et al.[113]also explored the changes in conductivity when the number of electrode pairs was changed. They found that the conductivity decreased substantially as the number of electrode pairs increased from 2 to 4, but the desorbed ions increased too. However, with the electrode pairs increased from 4 to 8, which had little influence on the ion removal efficiency.
The flow rate and initial feed concentration are other important factors that determine CDI performance. Huang et al.[114]and Pastushok et al.[115]found that the ion removal effect decreased with increasing flow rate (Fig. 7c). This was because as the flow rate increased, the contact time between the solution and the electrode surface decreased. Kim et al.[31]and Mi et al.[116]concluded that increasing the initial concentration could enhance the electrosorption capacity(Fig. 7d). This was because a higher concentration facilitating the establishment of EDL, resulting in a reduction in the overlap effect and the mass transfer rate was higher[70,117].
In summary, the operation parameters have different influences on CDI performance. Hence, it is necessary to comprehensively consider various parameters to achieve optimal experimental conditions.
3.4 Selective adsorption of the ions
Carbon electrodes have applications in diverse areas such as desalination, water softening, and heavy metal removal from wastewater. Generally, the actual influent composition is complex and contains various ions. These ions can interact with each other and lead to competitive adsorption.
The physicochemical properties of the ions can affect their adsorption. Zhang et al.[112]illustrated the adsorption effects of different cations using bamboo carbon electrodes. This study showed that the priority of ion electrosorption was as follows: Cu2+> Pb2+>Cr3+> Cd2+> Mg2+> Ca2+and Na+> K+. When the ionic radius was small, it was usually easy to enter the electrode structure and then be adsorbed and removed.Meanwhile, as the charge number of the ions increased, the electrostatic attraction also increased.Furthermore, Ca2+showed slower adsorption and desorption rates than Na+and K+owing to its larger size and slower diffusion rate[118]. Hao et al.[119]investigated the adsorption effect of various ions in binary and ternary systems (Fig. 8a). The results showed that the removal of Cu2+was excellent in both systems. This was primarily because Na+was a hard acid, whereas Cu2+was a soft acid that could preferentially bind to the electrode surface. Although Fe3+, with high valence, had a higher electrostatic force towards the electrode, inhibiting the adsorption of Cu2+, the adsorption capacity of electrodes could reach up to 1 048.2 mg g-1towards Cu2+. For the ternary system,the adsorption efficiency of Cu2+reduced in the presence of Fe3+and Na+. Based on the soft-soft interaction, the electrode could still adsorb Cu2+efficiently and selectively. In addition, the properties of the electrode materials also affect ion adsorption and desorption. For example, Sun et al.[120]found that oxidizing anions, such as ReO4-could form hydrogen bonds with functional groups on the carbon surface, and adsorbed anion basically fail to desorb at the equal conditions with Cl-(Fig. 8b). According to the Louis acid-base theory, pyridine N, as a hard base, has higher electronegativity and lower polarizability and can preferentially adsorb the hard acid (Na+). In contrast,pyrrole N, as a soft base, interacts more strongly with the soft acid (Pb2+)[121].
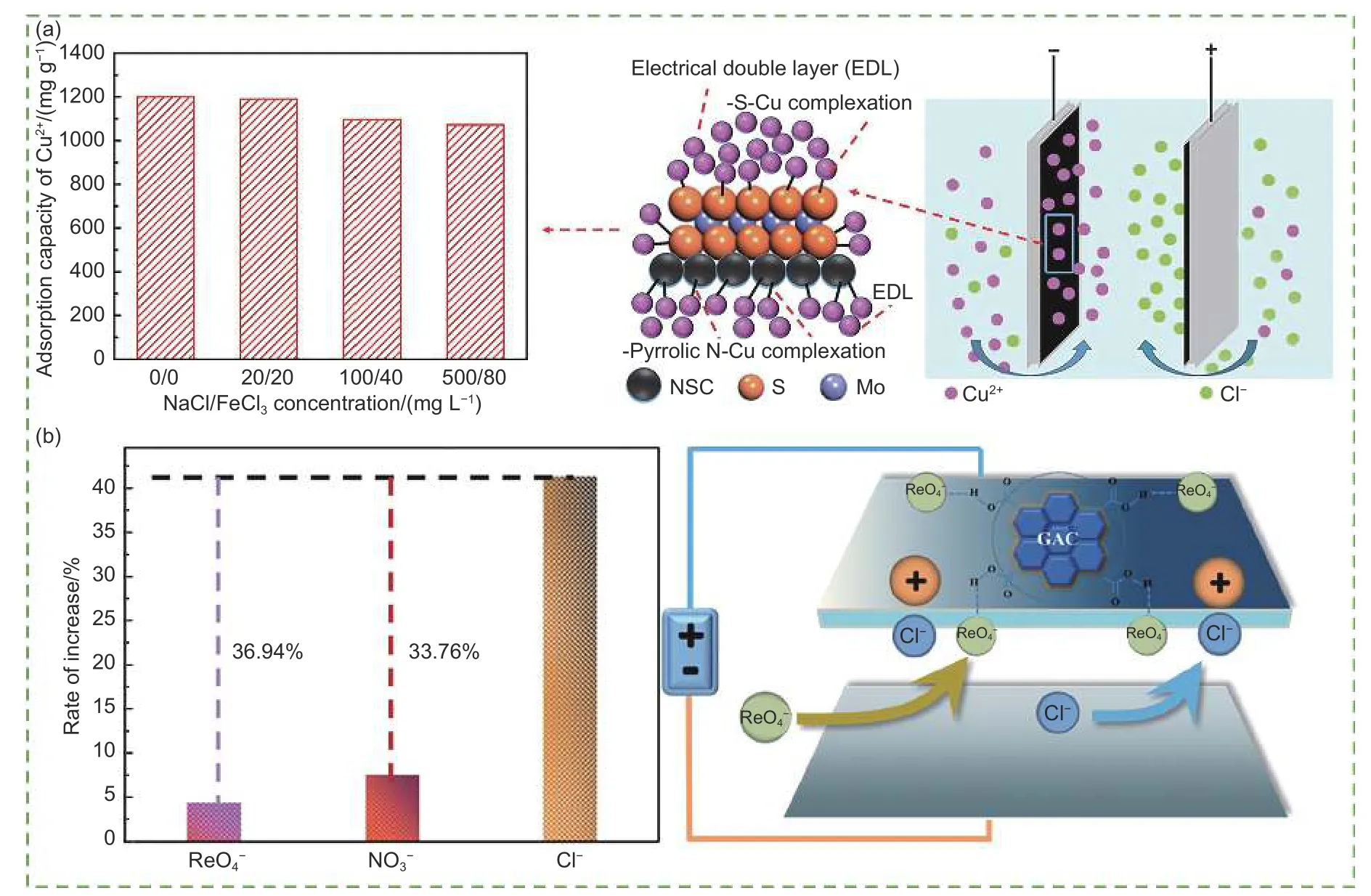
Fig. 8 Effects of (a) competitive ions on the adsorption of Cu2+ and adsorption mechanism diagram under 0.8 V[119], (b) the chemical bond on the electrosorption and desorption of anions in CDI and adsorption mechanism diagram[120]. (Reprinted with permission)
What’s more, compared with CDI, MCDI showed excellent salt removal and current efficiencies because of the selective transport of ions by the IEMs and no accumulation of ions in the EDL[122]. The selective removal of monovalent/divalent ions can be achieved by MCDI. For example, Sahin et al.[123]modified IEMs by adding polyelectrolyte multilayers,leading to the selective adsorption of monovalent ions.It is also necessary to select appropriate conditions based on the actual situation[124-125]. Zhang and Reible[126]found that Ca2+selectivity decreased by 20% as the applied voltage increased from 0.1 to 0.3 V, highlighting that electrical force affected the competitive electrosorption of ions kinetically. Similarly,Choi et al.[127]reported that the removal efficiency of monovalent cations increased under higher voltage conditions due to the increased mobility and the existence of monovalent cation selective exchange membranes. However, the flow rate had little influence on the selective electrosorption performance because the removal rates of monovalent and divalent cations remained similar with increasing flow rates in the case of both low and high total dissolved solids. Furthermore, Tsai et al.[128]observed that the NO3-selectivity coefficient increased as a function of charging time.Compared with Cl-, the selectivity coefficient of NO3-in CDI increased to 3.88 due to ion substitution as the charging time increased. Pang et al.[129]reported that dissolved inorganic carbon (DIC) hindered the removal of F-because DIC had lower hydration energy and higher ionic charges. In the NaF + NaHCO3solution,as the molar ratio of F-/DIC increased from 1∶2 to 1∶0.5, the SACFincreased by 2 times, indicating the importance of water characteristics in the selective removal of ions.
In summary, the selective adsorption of ions depends on the properties of the ions, electrode materials, and operational parameters. The selective adsorption mechanism is complicated, and the removal of a specific ion from a complex solution remains an urgent problem.
4 Regeneration of biochar electrodes
As the core part of the CDI system, the optimum electrode not only has the advantages of high-efficiency desalination but also maintains its long-term stability. Currently, most electrodes can maintain high stability after 5-20 cycles, and some can reach approximately 30-100 cycles after modification treatment. The primary problems include the electrode material itself and the regeneration method, which results in a reduction in the desalination effect[91,130].
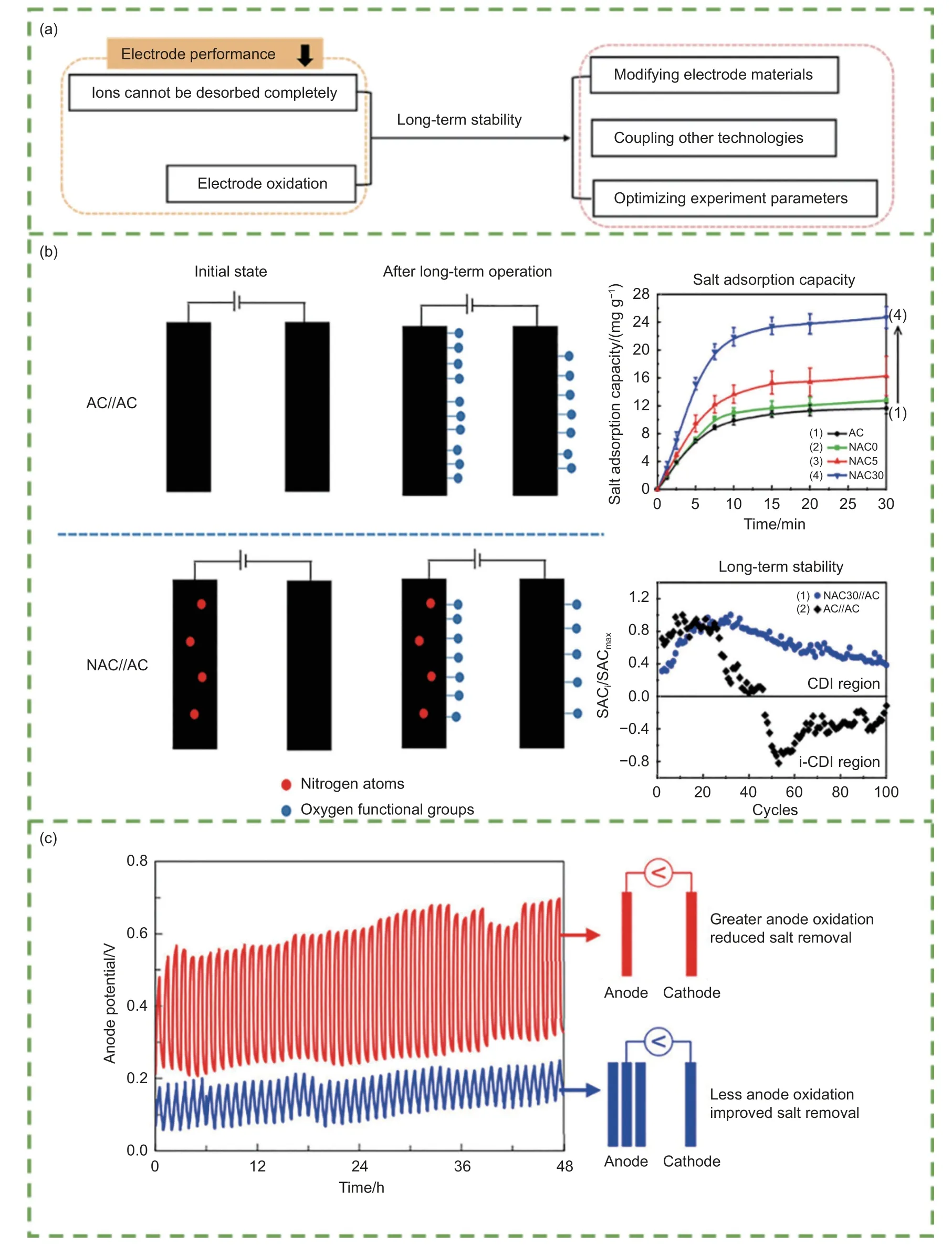
In terms of materials, two main aspects affect electrode regeneration (Fig. 9a). First, some ions are adsorbed on the electrode surface cannot be desorbed after regeneration, causing the occupation of some adsorption sites. Second, biochar electrodes are oxidized during long-term tests, leading an increase in resistance and a shift in the zero-charge potential, which damages the electrode itself and decreases the ion storage capacity[44,131-132]. Modification or combination with other materials and the use of asymmetric electrodes have been proposed to overcome the co-ion effect and carbon oxidation, enhance electrode performance, and maintain long-term stability in CDI[133].Liu et al.[134]decorated graphene-coated NPC electrodes using soybean and graphene. Soybean carbon containing 7.25% N enhanced the hydrophilicity of the electrode, whereas the graphene effectively improved the electrical conductivity, lowered the potential diffusion barrier of ions, and accelerated the ion transport rate. Moreover, the electrode exhibited excellent cycle stability, the desalination performance could remain at 93.5% after 50 cycles. Hsu et al.[131]observed the cycling stability of both symmetric and asymmetric electrodes. After 100 cycles, the asymmetric electrode still maintained 40% of its maximum salt adsorption (Fig. 9b). In contrast, the symmetric electrode only had 10% of the maximum salt adsorption in the 47thcycle. Furthermore, Algurainy and Call[135]improved anode stability by changing the mass ratio of the electrodes in continuous-flow CDI.Multiple anodes significantly lowered the charge transfer resistance and oxygen-to-carbon ratio compared with single anodes. After 48 cycles, SAC decreased by 68% for symmetric CDI, whereas the capacity remained stable for asymmetric CDI, with a 47%reduction for the double anode and only a 17% reduction for the triple anode (Fig. 9c). Ren et al.[136]prepared composite electrodes using biochar and silicon nanoparticles as a long-life energy storage electrode material with excellent cyclic stability and a capacity retention of 105.7% after 1 000 cycles. Yu et al.[137]fabricated an ionic liquid-coupled biochar/TiO2electrode to obtain chemically bonded interfaces between TiO2and sawdust biochar, generating an electrode with a long-term cyclic stability of 2 500 cycles.
The electrolyte concentration, current strength,time, and pH also affect electrode regeneration. The reverse electrode regeneration method is more conducive to ion desorption and reduces the rinsing water volume but may produce a resorption effect, which can be reduced by increasing the flow rate[138]. Zhang et al.[139]studied the effects of electrolyte concentration, regeneration current intensity, and time on regeneration efficiency. The results showed that the regeneration efficiency increased as the electrolyte concentration and regeneration current strength increased.Similarly, a regeneration time of no more than 5 h could enhance the regeneration efficiency. Chen et al.[140]observed that the regeneration efficiency was approximately 86% in constant current mode and 64%in constant voltage mode at the same discharge current owing to more energy loss occurring in constant voltage mode. The pH value is also a key factor affecting the regeneration of electrodes. This was because the positive and negative charges on the electrode surface could be adjusted. When the pH value was 7.5, regenerative desalination significantly increased from 23.5 to 103 mg g-1because of the more negative charges on the electrodes[131].
Furthermore, it is a feasible method for electrode regenerationviacoupling with other techniques. Zou et al.[141]regenerated an electrode using ultrasound washing, which increased the conductivity of the electrode by approximately 3 times compared with simpledeionized water washing. Jiang et al.[142]proposed a magnetic-assisted strategy to enhance CDI performance. With the synergetic manipulation effect of the electric field force and Lorentz force, the maximum conductivity of the 4thcycle was largely consistent with that of the 1stcycle, indicating good stability of the CDI device.
With the rapid development of CDI technology,research on the mechanisms and methods of electrode regeneration has gradually increased. Electrode regeneration is not only dependent on experimental parameters but can also be coupled with other materials and techniques. However, there are still some limitations that restrict long-cycle operations. Therefore, it is essential to develop more comprehensive, simple,and low-cost regeneration strategies based on the current research.
5 Difficulties and challenges
Carbon electrodes have made great progress in recent years. Biochar has played an important role in CDI owing to its advantages of low price, broad raw material resources, and simple preparation. Fig. 10 shows the bibliographic data of research articles related to carbon materials as electrodes for the application in CDI published in the last decade. Publications display a common trend of gradually increasing, especially in the case of biochar which has become a hotspot in the field of CDI. Nevertheless, some critical issues still restrict its practical application in CDI. Currently, the electrochemical properties of the obtained biochar are maintained within a particular range, metal oxide modification and heteroatom doping can improve these but there still exist problems. Carbon-metal composites can maximize the synergistic effect of Faraday and non-Faraday adsorption mechanisms to provide the driving force for the reaction, but the existence of metal oxides can lead to partial blockage of the pore structure[143]. Heteroatom doping can enhance the electrical conductivity and wettability of biochar to improve its ion mass transfer rate and electrosorption capacity. The interaction mechanism has not yet been systematically elaborated, and there are increasing costs and environmental issues. In terms of long-term economic benefits, the future application prospects of CDI systems are related to ion-selective adsorption and long-term stability. However, the selective adsorption mechanism for ions remains unclear and requires further research. Meanwhile, the poor stability of the electrode limits its long-term operation in CDI. Many methods have been proposed to reduce the oxidation of the electrodes and improve their long-term stability. Consequently, the electrodes with high electrochemical properties and long-term economic benefits remains a significant challenge.
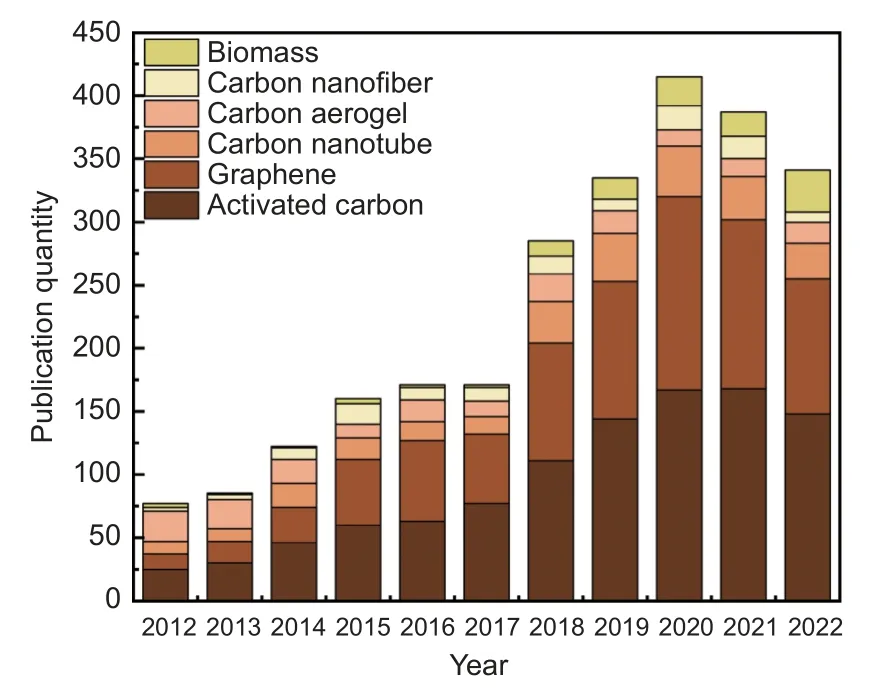
Fig. 10 Statistics of research articles on the use of biomass, carbon nanofibers, carbon aerogels, carbon nanotubes, graphene, and activated carbon,for preparing electrodes for the application of CDI, published in the last 10 years (data obtained from Web of Science)
Addressing the challenges listed above, future research on biochar materials should focus on the following aspects: (1) Construction of new carbon electrodes by developing effective modified or composite technology[144]. (2) Efficient removal of specific ions from complex systems by deeply exploring the selective adsorption mechanism of ions. (3) Synthesis of biochar with an excellent structure, rich functional groups, and desired pore size distribution for more effective adsorption and long-term stability. (4) Realization of highly efficient regeneration of electrodes and improvement of their long-term stability by combining CDI with other technologies. (5) Its economic and environmental feasibility assessments in CDI.
6 Conclusion
Compared with conventional desalination techniques, CDI technology has excellent application prospects. This review presented the preparation of biochar electrodes and their applications in CDI in recent years. It was concluded that the factors inferring the excellent performance of biochar electrodes were mainly related to their richer pore structure, higher electrical conductivity and wettability, and faster ion mass transfer rate. Moreover, the introduction of metal and heteroatomic groups facilitated the preparation of biochar electrodes with a graded pore structure and high electrochemical performance. Noteworthy, the desalination capacity was positively correlated with the mesopore content of biochar. Also, the CDI parameters influenced the desalination capacity of the system, whose performance could be improved comprehensively by optimizing the experimental parameters.Additionally, we summarized the mechanism and influencing factors of ion-selective adsorption as well as the reasons affecting the regeneration of electrodes and corresponding methods to provide ideas for the preparation of highly stable biochar electrodes in the future. Finally, the difficulties and challenges of biochar electrode materials were proposed. Overall,this comprehensive review established a foundation for the promising field of CDI in terms of preparation,performance, long-term stability of biochar electrodes.
Acknowledgements
This work was supported by Capacity Building Project of Some Local Colleges and Universities in Shanghai (21010501400) and Shanghai Sailing Program (23YF1415400).
- 新型炭材料的其它文章
- Large-scale synthesis of 3D ordered microporous carbon at low temperature using cobalt ions exchanged zeolite Y as a template
- Highly efficient Co—N—C electrocatalysts with a porous structure for the oxygen reduction reaction
- Reversible surface modification of PAN-based carbon fibers by a ferrocene-based surfactant
- Recent advances in 3D interconnected carbon/metal high thermal conductivity composites
- Synthesis and electrochemical properties of nano-Si/C composite anodes for lithium-ion batteries
- Factors that influence the performance of hydrogen detectors based on single-wall carbon nanotubes

