Nickel-catalyzed reductive coupling reaction of monofluoroalkyl triflates with alkyl carboxylic acids toward the synthesis of α-alkyl-α-fluoro-alkylketones✩
Rui Wang, Jie Xu, Jin-Xiao Li, Bing-Bing Wu, Ruo-Xing Jin, Yu-Xiang Bi, Xi-Sheng Wang
Key Laboratory of Precision and Intelligent Chemistry, University of Science and Technology of China, Hefei 230026, China
Keywords:Ni-catalyzed Reductive coupling α-Fluoroketones α-Alkyl-α-fluoro-alkylketones Monofluoroalkyl triflates
ABSTRACT The synthesis methods of α-fluoro-arylketones were well-established through electrophilic/nucleophilic fluorination and transition metal catalyzed cross-coupling.However, due to the site selectivity and substrate restriction, only a few cases have been developed to afford α-alkyl-α-fluoro-alkylketones.Herein,we report a general and efficient method of preparing diverse α-alkyl-α-fluoro-alkylketones via nickelcatalyzed reductive coupling reaction of monofluoroalkyl triflates with low-cost industrial raw material alkyl carboxylic acids.These transformations demonstrate high efficiency, mild conditions, and excellent functional group compatibility.This strategy provides a general and efficient method for the synthesis of α-alkyl-α-fluoro-alkylketones.
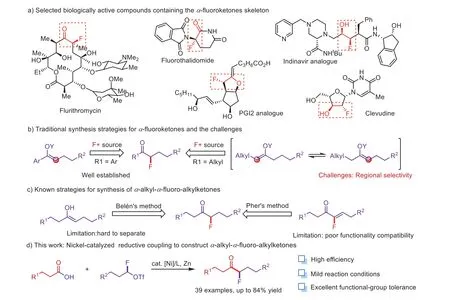
As a widely applied strategy to significantly modulate the lipophilic properties, electronic, metabolic stability and bioavailability of functional molecules [1–6], the introduction of fluorine atom into organic compounds has been found widespread applications in almost all aspects of the chemical industry, ranging from pharmaceuticals, agrochemicals and materials [7–13].However, due to the limited variety of monofluorinating agents [14–33], compared with the construction of trifluoromethyl compounds[34–46] and difluoroalkyl compounds [47–58], monofluoroalkylation especially the production ofα-fluoroketones have been less intensively investigated.
α-Fluoroketones as valuable building blocks are easily found in the pharmaceutical and bioactive molecules (Scheme 1a)[59,60].Accordingly, several methods for the synthesis ofα-fluoro-arylketones have been developedviadirect electrophilic/nucleophilic fluorination methods (Scheme 1b) [61–63].The synthesis ofα-fluoro-arylketones usingα-fluorocarbonyl compounds as building block through the transition metals catalyzed cross-coupling was another alter alternative choice.Recently,Qing [64,65], Shreeve [66], and Wu [67] have accomplished the synthesis ofα-fluoro-arylketonesviapalladium-catalyzedαfluorocarbonyl compounds coupling reactions with phenylboronic acids or bromobenzenes.Moreover, Negishi and Suzuki crosscoupling ofα-halo-α-fluoroketones have been shown to be effective strategies to obtainα-fluoro-arylketones [68,69].However,to the best of our knowledge, only a few cases of directly constructingα-alkyl-α-fluoro-alkylketones have been reported.Belén has accomplished iridium-catalysed tandem isomerisation/C–F bond formation from allylic alcohols and selectfluors to prepareα-fluorinated ketones as single constitutional isomers [70].Then Pher.G.Andersson has disclosed a straightforward method for the preparation of chiralα-alkyl-α-fluoro-alkylketones (Scheme 1c)[71].However due to the site selectivity and substrate restriction,it still remains huge challenge to synthesizeα-alkyl-α-fluoroalkylketones easily and efficiently.
Monofluoroalkyl triflates developed by our group could be used as a leveraging modular synthetic scaffold in divergent syntheses of aliphatic monofluorides [72].Meanwhile we have simplified the previous synthetic route in a one-step procedure by sequential addition of trifluoromethylsulfonic anhydride and Et3N·HF to the mixture of aldehydes and lutidine in dichloromethane.As expected, a series of alkyl aldehydes were smoothly transformed to the desired monofluoroalkyl triflates in a rapid and efficient fashion with high yields.To address the issues and challenges in synthesizingα-alkyl-α-fluoro-alkylketones, we envisaged monofluoroalkyl triflates as the monofluoroalkylating reagent for synthesis the desired productsvianickel-catalyzed reductive coupling reactions with cheap and readily available alkyl carboxylic acids.
Scheme 1.Synthesis of α-fluoroketones: Status quo & challenges.
Herein, we describe a novel and efficient nickel-catalyzed reductive coupling reaction of monofluoroalkyl triflates with alkyl carboxylic acids, giving a series ofα-alkyl-α-fluoro-alkylketones in moderate to high yields (Scheme 1d).The transformation demonstrated broad functional group compatibility and mild conditions.This method could provide a highly efficient and selective synthetic route toα-fluoroketones-containing pharmaceutical design and development.
Our initial studies commenced with 3-phenylpropionic acid (1a)as the pilot substrate, 1-fluoro-3-phenylpropyl trifluoromethanesulfonate (2a) as the mono-fluoroalkylating reagent and Boc2O (2.0 equiv.) was chosen as the activating agent to generate mixed anhydridein situfrom carboxylic acids (Table 1).First, using Ni(acac)2(10 mol%), bipyridineL1(15 mol%), Zn (3.0 equiv.) as the reductant, MgCl2(1.5 equiv.) as the additive and THF (0.2 mol/L) as the solvent, the desired product3was obtained in 14% yield (entry 1).Unfortunately, the yield of3could not been improved when polar solvents such as DMF, CH3CN and NMP were used (<10%, entries 2–4).Then a series of mixed solvents were tested (for details,see Supporting information).To our delight, THF and CH3CN component solvent could afford the desired product with 38% yield(entries 5–7).To further improved the yield, various nickel catalysts were investigated, which indicated that Ni(NO3)2·6H2O was the best choice to catalyze the reaction (entries 7–11).Considered the importance of the ligand for this transformation, ligands screening was then tested.The results showed that the conversion efficiency was promoted when the electron-donating substituents were introduced on the C4-position of bipyridine, the corresponding product3with 62% yield could be provided by usingL2(entry 12).WhenL3replacing the Me- on the ligand with more electrondonating group MeO- was investigated, the yield of3was enhanced to 67% (entry 13).L4with an electron-withdrawing substituent (4-CF3) would lead to a significantly lower yield (entry 14).And higher yield could not be obtained by using phenanthroline ligands (entries 15 and 16).We then reduced TBAI to 1.2 equiv., which could further increase the yield to 75% (entry 17).It was a critical factor that TBAI and 1-fluoro-3-phenylpropyl trifluoromethanesulfonate (2a) were added after the stirring of the other reactants for 30 min.Finally, theα-alkyl-α-fluoro-alkylketone3could be obtained with a separation yield of 80% by step feeding(entries 18 and 19).
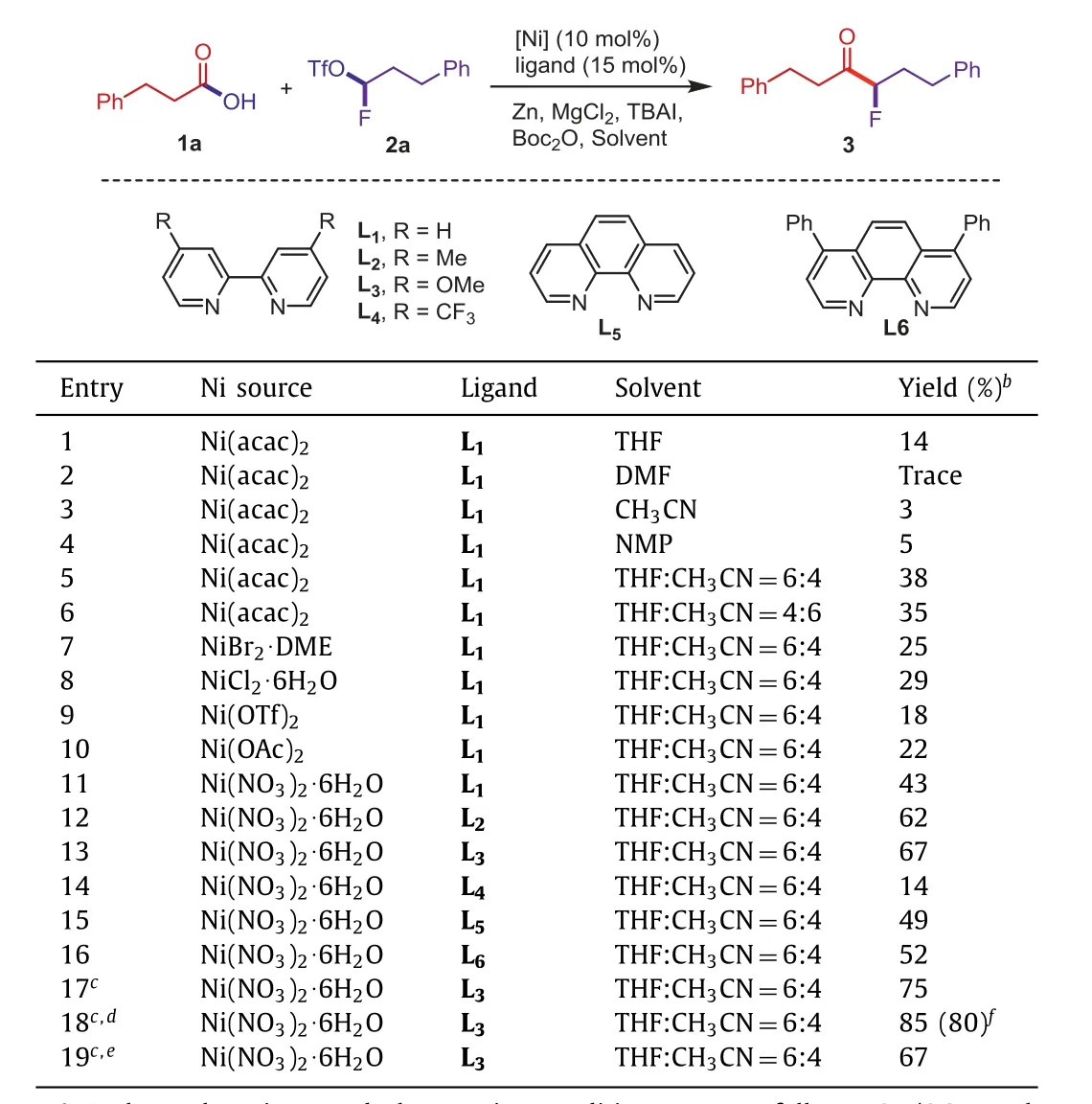
Table 1 Optimization of reaction conditions of nickel-catalyzed reductive coupling reaction of monofluoroalkyl triflates with alkyl carboxylic acids. a

Scheme 2.Scope of nickel-catalyzed reductive coupling reaction of monofluoroalkyl triflates with alkyl carboxylic acids.Reaction conditions were as follows: 1 (0.2 mmol), 2(0.3 mmol), [Ni] (10 mol%), L3 (15 mol%), TBAI (1.2 equiv.), Boc2O (0.4 mmol, 2.0 equiv.), MgCl2 (0.3 mmol, 1.5 equiv.), Zn (0.6 mmol, 3.0 equiv.), THF:CH3CN (0.6 mL: 0.4 mL),35°C, 12 h.
With the optimized conditions established for this nickelcatalyzed reductive mono-fluoroalkylation in hand, we next started to test the substrate tolerance of this transformation (Scheme 2)First, a series of alkyl carboxylic acids were well compatible with this catalytic system for cross-coupling with 1-fluoro-3-phenylpropyl trifluoromethanesulfonate (2a).Those carboxylicacids bearing different steric properties or long chains alkyl furnished the correspondingα-alkyl-α-fluoro-alkylketone with 50%-84%yields (4–7).It should be mentioned that a series of functional groups including alkene (8, 9), chloro (10) and ester (11) were well tolerated in this catalytic method.Remarkably, the alkyl halide,alkyl ester, and olefin can serve as versatile synthetic handles for further structural elaborations.This monofluoroalkylation proceeded well with 1-naphthyl (12) and 1-pyrenyl (13) substituted alkyl carboxylic acids and gave 64%-78% yields.The substituent effect on the phenyl rings was next examined, and good yields were observed with both electron-withdrawing and electron-donating groups, such as fluoro (14), chloro (15), bromo (16), methoxy (17),3,4-methylenedioxy (18), ester (19) and nitrile (20) were all well tolerated, which furnished the corresponding products in 43%-73%yields.To our delight, different heterocycles such as furan (21) and thiofuran (22) were also compatible and gave 78%-82% yields.
Next, we moved on to the scope of monofluoroalkylating reagents (Scheme 2).It should be noted that monofluoroalkyl triflates containing simple alkyl chains or cyclohexyl could also be successfully transformed into corresponding products with 71%-80% yields (23, 24, 38, 39).Notably, monofluoroalkyl triflates installed with a terminal chloro group on the alkyl chains were also applied onto different alkyl carboxylic acids and moderate yields ofα-alkyl-α-fluoro-alkylketones were accessed(25, 36, 37).To our delight, both electron-donating groups such as methoxy (28, 29) and electron-withdrawing groups such as fluoro(26), chloro (27), ester (30) on the aryl rings were well compatible with this transformation, affordingα-alkyl-α-fluoro-alkylketones with 60%-82% yields.Meanwhile, it was also suitable for the 1-naphthalene derived monofluoroalkyl triflates, which gave the de-
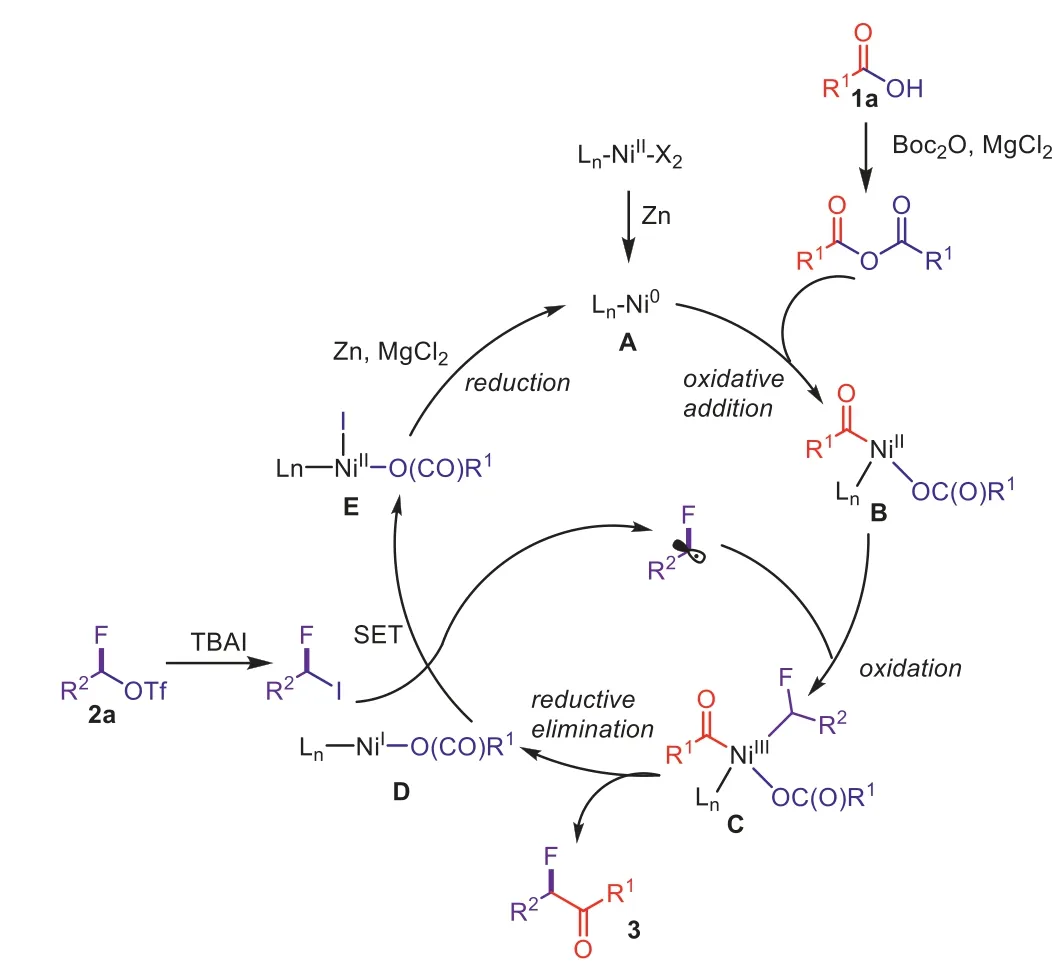
Scheme 3.Suggested catalytic cycle.
Referencessired products31–35in 56%-76% yields.And different alkyl carboxylic acids and monofluoroalkyl triflates can be coupled well under this strategy to obtain corresponding products (39–41).
Base on the previous reports [73–75], a propose a plausible mechanism have been depicted in Scheme 3.In the presence of Zn powder, Ni(NO3)2·6H2O can be reduced to Ni(0) species A to start the catalytic process.Acid anhydridein situformation from Boc2O and alkyl carboxylic acids followed by oxidative addition to
A, giving Ni(II) speciesB.Then radical oxidation to afford R1COLnNi(III)-Rf(intermediateC) and subsequent afford desiredα-alkylα-fluoro-alkylketones and R1(CO)O-LnNi(I) speciesDviareductive elimination.The alkyl radical and R1(CO)O-LnNi(II)-X can be generated by Ni(I) speciesDwhich undergo a SET process with Rf-I.Finally, intermediateEcan be reduced to Ni(0) speciesAby zinc powder in the presence of MgCl2to complete the cycle.
In summary, we have developed a practical and creationary strategy for the fast synthesis ofα-alkyl-α-fluoro-alkylketones.With alkyl carboxylic acids a low-cost industrial raw material, served as the acyl source, a general and efficient nickelcatalyzed reductive cross-coupling with monofluoroalkyl triflates has been established.this method demonstrated mild conditions and the excellent functional-group tolerance.α-Fluoroketonescontaining pharmaceutical design and development could be completed though this efficient strategy.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge the financial support of the National Key R&D Program of China (No.2021YFF0701700), the National Science Foundation of China (Nos.22271264, 21971228).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.cclet.2023.108490.
[1] J.Wang, M.Sánchez-Roselló.J.L.Aceña, et al., Chem.Rev.114 (2014)2432–2506.
[2] D.O’Hagan, Chem.Soc.Rev.37 (2008) 308–319.
[3] K.Mgller, C.Faeh, F.Diederich, Science 317 (2007) 1881–1886.
[4] S.Purser, P.R.Moore, S.Swallow, V.Gouverneur, Chem.Soc.Rev.37 (2008)320–330.
[5] W.K.Hagmann, J.Med.Chem.51 (2008) 4359–4369.
[6] C.Ni, J.Hu, Chem.Soc.Rev.45 (2016) 5441–5454.
[7] P.Kirsch, Modern Fluoroorganic Chemistry: Synthesis Reactivity, Applications,2nd Ed., Wiley-VCH, Weinheim, 2013.
[8] M.B.van Niel, I.Collins, M.S.Beer, et al., J.Med.Chem.42 (1999) 2087–2104.
[9] R.Berger, G.Resnati, P.Metrangolo, E.Weber, J.Hulliger, Chem.Soc.Rev.40(2011) 3496–3508.
[10] Y.Zhou, J.Wang, Z.Gu, et al., Chem.Rev.116 (2016) 422–518.
[11] N.A.Meanwell, J.Med.Chem.61 (2018) 5822–5880.
[12] F.Ibba, T.D.W.Claridge, V.Gouverneur, et al., J.Am.Chem.Soc.142 (2020)19731–19744.
[13] J.He, Z.Li, G.Dhawan, et al., Chin.Chem.Lett.34 (2023) 107578.
[14] Y.Zhao, B.Gao, C.Ni, J.Hu, Org.Lett.14 (2012) 6080–6083.
[15] C.Guo, X.Yue, F.L.Qing, Synthesis 11 (2010) 1837–1844.
[16] X.Zhang, W.M.Qiu, D.J.Burton, Tetrahedron Lett.40 (1999) 2681–2684.
[17] W.K.Tang, Z.W.Xu, J.Xu, et al., Org.Lett.21 (2019) 196–200.
[18] Y.Zhao, C.Ni, F.Jiang, et al., ACS Catal.3 (2013) 631–634.
[19] X.Jiang, S.Sakthivel, K.Kulbitski, G.Nisnevich, M.Gandelman, J.Am.Chem.Soc.136 (2014) 9548–9551.
[20] J.Hu, B.Gao, L.Li, C.Ni, J.Hu, Org.Lett.17 (2015) 3086–3089.
[21] Y.M.Su, G.S.Feng, Z.Y.Wang, Q.Lan, X.S.Wang, Angew.Chem.Int.Ed.54(2015) 6003–6007.
[22] L.An, Y.L.Xiao, Q.Q.Min, X.Zhang, Angew.Chem.Int.Ed.54 (2015)9079–9083.
[23] Y.Wu, H.R.Zhang, Y.X.Cao, Q.Lan, X.S.Wang, Org.Lett.18 (2016) 5564–5567.
[24] J.Sheng, H.Q.Ni, G.Liu, Y.Li, X.S.Wang, Org.Lett.19 (2017) 4480–4483.
[25] N.Y.Wu, X.H.Xu, F.L.Qing, ACS Catal.9 (2019) 5726–5731.
[26] H.Yin, J.Sheng, K.F.Zhang, et al., Chem.Commun.55 (2019) 7635–7638.
[27] N.A.Beare, J.F.Hartwig, J.Org.Chem.67 (2002) 541–555.
[28] E.Cosimi, J.Saadi, H.Wennemers, Org.Lett.18 (2016) 6014–6017.
[29] J.Sheng, H.Q.Ni, S.X.Ni, et al., Angew.Chem.Int.Ed.60 (2021) 15020–15027.
Six months had passed but the kingdom was not attacked. The king regretted his decision and told the grey goose and gander to bring home his daughter. Then the king understood that he had to be careful before taking actions based up the rumors which need not be true. The king was aware of his discretion3 before taking any decision.
[30] S.X.Ni, Y.L.Li, H.Q.Ni, et al., Chin.Chem.Lett.34 (2023) 107614.
[31] F.L.Qing, X.Y.Liu, J.A.Ma, et al., CCS Chem.4 (2022) 2518–2549.
[32] Y.Li, W.Liu, Z.Y.Liu, et al., CCS Chem.4 (2022) 2888–2896.
[33] Y.Hu, J.Luo, C.Lü, Chin.Chem.Lett.21 (2010) 151–154.
[34] Y.Z.Cheng, J.Ma, Y.Zhang, S.Y.Yu, Adv.Synth.Catal.356 (2014) 2859–2866.
[35] L.Li, Q.Y.Chen, Y.Guo, J.Fluorine Chem.167 (2014) 79–83.
[36] Y.Y.Yu, G.I.Georg, Adv.Synth.Catal.356 (2014) 3510–3518.
[37] B.Sahoo, J.L.Li, F.Glorius, Angew.Chem.Int.Ed.54 (2015) 11577–11580.
[38] R.Tomita, T.Koike, M.Akita, Angew.Chem.Int.Ed.54 (2015) 12923–12927.
[40] J.S.Lin, B.Tan, X.Y.Liu, J.Am.Chem.Soc.138 (2016) 9357–9360.
[41] H.Xiao, Z.Liu, H.Shen, et al., Chem 5 (2019) 940–949.
[42] H.Wang, Y.Xie, Y.Zhou, N.Cen, W.Chen, Chin.Chem.Lett.33 (2022) 221–224.
[43] B.B.Wu, J.Xu, K.J.Bian, Q.Gao, X.S.Wang, J.Am.Chem.Soc.144 (2022)6543–6550.
[44] H.Luo, Y.Zhao, D.Wang, M.Wang, Z.Shi, Green Synth.Catal.1 (2020)134–142.
[45] Y.Dai, F.Wang, S.Zhu, L.Chu, Chin.Chem.Lett.33 (2022) 4074–4078.
[46] N.Meng, Y.Lv, X.Zhao, W.Wei, Chin.Chem.Lett.32 (2021) 258–262.
[47] Z.Feng, Q.Q.Min, Y.L.Xiao, B.Zhang, X.Zhang, Angew.Chem.Int.Ed.53 (2014)1669–1673.
[48] M.K.Schwaebe, J.R.McCarthy, J.P.Whitten, Tetrahedron Lett.41 (2000)791–794.
[49] Y.J.Chen, L.K.Li, Y.Y.Ma, Z.P.Li, J.Org.Chem.84 (2019) 5328–5338.
[50] X.Huang, Y.Zhang, C.Zhang, et al., Angew.Chem.Int.Ed.58 (2019)5956–5961.
[51] H.Y.Zhao, X.Gao, S.Zhang, X.Zhang, Org.Lett.21 (2019) 1031–1036.
[52] H.Chen, J.Wang, J.Wu, Y.Kuang, F.Wu, J.Fluorine Chem.200 (2017) 41–46.
[53] J.X.Wang, J.J.Wu, H.Chen, S.W.Zhang, F.H.Wu, Chin.Chem.Lett.26 (2015)1381–1384.
[54] D.Wang, J.Wu, J.Huang, et al., Tetrahedron 73 (2017) 3478–3484.
[55] J.Liang, G.Huang, P.Peng, et al., Adv.Synth.Catal.360 (2018) 2221–2227.
[56] X.Chen, Z.Zhu, S.Liu, Y.H.Chen, X.Shen, Chin.Chem.Lett.33 (2022)2391–2396.
[57] W.Wu, Y.You, Z.Weng, Chin.Chem.Lett.33 (2022) 4517–4530.
[58] J.Li, W.Xi, S.Liu, et al., Chin.Chem.Lett.33 (2022) 3007–3011.
[59] S.D.Putnam, M.Castanheira, G.J.Moet, D.J.Farrell, R.N.Jones, Diagn.Microbiol.Infect.Dis.66 (2010) 393–401.
[60] P.A.Champagne, J.F.Paquin, Chem.Rev.115 (2015) 9073–9174.
[61] T.Ishimaru, N.Shibata, T.Horikawa, et al., Angew.Chem.Int.Ed.47 (2008)4157–4161.
[62] H.Teare, E.G.Robins, E.˚Arstad, S.K.Luthra, V.Gouverneur, Chem.Commun.(2007) 2330–2332.
[63] W.Zhang, J.Hu, Adv.Synth.Catal.352 (2010) 2799–2804.
[64] C.Guo, R.W.Wang, Y.Guo, F.L.Qing, J.Fluorine Chem.133 (2012) 86–96.
[65] C.Guo, R.W.Wang, F.L.Qing, J.Fluorine Chem.143 (2012) 135–142.
[66] Y.Guo, B.Twamley, J.M.Shreeve, Org.Biomol.Chem.7 (2009) 1716–1722.
[67] J.Zhou, X.Fang, T.L.Shao, X.Y.Yang, F.H.Wu, J.Fluorine Chem.191 (2016)54–62.
[68] Y.F.Liang, G.C.Fu, J.Am.Chem.Soc.136 (2014) 5520–5524.
[69] J.Liang, J.Han, J.Wu, et al., Org.Lett.21 (2019) 6844–6849.
[70] N.Ahlsten, B.Martín-Matute, Chem.Commun.47 (2011) 8331–8333.
[71] S.Ponra, J.Yang, S.Kerdphon, P.G.Andersson, Angew.Chem.Int.Ed.58 (2019)9282–9287.
[72] B.B.Wu, J.Xu, Q.Gao, et al., Angew.Chem.Int.Ed.61 (2022) e202208938.
[73] C.Zhao, X.Jia, X.Wang, H.Gong, J.Am.Chem.Soc.136 (2014) 17645–17651.
[74] J.B.Diccianni, T.Diao, Trends Chem.1 (2019) 830–844.
[75] C.S.Day, Á.Rentería-Gómez, S.J.Ton, et al., Nat.Catal.6 (2023) 244–253.
 Chinese Chemical Letters2023年10期
Chinese Chemical Letters2023年10期
- Chinese Chemical Letters的其它文章
- Tribute text in memoriam of James N.Seiber (1940–2023)
- Recent advances in MXenes-based glucose biosensors
- Oxidative cyclopalladation triggers the hydroalkylation of alkynes✩
- An integrated supramolecular fungicide nanoplatform based on pH-sensitive metal–organic frameworks
- Probing the effect of nitrate anion in CAN: An additional opportunity to reduce the catalyst loading for aerobic oxidations✩
- A highly selective fluorescent probe for visualizing dry eye disease-associated viscosity variations
