Development of sorghum mutants with improved in vitro protein digestibility by CRISPR/Cas9 editing of kafirin genes
Lev A.Elkonin,Grigoriy A.Gershchenkov ,Ntlie V.Borisenko ,Odyssey A.Kenzhegulov ,Sule Kh.Srsenov ,Ntly A.Rozhnov ,Vlery M.Pnin
a Federal Centre of Agriculture Research of South-East Region,Saratov 410010,Russia
b Institute of Biochemistry and Genetics -Subdivision of the Ufa Federal Research Centre of the Russian Academy of Sciences,Ufa 450054,Russia
Keywords: Sorghum CRISPR/Cas Kafirins In vitro protein digestibility Vitreous endosperm
ABSTRACT Sorghum(Sorghum bicolor(L.)Moench)is a major world crop that is a reliable source of fodder and food grain in arid regions.However,unlike other cereals,sorghum grain has low nutritional value,owing mainly to the resistance of its storage proteins(kafirins)to protease digestion.Changing the composition of kafirins or their primary structure may address this problem.To induce mutations in kafirin-encoding genes that were expected to disturb their accumulation in endosperm cells,we used a genome-editing approach.By Agrobacterium-mediated genetic transformation of immature embryos of cv.Avans,we obtained 14 transgenic plants with genetic constructs for site-directed mutagenesis of the k1C5 and gKAF1 genes encoding 22 kDa α-and 28 kDa γ-kafirins,respectively.Sequencing of 5 regenerants obtained by using k1C5-addressing vector revealed two plants with mutations.T1 progeny of these mutants had higher in vitro digestibility of endosperm proteins(86%-92%),in comparison with the donor Avans (63%-67%).The kernels of these plants had a thick vitreous endosperm.A mutant with increased in vitro protein digestibility and vitreous endosperm,carrying a mutation in the target sequence,was also obtained by use of the gKAF1-addressing vector.Thus,using genome editing technology,we have obtained mutants with improved kafirin digestibility that can be used in sorghum breeding.
1.Introduction
Development of cultivars and hybrids that ensure sustainable grain production in the face of global warming is the main task of plant breeding.In this regard,sorghum is of particular importance.Sorghum is a high-yielding,drought-resistant grain crop,a reliable source of feed and food grain for regions that regularly suffer from drought.In global agriculture,sorghum is one of the five most widely cultivated cereals on Earth.It feeds more than 500 million people in 30 countries in Africa and Asia [1].Given global warming and the expansion of drought-prone acreage,where sustainable production of other grains such as wheat,corn,and barley is difficult,the importance of sorghum in world grain production will steadily increase.
Sorghum grain is rich in various bioactive compounds such as phenolic acids,procyanidins,flavonoids,and anthocyanins,which have been shown to inhibit oxidative stress and have anti-cancer potential [2-5].Sorghum grain does not contain gluten and can serve as a source of protein for people with gluten intolerance,who must follow a gluten-free diet.The frequency of celiac disease,depending on the risk group,varies from 6.6% to 16.3% [6].
One shortcoming of sorghum is the resistance of its storage proteins (kafirins) to proteolytic digestion.Protein digestibility in the vast majority of varieties and hybrids varies from 40%to 60%[7-9].Poor protein digestibility reduces starch digestibility because undigested kafirins prevent access of amylolytic enzymes to starch granules [9-11].Development of grain sorghum lines with improved digestibility of kafirins approaching the digestibility of storage proteins in other cereals will increase the grain nutritional value and widen the distribution of sorghum varieties and hybrids.
It is believed that the resistance of kafirins to proteolytic digestion is due primarily to their chemical structure [12,13].Kafirins have a high content of cysteine,which forms intra-and intermolecular disulfide bonds leading to the formation of oligo-and polymers of kafirins that are resistant to protease digestion.Resistance depends also on other factors:the structural organization of protein bodies in sorghum endosperm cells,in which γ-kafirins,which are more resistant to proteolytic digestion,form the outer shell of protein bodies and hinder the access of proteases to more easily digestible α-kafirins located inside protein bodies;interaction of kafirins with non-protein components,in particular with tannins that reduce the activity of proteases,and with polysaccharides of the starch granules;and interaction of kafirins with non-kafirin proteins (glutelins),which are also capable of forming polymers linked by intramolecular disulfide bonds [12,13].
Several approaches have been used for addressing kafirin resistance to proteolytic digestion.These include experimental induction of mutants with altered synthesis of kafirins [14],identification of naturally occurring allelic variants of kafirins[15],RNA interference (RNAi) for suppression synthesis of αand/or γ-kafirins [16-19],and introduction of a modified gene encoding β-kafirin with additional proteolytic cleavage sites [20].
Site-directed mutagenesis using genetic constructs carrying the CRISPR/Cas system is actively used to solve a variety of problems in genetics and plant breeding [21].This approach allows one to change the structure of plant genes,and thereby,practically without introducing foreign genetic information,to change the plant metabolism in a desired direction [22-24].In the offspring of mutants,owing to recombination,the system can generate plants that carry the induced mutation but are free of the genetic construct that induced it.As a result,the resulting mutants are free of foreign genetic information;i.e.,are not transgenic organisms.
The CRISPR/Cas9 system includes thecas9endonuclease gene and guide RNA (gRNA),which directs the CAS9 endonuclease to a target nucleotide sequence [23-25].The classical variant of the CAS9 nuclease recognizes the NGG-3′(Protospacer Adjacent Motif,PAM) sequence adjacent to the target (protospacer),which thus serves as the identification mark of the target in the edited genomic DNA.CAS9 endonuclease produces double-strand breaks in the target DNA located three nucleotides 5′to the PAM sequence.These breaks result in insertions or deletions at the target site,which can lead to frameshifts and null mutations.
The CRISPR/Cas system was successfully used to induce mutations in the nucleotide sequence of a signal polypeptide of the 22 kDa α-kafirin and to obtain plants with improvedin vitroprotein digestibility [26].However,because most of these mutants had floury endosperm with distorted vitreous-layer development,they were not useful for practical sorghum breeding.Gene editing was also used to knock out the β-kafirin gene[27].Although these mutants were characterized by unique protein composition and changed protein body morphology,there was no improvement of grain quality in the cultivar used in the experiment.A report [28]describing edited sorghum plants with mutations in the nucleotide sequences of the γ-kafirin gene did not describe the effect of these mutations on protein digestibility.
Previously[29],we have created a series of binary vectors(p1Cp4C) for site-directed mutagenesis of genes encoding α-and γkafirins.The purpose of this work was to obtain sorghum mutants with improvedin vitroprotein digestibility by using site-directed mutagenesis of nucleotide sequences encoding α-and γ-kafirins that may be useful for sorghum breeding.
2.Material and methods
2.1.Plant material and growth conditions
To obtain transgenic sorghum plants carrying genetic constructs for editing thek1C5orgKAF1genes,immature embryos of the grain sorghum variety Avans were used.This is a new commercial variety of grain sorghum,approved for cultivation in several regions of the Russian Federation,characterized by high yield and a large oval panicle with white grain.Donor plants were grown in 2020 in the field and in 2021 in a greenhouse equipped with metal halide lamps (photoperiod 16 h day/8 h night,24-26 °C in the daytime,16-18 °C at night).Regenerants (T0) were grown in a greenhouse.T1generation was grown in 2021 in outdoor pots.
2.2.Construction of the CRISPR/Cas9 system and sorghum transformation
Previously created [29] binary vectors p1C,p2C,and p3C for site-directed mutagenesis of the α-kafirin encoding genek1C5(one of the genes of the gene family encoding the synthesis of 22 kDa α-kafirin) and 28 kDa γ-kafirin encoding genegKAF1were used.These vectors contain the maize-codon-optimizedcas9endonuclease gene under the control of theubi1promoter,the guided RNA (gRNA) scaffold under the control of a OsU3-promoter,and thebarmarker gene under the control of the maizeubi1-promoter(Fig.1).Vectors for editing the nucleotide sequence of the signal polypeptide of the 22 kDa α-kafirin in the gRNA scaffold carried oligonucleotides that were complementary to target motifs 1C or 2C(vectors p1C or p2C,respectively).A vector for editing the nucleotide sequence of the signal polypeptide of the 28 kDa γ-kafirin in the gRNA scaffold carried oligonucleotides complementary to target motif 3C (vector p3C).These target motifs were choose using the CRISPOR(https://crispor.tefor.net/crispor.py)and CHOPCHOP (https://chopchop.cbu.uib.no) online tools based on such features as specificity score,predicted efficiency,outcome of out-of-frame mutations,and number of off-targets.The positions of selected target motifs in thek1C5andgKAF1genes are shown in Fig.1.These vectors were introduced by electroporation into the cells ofAgrobacterium tumefaciens,strain AGL0,and with the newly created strains AGL0/p1C,AGL0/p2C,and AGL0/p3C experiments onAgrobacterium-mediated genetic transformation of immature sorghum embryos were carried out according to protocol [30] with modifications [19].
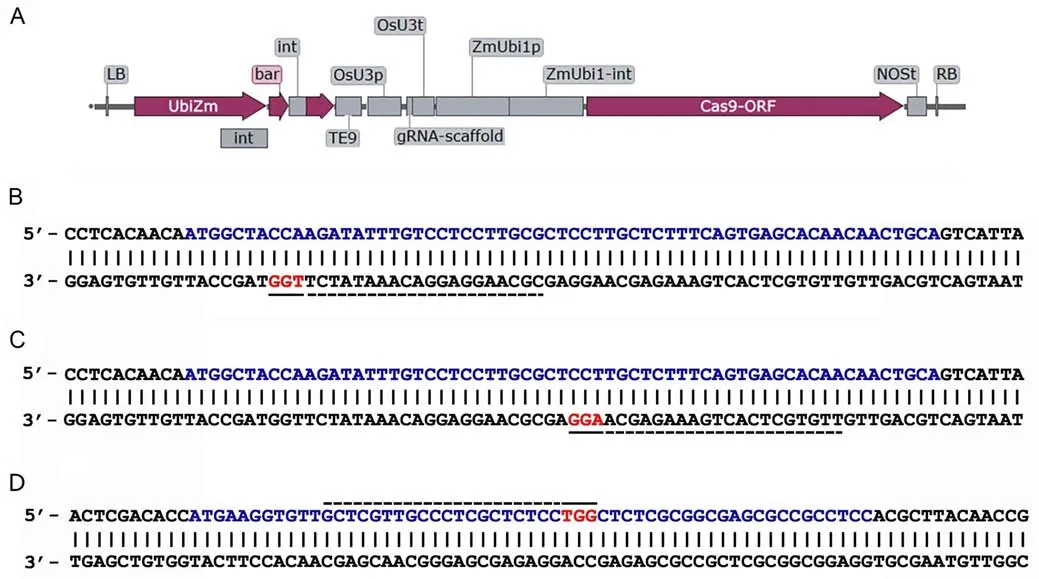
Fig.1.T-DNA structure of the p1C-p3C binary vectors(A)and positions of the targets on the nucleotide sequences of the k1C5 gene(B,C)and gKAF1 gene(D)encoding signal polypeptide of 22 kDa α-and γ-kafirins,respectively.In the p1C vector,the gRNA scaffold contains oligonucleotides complementary to the target 1C:11-21 nucleotides of the k1C5 gene sequence encoding the signal polypeptide 22 kDa of α-kafirin(B);in the p2C vector,the gRNA scaffold contains oligonucleotides complementary to the target 2C:33-36 nucleotides of the k1C5 gene sequence encoding the signal polypeptide 22 kDa of α-kafirin (C);in the p3C vector,the gRNA scaffold contains oligonucleotides complementary to the target 3C: 12-32 nucleotides of the gKAF1 gene sequence encoding the signal polypeptide of the γ-kafirin (D).
Briefly,immature embryos pre-cultivated for 3 days on M11 medium [31] were inoculated with agrobacterial suspension,which was grown in AB medium for activation ofvirgenes as described by Gelvin [32].The embryos were then co-cultivated for 3 days on filter paper wetted with co-cultivation medium,after which they were transferred to resting medium for 7 days.The description of the media composition is given in [19].Selection of transformed calli was applied on the media (M11 with 2,4-D and amino acids,or MS with 2,4-D and 6-BAP) containing 2.5 mg L-1glufosinate-ammonium(GA).For plant regeneration,MS medium with 6-BAP,kinetin,IAA,and addition of CuSO4was used.For rooting,regenerated shoots were transferred to hormone-free ½-MS medium.For acclimatization,plants with roots were transferred into tubes with tap water.Regenerated plants with welldeveloped root systems were transplanted into vessels with soil and grown in a greenhouse.
2.3.Detection of transgenic plants contained CRISPR/Cas9 system
For identification of transgenic plants carrying genetic constructs for site-directed mutagenesis of kafirin genes,DNA was isolated from leaves of T0plants using the modified CTAB method.The presence of the genetic construct was tested by PCR with primers amplified the 436-bp fragment of thecas9gene(F(5′-3′):tgagactctaattggataccgaggg;R (5′-3′): tttggaactgacagaaccgcaac) (Evrogen,Moscow,Russia) and the 444-bp fragment of thebargene (F:tgtcttggttgtgatgatgtggtc;R: gcgtatgaaggcagggctaaa) (Evrogen).PCR analysis was performed using the MasterCycler Personal(Eppendorf,Hamburg,Germany) and T100 (Bio-Rad,Singapore).The reaction mixture contained 50 ng DNA,0.03 U μL-1SynTaq DNA polymerase (Synthol,Moscow,Russia),0.6 pmol of each primer,×onefold PCR buffer(Synthol),2.5 mmol L-1MgCl2,0.2 mmol L-1dNTP mixture (Synthol).The total volume of the reaction mixture was 25 μL.PCR conditions were as follows: for thecas9gene fragment: 95 °C (2 min),then 40 cycles [95 °C (30 s),64 °C(30 s),72 °C (1 min 10 s)],and 72 °C (7 min).For thebargene:95 °C (2 min),then 40 cycles [95 °C (1 min),56° (1 min),72 °C(1 min 30 s)],and 72 °C (10 min).Amplified fragments were visualized by 2.0% agarose gel electrophoresis.
2.4.Sequencing of edited targets in signal sequence
Primer selection for the amplification of PCR products was performed using the CRISPOR (https://crispor.tefor.net/crispor.py)online tool.Selection of target motifs has been described [29].The size of the PCR product containing the amplified sequence of thek1C5gene was 729 bp and the size of the amplified sequence of thegKAF1gene was 773 bp.The primers used for amplification and sequencing are listed in Table S1.The PCR products were cloned into the pAL2-T vector(Evrogen).The correctness of cloning was checked by restriction analysis with ApaI,EcoRI,NcoI,NotI,and PstI (all enzymes from Thermo Scientific,USA).For mutation detection in T0and T1plants,cloned PCR products were subjected to Sanger sequencing with an ABI 3130 genetic analyzer,performed by Synthol.Identification of edited targets in sequenced clones was performed with Chromas (https://www.technelysium.com.au) and SnapGene Viewer 5.2.4 (https://www.snapgene.com/).
2.5.In vitro digestibility of endosperm proteins
To studyin vitroprotein digestibility,flour (20 mg) of experimental samples and of original cv.Avans was treated with 5 mL of 0.15% pepsin solution (Carl Roth,Karlsruhe,Germany;Art.-Nr.KK38.1,2000 FIP-U/g)in a 0.1 mol L-1potassium phosphate buffer(pH 2.0)for 120 min at 37 °C with repeated shaking.Control samples were incubated in potassium phosphate buffer without pepsin addition under the same conditions.The experiments were performed with two replications.
For quantitative estimation of protein digestibility,we used the efficient and informative method of analysis of electrophoretic spectra of proteins in SDS-polyacrylamide gel electrophoresis(SDS-PAGE)after pepsin treatment of the flour[33]and used since then in several studies [34,7,8].For this purpose,the digested and control samples were centrifuged at 13,000 r min-1and the pellet was incubated with a sample buffer (0.0625 mol L-1Tris·HCl,pH 6.8) under reducing conditions (2% SDS,5% 2-mercaptoethanol)at 100°C for 90 s.The samples were centrifuged at 13,000 r min-1and separated by SDS-PAGE in 13.0% (w/v) polyacrylamide gel according to the modified Laemmli method [35].In each lane,20.7 μL of extract was loaded.Separation was monitored with protein molecular-weight markers,14-116 kDa(Thermo Fisher Scientific,Waltham,MA,USA),or 10-200 kDa (Servicebio,Wuhan,Hubei,China).Gels were stained with Coomassie Brilliant Blue R-250.After electrophoresis,gels were scanned with a ChemiDoc Imaging System (Bio-Rad Laboratories,Hercules,CA,USA) and the amount of protein was quantified with Image Lab 6.1 (Bio-Rad).The digestibility value was counted as the percent ratio of the protein volume in the digested sample to that in the control sample.As a standard of highin vitroprotein digestibility,we used the previously obtained RNAi mutant Avans-1/18 with a genetic construct for RNAi silencing of thegKAF1gene [36].
2.6.Statistical analysis
To evaluate differences inin vitroprotein digestibility of studied samples,variance analysis using the AGROS software package,version 2.09 (S.P.Martynov,Institute of General Genetics,Russian Academy of Sciences) with Duncan’s multiple range test was performed.
3.Results and discussion
3.1.Obtaining CRISPR/Cas9-induced mutants
In the experiments on theAgrobacterium-mediated genetic transformation of immature embryos of cv.Avans,14 regenerants PCR-positive forcas9were obtained: five in the experiment with p2C,one with p1C,and 8 with p3C (Table 1).Testing these plants for the presence of agrobacterial contamination using PCR for theA.tumefaciens chvAchromosomal gene gave a negative result,with the exception of one regenerant,which was excluded from further analysis.The frequency of independent transgenic events varied from 4.5% to 9.2% in individual experiments.Such a frequency is usually observed in sorghumAgrobacterium-mediated genetic transformation when standard binary vectors and regularAgrobacteriumstrains are used [37].
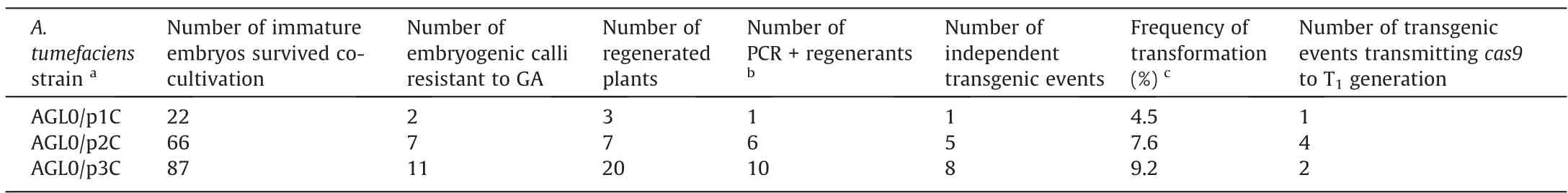
Table 1Obtaining plants carrying genetic constructs for site-directed mutagenesis of kafirin genes in experiments on Agrobacterium-mediated genetic transformation of immature embryos of grain sorghum,cv.Avans.
PCR analysis of T1plants revealed the inheritance ofcas9in four families of T0plants carrying genetic constructs for editing thek1C5gene(targets 1C and 2C)and in two of three investigated families of T0plants carrying genetic constructs for editing thegKAF1gene (target 3C).This finding confirms the integration of these genetic constructs into the genomes of T0plants.Segregation forcas9in individual T1families varied (Table 2),with predominance of PCR-negative plants.Remarkably,in the progeny of two T0plants PCR-positive forcas9(2C-2.1.1 and 3C-58),no plants carrying genetic constructs for genome editing were observed,although plant 2C-2.1.1 had mutations in the target sequence (see below).This finding suggests a tendency for elimination of the CRISPR/Cas genetic construct from the genome of transgenic sorghum plants.
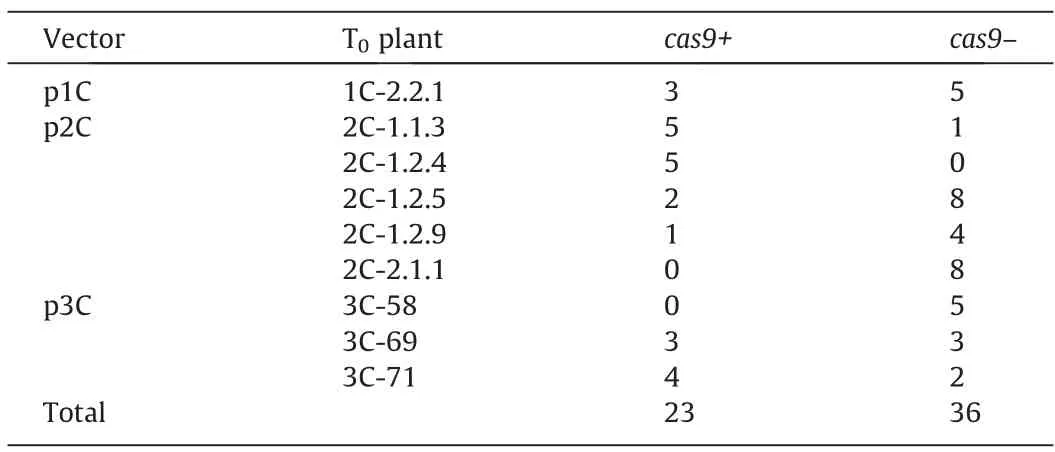
Table 2Inheritance of the genetic constructs for editing the k1C5 genes(p1C and p2C vectors)and gKAF1 (p3C vector) in the T1 generation.
DNA sequencing of four T0plants,PCR-positive forcas9,obtained in an experiment with the AGL0/p2C strain (target 2C in the R-chain of thek1C5gene,Fig.1),showed that two of them(2C-1.2.5 and 2C-2.1.1) have mutations in the target motif: the nucleotide sequence,encoding the 22 kDa α-kafirin signaling polypeptide.In plant 2C-1.2.5,point mutations of 11th nucleotide and 19th nucleotide,5′to the PAM sequence (5′-AGG),C →A and T →A,respectively,were observed (Fig.2A).In this plant,mutations in the signaling polypeptide-encoding sequence,outside the target sequence,were also observed: T →C (20th nucleotide 3′to the PAM)(Fig.2B).Different mutation events revealed in different DNA strands suggest the biallelic nature of the 2C-1.2.5 mutant.In silicoanalysis showed that the point mutation of 11th nucleotide of the target can lead to the replacement of valine by leucine,while the point mutation of 20th nucleotide 3′to the PAM can lead to the replacement of isoleucine by valine.
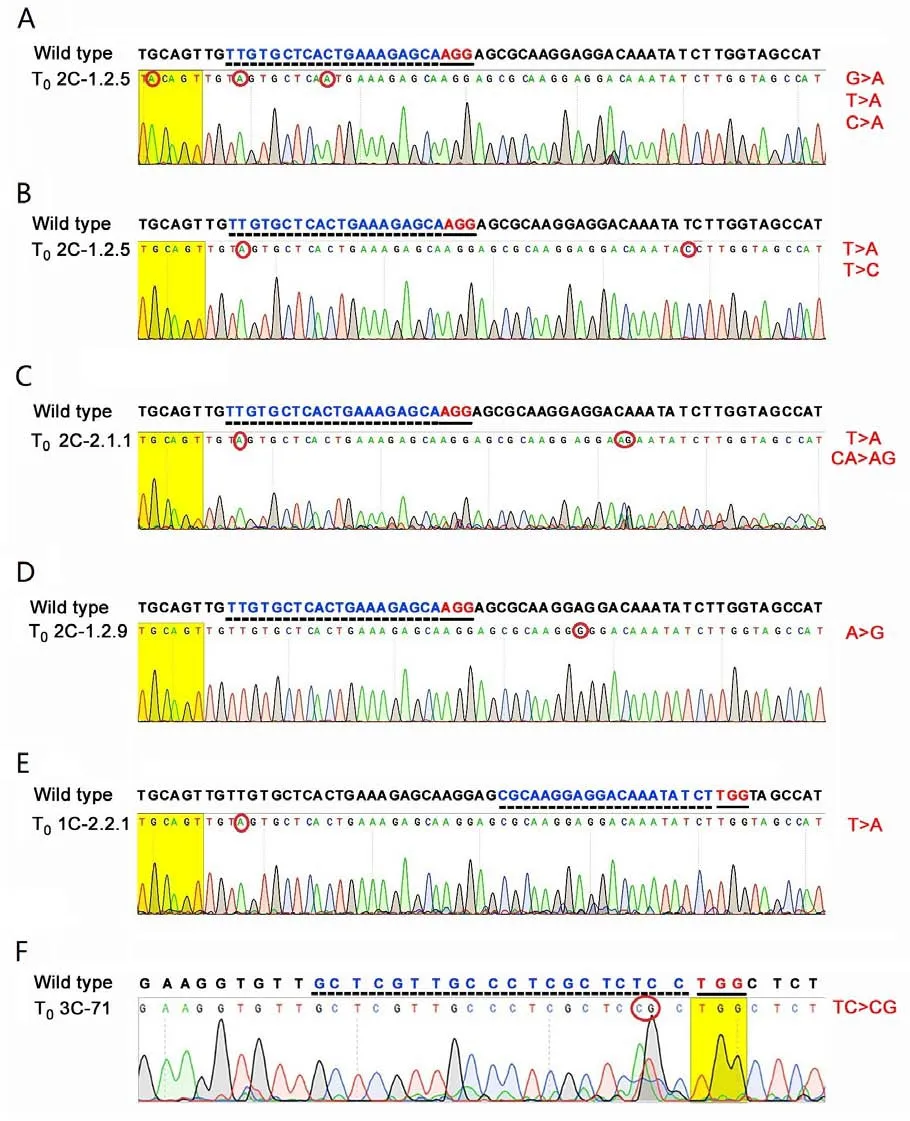
Fig.2.Mutations identified in the nucleotide sequences of the k1C5 gene(A-E)and the gKAF1 gene(F)encoding signal polypeptide of 22 kDa α-kafirin and 28 kDa γ-kafirin,respectively,in sorghum plants obtained in experiments on site-directed mutagenesis of kafirin genes.PAM sequences are in red and underlined;target sequences are in blue font and underlined with dotted lines;mutations are indicated by red circles.The nucleotide sequences encoding the 22 kDa α-kafirin and the 28 kDa γ-kafirin signaling polypeptides were retrieved from Phytozome,https://phytozome.jgi.doe.gov: Sobic.005G193100,Chr05: 67654898-67655764 reverse;and Sobic.002G211700.1,Chr02:60423442-60424313 forward,respectively.
In the plant 2C-2.1.1,a point mutation in the 19th nucleotide,5′to the PAM sequence (5′-AGG),T →A,was also found,as well as the mutation outside the target sequence: replacement ofCA →AG(14th and 15th nucleotides 3′to the PAM)(Fig.2C).In silicoanalysis showed that this mutation could lead to the replacement of leucine,a nonpolar amino acid,by serine,a polar amino acid.
No mutations were found in the signaling polypeptideencoding sequence of T0plant 2C-1.2.4,despite the fact that thecas9gene was inherited in its T1progeny (Table 2).
In plant 2C-1.2.9 (Fig.2D),a point mutation was found outside the target sequence,in the 10th nucleotide 3′to the PAM sequence:A →G.This mutation could lead to the replacement of leucine,a hydrophobic amino acid,by proline,a polar amino acid with conformationally rigid structure,strongly bending the peptide chain.
Thus,among four sequenced mutants obtained in the experiment with the vector carrying the 2C target,three carried mutations located 3′-end to the PAM sequence.This finding can be explained by the presence of additional PAM sequences located 3′-end to the 2C target(two 5′-AGG and one 5′-TGG).It is possible that owing to such proximity,the CAS9 nuclease could err,leading to these mutations.Deletions 3′-end to the target,including PAM,have been described in maize [38,39] and rice [40].
No mutations were found in the signaling polypeptide-encoding sequence of the T0cas9positive plant 1C-2.2.1 obtained with a genetic construct harboring the 1C target motif (Fig.2E),except the same T →A substitution that was observed in the mutants 2C-2.1.1 and 2C-1.2.5.However,this substitution is located far from the 1C target sequence and perhaps represents natural polymorphism rather than an induced mutation.In support of this inference,three of the five plants in which the signaling polypeptide-encoding sequence was sequenced carried adenine in this position,while two carried thymine,the same as the Phytozome reference sequence(Sobic.005G193100,Chr05: 67654898-67655764 reverse).
Among five sequencedcas9-positive plants from T0generation obtained in the experiment with the p3C vector,the mutation in the target sequence was found in one,3C-71: TC →CG (second and third nucleotide of the target sequence,5′to the PAM)(Fig.2F).In silicoanalysis showed that this mutation would result in an amino acid substitution in the γ-kafirin signaling polypeptide: replacement of leucine by proline at position 10 of the signaling polypeptide in the 3C-71 mutant.
3.2.In vitro digestibility of endosperm proteins and endosperm type
Investigation ofin vitroprotein digestibility of endosperm proteins showed a significantly higher digestibility of kafirins in the T1progeny of all mutants carrying mutations within the target motifs 1C,2C and 3C,in comparison with donor cv.Avans (Fig.3).These differences are clearly visible in plant #2 from the progeny of the mutant 2C-2.1.1 (Fig.3A-lanes 14,15).The level of protein digestibility in this mutant was 85.6%,whereas the digestibility of the donor cv.Avans in this experiment was 66% (Tables 3,S2).Plants #2,#12,and #14 from the progeny of the mutant 2C-1.2.5 also showed sharp differences in protein digestibility from the donor cv.Avans (Fig.3B-lanes 13,14,3D-lanes 7,8,10,and 11),with digestibility reaching 88%-92% (Tables 3,S2).These values match the protein digestibility level of the RNAi mutant Avans-1/18 with a genetic construct for RNAi silencing of thegKAF1gene[36] (Fig.3A-lanes 4,5,3B-lane 5,3D-lane 5;Table S2).

Fig.3.Electrophoretic spectra of flour proteins from kernels of T1 sorghum mutants obtained by site-directed mutagenesis of k1C5 and gKAF1 genes.The spectra of mutants with significantly higher digestibility than the donor cultivar Avans(Tables 3,S2),are marked with asterisks.(A)1,17:protein molecular mass markers;2-4:donor cv.Avans;5-7:RNAi mutant Avans-1/18 with a genetic construct for RNAi silencing of the gKAF1 gene[36];8-10 and 11-13:plants#1(cas9+)and#3(cas9+),respectively,from the progeny of non-mutated regenerant 2C-1.1.3;14-16: plant #2 (cas9-) from the progeny of the mutant 2C-2.1.1;2,3,5,6,8,9,11,12,14,15: the flour treated with pepsin(two replications of digestion);4,7,10,13,16: the flour not treated with pepsin (control).(B) 1:protein molecular mass markers;2-4:donor cv.Avans;5,6: RNAi mutant Avans-1/18;7-9 and 10-12:plants#4(cas9+)and#5(cas9+),respectively,from the progeny of the mutant 1C-2.2.1;13-15:plant#2(cas9-)from the progeny of mutant 2C-1.2.5;2,3,5,7,8,10,11,13,14: the flour treated with pepsin (two replications of digestion);4,6,9,12,15: the flour not treated with pepsin (control).(C) 1: protein molecular mass markers;2-4: donor cv.Avans;5-7: RNAi mutant Avans-1/18;8-15: plants from the progeny of the mutant 3C-71 produced using a genetic construct to mutate gKAF1 gene: 8,9: plant #1 (cas9-);10-12: plant #4 (cas9 +);13-15: plant #6 (cas9 +);2,3,5,6,8,10,11,13,14: flour treated with pepsin (two replications of digestion);4,7,9,12,15:the flour not treated with pepsin (control).(D)1:protein molecular mass markers;2-4:donor cv.Avans;5,6:RNAi mutant Avans-1/18;7-9 and 10-12: plants #12 (cas9-) and #14 (cas9-),respectively,from the progeny of the mutant 2C-1.2.5;2,3,5,6,7,8,10,11: flour treated with pepsin (two replications of digestion);4,6,9,12:the flour not treated with pepsin(control).(E)Plant#4 from T2 progeny of the mutant 2C-1.2.9-2;1,2:flour treated with pepsin(two replications of digestion);3: flour not treated with pepsin (control).
A high level of kafirin digestibility was also observed in the plants from the T1generation of the mutant 1C-2.2.1 obtained by using the genetic construct with another target motif (1C).Plant#4 showed 85.8% protein digestibility,differing significantly from the donor cv.Avans (Fig.3B-lanes 7,8;Tables 3,S2).Given that sequencing of the nucleotide sequence encoding the signaling polypeptide of thek1C5gene did not reveal induced genetic changes,it is possible that the mutation responsible for the high digestibility of kafirins in this mutant is located not in thek1C5gene but in another gene of this gene family with a similar nucleotide sequence.
Plants #1 and #6 from the progeny of the mutant 3C-71 that had mutation in thegKAF1gene encoding the 28 kDa γ-kafirin gene also displayed improvedin vitroprotein digestibility (Table S2;Fig.3C-lanes 8,13,and 14) but this improvement was less visible(70% and 76%,respectively) than in the mutants with mutations in the 22 kDa α-kafirin-encoding gene.As can be seen from the electrophoresis pattern (Fig.3C),this mutation reduced the content of kafirin monomers in the 3C-71 mutant in comparison with donor cv.Avans,although flour weight and extraction conditions in the mutant and donor cultivars were the same.
In the T0generation of mutants 2C-1.2.5 and 2C-2.1.1,we observed kernels with reduced vitreous endosperm[29].However,in T1plants with high digestibility of kafirins from the progeny of these mutants,as well as from the progeny of mutant 3C-71,a majority of kernels had a thick vitreous layer(Fig.4).Perhaps they were caused by environmental factors,given that T1was grown in pots in the open air whereas T0was grown in the greenhouse under conditions of higher air humidity and weaker illumination spectrally differing from sunlight.The influence of environmental factors such as air humidity,air temperature,precipitation,and sunshine hours on kernel vitreousness is well known in durum wheat [42,43].

Fig.4.Cross sections of kernels of sorghum mutants,T1 generation,and donor cultivar.(A)2C-2.1.1;(B)2C-1.2.5;(C)3C-71;(D) Avans.Scale bars,1.0 mm.Fig.1D is taken from Gerashchenkov et al.[29].
The presence of thick vitreous endosperm distinguishes our mutants from many previously obtained[14,16-18,36,41]mutants with improved kafirin digestibility,including those produced by editing the nucleotide sequences of 22 kDa α-kafirin [26].Such a difference might be explained by the specific effect of mutations in thek1C5gene induced in our experiments.It is possible that some mutations cause conformational changes in α-kafirin that improve protein digestibility without affecting their interaction with starch granules characteristic of vitreous endosperm.Recently [15],screening of a large number of sorghum lines,including those with high digestibility,revealed the presence of alleles of α-kafirin-encoding genes that are not associated with the formation of floury endosperm but determine increased digestibility level.
Base substitution in the sequence of a single α-kafirin-encoding gene resulting in single amino acid substitution strongly affectedin vitroprotein digestibility contrary to Indel mutations in a βkafirin encoding gene [27],which had no effect on protein digestibility.The induction of mutations in the gene encoding αkafirin affected protein digestibility more than did a mutation in the gene encoding γ-kafirin.These differences must stem from the different contributions of different genes to the formation of protein bodies in endosperm cells.They invite a study of the ultrastructure of the protein bodies in these mutants.
In the plants with high protein digestibility from the progeny of the mutant 2C-2.1.1 and in some plants from the progeny of 2C-1.2.5 mutants,there was no amplification of thecas9-specific fragment or of the fragment of thebarmarker gene (Table 3),indicating the production of a mutant sorghum line with an editedk1C5gene sequence that does not carry a genetic construct that induced this mutation.Thus,the genetic construct we used,aimed at inducing mutations in the nucleotide sequence encoding the α-kafirin signal polypeptide,was effective for obtaining sorghum mutants with improved kafirin digestibility.
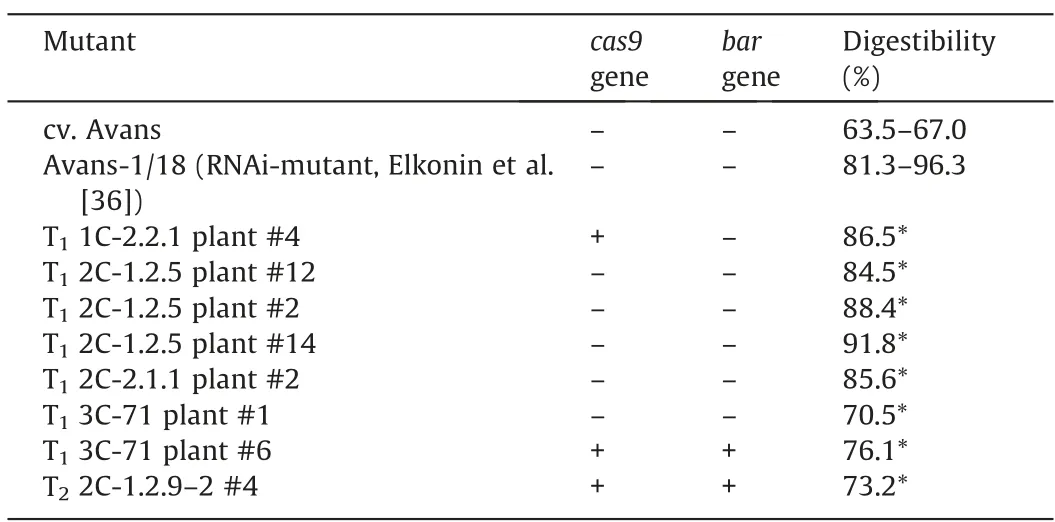
Table 3In vitro digestibility of flour proteins in some plants from the progeny of sorghum mutants obtained by using p1C,p2C and p3C vectors for site-directed mutagenesis of k1C5 and gKAF1 genes.
4.Conclusions
Using genome editing technology,we obtained grain sorghum mutants with improved kafirin digestibility that do not harbor the genetic construct that induced these mutations or the selectable genetic marker linked with this construct.These mutants are characterized by substitutions in the nucleotide sequence encoding the signaling polypeptide of 22 kDa α-and 28 kDa γ-kafirins.This is the first report of sorghum mutants with both improved digestibility of kafirins and hard vitreous endosperm,which should be of value in sorghum breeding.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
CRediT authorship contribution statement
Lev A.Elkonin:Conceptualization,Funding acquisition,Project administration,Methodology,Formal analysis.Grigoriy A.Gerashchenkov:Methodology,Investigation,Formal analysis.Natalie V.Borisenko:Investigation.Odyssey A.Kenzhegulov:Investigation.Saule Kh.Sarsenova:Investigation.Natalya A.Rozhnova:Investigation.Valery M.Panin:Investigation,Formal analysis.
Acknowledgments
This work was supported by the Russian Foundation for Basic Research (grant#19-016-00117).We thank Dr.Jochen Kumlehn from Leibniz Institute of Plant Genetics and Crop Plant Research(IPK) (Gatersleben,Germany) for providing the generic vector pSH121 and valuable advice for our work,and Prof.James C.Nelson from Kansas State University(USA)for editing our manuscript.
Appendix A.Supplementary data
Supplementary data for this article can be found online at https://doi.org/10.1016/j.cj.2023.02.005.
- The Crop Journal的其它文章
- Reversible protein phosphorylation,a central signaling hub to regulate carbohydrate metabolic networks
- Genetic and environmental control of rice tillering
- High-throughput phenotyping of plant leaf morphological,physiological,and biochemical traits on multiple scales using optical sensing
- The R2R3-MYB transcription factor GaPC controls petal coloration in cotton
- The photosensory function of Zmphot1 differs from that of Atphot1 due to the C-terminus of Zmphot1 during phototropic response
- Disruption of LEAF LESION MIMIC 4 affects ABA synthesis and ROS accumulation in rice

