Albumin-bound kynurenic acid is an appropriate endogenous biomarker for assessment of the renal tubular OATs-MRP4 channel
Ynrong M ,Fenglin Rn ,Mingyn Xin ,Xueyn Gou ,Xinyi Wng ,Xinn Wu ,,*
a The First School of Clinical Medicine, Lanzhou University, Lanzhou, 730000, China
b School of Pharmacy, Lanzhou University, Lanzhou, 730000, China
Keywords:Transporter Biomarker Kynurenic acid Renal tubular excretion
ABSTRACT Renal tubular secretion mediated by organic anion transporters (OATs) and the multidrug resistanceassociated protein 4 (MRP4) is an important means of drug and toxin excretion.Unfortunately,there are no biomarkers to evaluate their function.The aim of this study was to identify and characterize an endogenous biomarker of the renal tubular OATs-MRP4 channel.Twenty-six uremic toxins were selected as candidate compounds,of which kynurenic acid was identified as a potential biomarker by assessing the protein-binding ratio and the uptake in OAT1-,OAT3-,and MRP4-overexpressing cell lines.OAT1/3 and MRP4 mediated the transcellular vectorial transport of kynurenic acid in vitro.Serum kynurenic acid concentration was dramatically increased in rats treated with a rat OAT1/3 (rOAT1/3) inhibitor and in rOAT1/3 double knockout(rOAT1/3-/-)rats,and the renal concentrations were markedly elevated by the rat MRP4(rMRP4)inhibitor.Kynurenic acid was not filtered at the glomerulus(99%of albumin binding),and was specifically secreted in renal tubules through the OAT1/3-MRP4 channel with an appropriate affinity(Km)(496.7 μM and 382.2 μM for OAT1 and OAT3,respectively)and renal clearance half-life(t1/2)in vivo(3.7±0.7 h).There is a strong correlation in area under the plasma drug concentration-time curve(AUC0-t) between cefmetazole and kynurenic acid,but not with creatinine,after inhibition of rOATs.In addition,the phase of increased kynurenic acid level is earlier than that of creatinine in acute kidney injury process.These results suggest that albumin-bound kynurenic acid is an appropriate endogenous biomarker for adjusting the dosage of drugs secreted by this channel or predicting kidney injury.
1.Introduction
The kidney ensures the removal of numerous drugs and their metabolites from the body.Dysregulation or decompensation of kidney function directly affect drug pharmacokinetics,pharmacodynamics,and toxicity.Thus,it is essential to assess renal capacity for guiding drug treatment in patients with renal malfunction.Serum creatinine and creatinine clearance are routinely used as measurements of renal capacity and for making dosage adjustments for drugs excreted by the kidney.Unfortunately,however,increasing evidences indicate that the correlation between elevated serum creatinine and the deterioration of kidney function or reduction of drug excretion is not absolute [1-5].Together,creatinine as a biomarker for adjusting drug dosage has limitations,resulting from differences in the excretion process between drugs and creatinine.
Glomerular filtration and tubular secretion constitute the major components of the renal excretion of drugs.About 32% of the top 200 prescribed drugs are predominantly cleared by the kidney,of which more than 90% are actively secreted by renal tubular transporters [6].Wang and Kestenbaum [7] pointed out that proximal tubular secretory clearance was a neglected partner of kidney function.So far,it is lack of effective method to evaluate the secretory function of renal tubular transporters.Endogenous biomarker is a valuable approach to facilitate transport activity and drug interaction risk assessment for a number of transporters[8,9].Therefore,it is important to identify biomarkers for assessing the secretory function of renal tubular transporters and accurately predicting renal excretion of drugs.
Renal tubular secretion is mediated by a variety of transporters expressed in renal tubular epithelial cells,with uptake transporters in the basolateral membrane and efflux transporters in the apical membrane constituting these vector“channels”[6,10].The organic anion transport channel is composed of organic anion transporters(OATs),with OAT1 and OAT3 expressed in the basolateral membrane and multidrug resistance-associated proteins (MRPs)expressed in the apical membrane.OATs-MRPs channel mediates the vectorial transport process of substrates that include nonsteroidal anti-inflammatory agents,antiviral drugs,antibiotics,methotrexate,and toxins [11-14].Therefore,the ability to assess the function of OATs-MRPs channel by specific and sensitive biomarkers is crucial to accurately predict the renal excretion of organic anion drugs.Although OAT1 and OAT3 have different affinities for different substrates,they share an overlapping spectrum of substrate specificities,and in many cases,when one transporter is dysfunctional,the function of the other is increased compensatively,so that the excretion of its substrates remains unchanged.As a result,the common substrates of OAT1 and OAT3 may be potential biomarkers for evaluating OATs.In recent years,some endogenous substrates of OATs have been uncovered,such as 6β-hydroxycortisol and hippuric acid [15,16].Unfortunately,6β-hydroxycortisol is excreted into the urine by the multidrug and toxin extrusion protein(MATE)1 and 2-K after uptake by OAT1/3,but not by MRPs,and hippuric acid has a protein-binding ratio of 48%,which is largely filtered by the glomerulus [17-19].Thus,an endogenous biomarker for evaluating the capacity of the OATs-MRPs channel has not yet been identified.
When the kidneys are injured,decreases in renal tubular function and glomerular filtration are independent of each other,with changes in organic anion transport often being greater than that of glomerular filtration in kidney disease [20,21],so endogenous compounds that are both filtered by the glomerulus and secreted by the renal tubules cannot be used to evaluate glomerular filtration or renal tubular secretion.The ideal endogenous biomarker for evaluating the function of the transport channel should not be filtered by the glomerulus,but should be transported specifically through this channel.Not being filtered by the glomerulus requires a molecular weight of the compound greater than 50 kDa,but these substances are typically proteins rather than substrates for transporter protein,which brings great obstacles to the discovery of biomarker of transport channels.Metabolomics technology is a common method for identifying endogenous substrates of transporters by using transporter-knockout models or specific transporter inhibitors [22].Obviously,small molecular substances(<1 kDa)identified by metabolomics typically undergo glomerular filtration and cannot be used as biomarkers for transport channels.Some studies have found that protein-bound drugs/endogenous compounds are not filtered by the glomerulus,and renal excretion is governed by tubular transporters [23,24].Therefore,screening protein-bound endogenous compounds holds the greatest potential for identifying specific biomarkers for renal tubular transport channels (Fig.1).
Uremic toxins(UTs)are a general term for nitrogen wastes that cannot be effectively eliminated from the body after renal injury.About 130 species have been found,of which more than 30 are protein-bound in the blood(The EUTox Work Group,https://www.uremic-toxins.org/).In vitro studies have found that many UTs are substrates of OATs,but they mainly exist in protein-bound forms in vivo,such as indole-3-acetic acid,3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF),p-cresol sulfate,and 3-indoxyl sulfate [25-29].These results support that the hypothesis that protein-bound UTs can serve as biomarkers for OATs-MRPs.Quantitative polymerase chain reaction (qPCR) analysis has shown that the expression of MRP4 in renal cortex is five times higher than that of MRP2,and the affinity (Km) of aminohippuric acid for MRP2 (Km= 2 mM) is lower than that for MRP4(Km=160 μM)[30].Therefore,our goal was to identify endogenous biomarker(s)that could be used to evaluate the capacity of OAT1/3-MRP4 channel by screening protein-bound UTs,and to provide a basis for predicting drug excretion mediated by this channel.
2.Materials and methods
2.1.Chemicals and materials
UTs and reference materials were purchased from the companies listed in Table S1.Human serum albumin(HSA)with a purity of 96%-98%was obtained from Solarbio Science&Technology Co.,Ltd.(Beijing,China).Ultrafiltration devices (Centrifree®Ultrafiltration membrane,molecular cutoff of 30 kDa)were obtained from EMD Millipore (Billerica,MA,USA).Cell culture dishes,6-and 12-well plates,and 6.5 mm transwell with 0.4-μm pores were obtained from Corning Inc.(Oneonta,NY,USA).
2.2.Animals

Fig.1.Renal excretion of free and albumin-bound compounds and screening strategies for biomarkers for the organic anion transporters (OATs)-multidrug resistance-associated protein 4 (MRP4) channel.ATP: adenosine triphosphate.
Male Sprague-Dawley rats were obtained from the experimental animal center of Lanzhou Institute of Biological Products Co.,Ltd.(Lanzhou,China).Rat OAT1 (rOAT1) and rOAT3 double knockout(rOAT1/3-/-),and rat MRP4(rMRP4)knockout(rMRP4-/-)Sprague-Dawley rats were constructed by Cyagen Biosciences Inc.(Suzhou,China).All rats were housed in the specific pathogen-free animal room at Lanzhou University and maintained under light-dark cycles at 22-25°C for 12 h with free access to food and water.All animal experimental protocols and human blood collection and use were authorized by the Ethics Committee of the First Hospital of Lanzhou University(Approval No.:LDYYLL2018-32).
2.3.Cell culture
Human embryonic kidney 293T (HEK293T) cells and Madin-Darby canine kidney (MDCK) cells were obtained from the National Collection of Authenticated Cell Cultures (Shanghai,China)and were cultured at 37°C under 5% CO2in Dulbecco’s modified Eagle’s medium (Sigma-Aldrich,St Louis,MO,USA) supplemented with 10% (V/V) fetal bovine serum (Gibco,Grand Island,NY,USA)and 1% (V/V) penicillin-streptomycin (Gibco).Lentivirus vectors were constructed by Shanghai Genechem Co.,Ltd.(Shanghai,China) and separately transfected into HEK293T or MDCK cells.HEK293T or MDCK cells transfected with vector alone were used to obtain the background activity (HEK293-MOCK or MDCK-MOCK).
2.4.Sample preparation and liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis
The method of sample pretreatment was as follows.Rat renal tissue was washed with saline to remove blood and other contaminants and then dried with absorbent paper.Renal tissue(300 mg) was added to 600 μL of saline and homogenized via TissueLyser,followed by centrifugation at 14,000 r/min at 4°C for 20 min to prepare the renal tissue extract.Whole blood samples of rats and humans were centrifuged at 1,200 r/min,4°C for 12 min immediately after collection for separation of serum from blood cells.50 μL of serum,renal tissue homogenate,or cell samples were added to 50 μL of internal standard solution and 100 μL of acetonitrile,and were vortex-mixed for 40 s and centrifuged at 14,000 r/min for 15 min.The internal standard solution included 10,680 ng/mL d3-creatinine (cation mode),22,230 ng/mL d5-hippuric acid,400 ng/mL d3-CMPF,750 ng/mL d5-kynurenic acid,and 780 ng/mL d7-N-(cinnamoyl)glycine,and those internal standard concentrations were 100 ng/mL when analyzing the cell samples.
UTs were determined by LC-MS/MS (Agilent Technology,Santa Clara,CA,USA)according to the methods described previously[31].In brief,an Agilent Hilic column (4.6 mm × 100 mm,2.7 μm) was used for chromatographic separation and the mobile phase consisted of water containing 0.1% (V/V) formic acid and acetonitrile(60%:40%,V/V) at a flow rate of 0.6 mL/min.The column temperature was 30°C,and the injection volume was 10 μL.The mass spectrometer was operated in positive and negative electrospray ionization mode,independently,and absolute quantification was carried out by multiple reaction monitoring (Table S2).
2.5.Determination of the protein-binding ratio of UTs
The determination of the protein-binding ratio of UTs was performed as described previously [31].Briefly,400 μL of serum samples were centrifuged at 3,500gfor 30 min at 37°C in ultrafiltration tubes for collecting the filtrate.50 μL of filtrate and fresh serum were added to 50 μL of internal standard and 100 μL of acetonitrile,and after vortex-mixing for 40 s and centrifugation at 14,000 r/min for 10 min,the supernatant was assayed for the free and total concentration of UTs,respectively.
To assess the protein-binding ratio of UTs in vitro,UT solution was mixed with 4% (V/V) HSA to obtain a final concentration of about 100 μM and incubated at 37°C for 2 h.After incubation,100 μL of sample was taken for determination of the total concentration,and another 300 μL was centrifugated at 14,000 r/min for 10 min at 37°C in an ultrafiltration tube for determination of the free concentration.The protein-binding ratio was calculated as follows: Protein-binding ratio =(total concentration -free concentration)/total concentration × 100%.
2.6.Cellular uptake and transport of UTs
2.6.1.Uptake of UTs
HEK293T-MOCK and HEK293T-OAT1,-OAT3 and -MRP4 cells were seeded in 12-well plates (5 × 105cells/mL,1 mL) for 24 h,after which the medium was replaced with buffer (118 mM NaCl,23.8 mM NaHCO3,4.8 mM KCl,1.0 mM KH2PO4,1.2 mM MgSO4,12.5 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid(HEPES),5 mM glucose,and 1.5 mM CaCl2,pH 7.4,37°C) [16]containing 100 μM UT to incubate for 30 min.After incubation,the cells were washed twice with buffer,and 200 μL of H2O was added for collecting cell samples.The protein was quantified with a protein quantification kit (Thermo Fisher Scientific Inc.,Waltham,MA,USA) and the concentrations of UTs were determined by LCMS/MS.In the process of specificity determination,UT uptake was investigated in organic cation transporter 2 (OCT2)-,multidrug and toxin extrusion protein (MATE) 1-,MATE2-K-,OAT2-,organic anion transporting polypeptide 4C1 (OATP4C1)-,P-glycoprotein(P-gp)-,peptide transporter 2(PEPT2)-,urate transporter 1(URAT1)-,and OAT4-overexpressing cell lines.Uptake buffer for MRP4-and P-gp-overexpressing cell lines contained 4 mM ATP,and the pH of uptake buffer in MATE1-and MATE2-K-overexpressing cell lines was adjusted to 8.0.
2.6.2.Uptake of UTs at 4°C and 37°C
Cells were seeded in 12-well plates(5×105cells/mL,1 mL)for 24 h,after which the medium was replaced with buffer containing 100 μM UT at 37°C to uptake for 30 min.Cells were placed in a 4°C environment for 30 min,then buffer containing UT was added at 4°C to uptake for 30 min.
2.6.3.Inhibition of uptake and efflux of UTs
HEK293T-MOCK and HEK293T-OAT1 and -OAT3 cells were seeded in 12-well plates(5×105cells/mL,inoculation volume was 1 mL) for 24 h,after which the medium was replaced with buffer containing 100 μM UT and 0,0.5,1,5,10,50,100 and 500 μM probenecid(PROB)to uptake for 30 min.Uptake was terminated by adding 1 mL/well of ice-cold buffer,followed by washing twice.Double-control or OAT1/3 and MRP4 double-transfected MDCK cells were seeded in 12-well plates(5×105cells/mL,1 mL)for 24 h,after which 100 μM UT and MK-571 (100 and 500 μM) in buffer were added and the incubation continued for 30 min.Half-maximal inhibitory concentration (IC50) of PROB inhibited OATs-mediated UTs uptake was calculated.
2.6.4.Transport kinetics of UTs
HEK293T-MOCK and HEK293T-OAT1/3 cells were incubated into 12-well plates (5 × 105cells/mL,1 mL) for 24 h.Uptake was initiated by adding 100 μM UT buffer and performed for 2,5,10,20,and 30 min,after which linear uptake times were determined.To obtain the uptake affinity and maximum transport rate of UTs,liner uptake time was selected as a representative of uptake rate and used to measure concentration-dependent uptake in HEK293TMOCK and HEK293T-OAT1/3 cells,the concentration of UTs was determined by LC-MS/MS,and theKmand maximum uptake rate(vmax) of UT uptake by OAT1/3 were calculated according to the Michaelis-Menten equation.
2.7.Transcellular transport study
Double-control and OAT1/3-MRP4 double-overexpressing MDCK cells (5 × 105cells/mL,500 μL) were seeded in 12-well Transwell inserts (6.5 mm,pore size 0.4 μM),and the transmembrane resistance was measured after culturing for 3-5 days to ensure the integrity of the monolayer.For A-B transcellular transport,500 μL buffer(containing 100 μM UT)and 700 μL buffer were added to the apical compartment and the basolateral compartment,respectively,and after 2 h the buffer in the basolateral compartment was collected for the determination of UTs transport.For B-A transcellular transport,700 μL of buffer (containing 100 μM UT or inhibitor of OATs and MRP4)and 500 μL of buffer were added to the basolateral compartment and apical compartment,respectively,and after 2 h apical compartment buffer was collected.
2.8.Animal experiments
2.8.1.rOATs inhibition experiment
Rats were randomly divided into control,PROB (30 mg/kg),PROB (60 mg/kg),and PROB (120 mg/kg) (n=7),and were orally given normal saline,30 mg/kg PROB,60 mg/kg PROB,and 120 mg/kg PROB for 7 days,respectively.Blood and renal tissues were collected for the determination of UTs by LC-MS/MS,and renal function-related indexes in serum were measured by the automatic biochemical analyzer.Some kidney tissues were fixed in a 10%(V/V)formaldehyde solution for hematoxylin and eosin (H&E) staining.The dosing scheme in the urine excretion experiment was the same as above.Urine samples were collected for 12 h and the cumulative urinary excretion of UTs was calculated.
2.8.2.rMRP4 inhibition experiment
Rats were randomly divided into control,MK-571 (5 mg/kg),MK-571 (10 mg/kg),and MK-571 (20 mg/kg) (n=6),and were orally treated with normal saline,5 mg/kg MK-571,10 mg/kg MK-571,and 20 mg/kg MK-571 for 7 days,respectively.An automatic biochemical analyzer was used to determine renal function-related indexes,and LC-MS/MS was used to determine the concentration of UTs in serum and renal tissue.Renal tissue samples were fixed in 10% (V/V) formaldehyde solution for H&E staining.Urine samples were collected to determine the cumulative urinary excretions of UTs.
2.8.3.rOAT1/3-/-and rMRP4-/-rat experiment
rOAT1/3-/-rats and rMRP4-/-rats were constructed by clustered regularly interspaced short palindromic repeats (CRISPR)/CRISPR-associated protein 9(Cas9)technology.The schemes for the generation of rOAT1/3-/-(n=7)and rMRP4-/-(n=6)are shown in Fig.S1.After obtaining homozygous knockout rats as confirmed sequencing and polymerase chain reaction (PCR),the messenger RNA(mRNA)levels of rOAT1/3 and rMRP4 were measured,and the serum levels,renal tissue concentration,and 12-h cumulative urinary excretion of UTs were determined.
2.8.4.Correlation between biomarkers and cefmetazole
Rats were randomly divided into control,PROB(60 mg/kg),and PROB(120 mg/kg)(n=5),and were administered normal saline,60 mg/kg PROB,and 120 mg/kg PROB for 7 days,respectively,and then 100 mg/kg cefmetazole was administered through the tail vein.Blood samples were collected from the femoral artery at 5,10,15,30,60,120 and 240 min.The concentration of cefmetazole in plasma was determined by high-performance liquid chromatography(HPLC),and concentrations of plasma creatinine and kynurenic acid were determined by LC-MS/MS to determine the correlation of area under the plasma drug concentration-time curve(AUC0-t)between the kynurenic acid and creatinine with cefmetazole.To further dissect the role of kynurenic acid in the prediction and dosage adjustment of cefmetazole under the abnormal renal tubular OATMRP4 channel,wide-type (WT) rats (WT group) were intraperitoneally injected with cefmetazole 100 mg/kg for 7 consecutive days,and rOAT1/3-/-rats were divided to knockout (KO) and KO dose adjustment (KO-D) group (n= 5) and were intraperitoneally administered 100 mg/kg and 60 mg/kg cefmetazole for 7 consecutive days.Blood samples were collected from the femoral artery at 5,10,15,30,60,120,240,360,and 480 min.
2.8.5.The role of biomarkers for evaluating acute renal tubular injury
Rats were randomly divided into control and cisplatin treatment group (n=6).All rats except the control group were given intraperitoneally cisplatin (1 mg/kg) for 1,2,3,5,and 7 days.Blood samples were collected for determining the concentration of creatinine,cystatin C,and kynurenic acid.
2.9.PCR and qPCR
2.9.1.PCR
Neonatal rats aged 10-14 days were numbered via the amputation method,and 2-5 mm tails were collected for DNA determination.DNA was extracted according to the instructions of the animal tissue DNA isolation kit (FOREGENE,Chengdu,China),and the concentration was detected by a nucleic acid detector and adjusted to 60-120 ng/μL.After the PCR product was cooled to room temperature,1.5% agarose gel electrophoresis was used to determine the genotype.The relevant primer information is shown in Table S3.For genotyping of rOAT1/3-/-rats,the 370 bp band of homozygous,the 613 bp band of WT,and the 370 bp and 613 bp bands of heterozygous rats were selected.For rMRP4-/-rat typing,the 890 bp band of homozygous,the 763 bp band of WT,and the 890 bp and 763 bp bands of heterozygous rats were used.
2.9.2.qPCR
RNA was extracted from rat renal tissues according to the Easte®Super total RNA extraction kit(Promega,Shanghai,China),and the concentration and purity(RNA concentration 1.5-3.0 μg/mg,A260/A280 1.9-2.1,A260/A230 2.0-2.5) were determined and adjusted to 50-150 ng/μL.RNA integrity was detected by 1.5% agarose gel electrophoresis.The relevant primer information is shown in Table S3.The RNA was reverse-transcribed into the corresponding complementary DNA (cDNA) according to the RevertAid first-strand cDNA synthesis kit (Thermo Fisher Scientific Inc.),and the PCR reaction system was used according to UltraSYBR Mixture instructions (Cowin Biotech Co.,Ltd.,Beijing,China),with the reaction carried out on a Bio-Rad CFX96 fluorescence quantitative PCR instrument (Bio-Rad,Hercules,CA,USA).
2.10.Renal elimination half-life (t1/2) of potential biomarker
2.10.1.The t1/2 of kynurenic acid
Rats were randomly divided into control,PROB,and MK-571 group,and were intravenously administered d5-kynurenic acid (2 mg/kg),d5-kynurenic acid (2 mg/kg) +PROB (60 mg/kg),and d5-kynurenic acid (2 mg/kg) +MK-571 (20 mg/kg) (n=6),respectively.Urine samples were collected at 0-2,2-4,4-6,6-8,8-10,and 10-12 h for determination of cumulative urinary excretion of d5-kynurenic acid,d4-xanthurenic acid,and kynurenic acid.
2.10.2.The t1/2 of CMPF
Rats were randomly divided into control,PROB,and MK-571 groups,and were intravenously given d3-CMPF (2 mg/kg),d3-CMPF (2 mg/kg) +PROB (60 mg/kg),and d3-CMPF (2 mg/kg) +MK-571 (20 mg/kg) (n=6),respectively.Urine samples were collected at 0-2,2-4,4-6,6-8,8-10,and 10-12 h to determine the cumulative urinary excretion of d3-CMPF and CMPF.
2.11.Correlation analysis of serum indexes with kynurenic acid and CMPF
A total of 824 samples were obtained from blood samples collected in Hospital of the Department of Nephrology,the First Hospital of Lanzhou University(Lanzhou,China)from June 2019 to December 2019.The correlation between kynurenic acid and CMPF with albumin,globulin,total protein (TP),aspartate aminotransferase (AST),alanine aminotransferase (ALT),total bilirubin (TBIL),alkaline phosphatase(ALP),γ-glutamyl transferase(GGT),total bile acid (TBA),glucose,total cholesterol (TC),triglyceride (TG),highdensity lipoprotein (HDL-C),low-density lipoprotein (LDL-C),lactate dehydrogenase (LDH),α-hydroxybutyrate dehydrogenase(α-HBDH),creatine kinase (CK),homocysteine,creatinine,cystatin C,and β2-microglobulin in serum was evaluated.
2.12.Serum concentration of kynurenic acid in patients
In intensive care unit(ICU)patients,we collected serum from 4 patients (1-4) with acute kidney injury and 5 patients (5-9)without acute kidney injury during treatment.In addition,blood samples from hospitalized patients with renal abnormalities were obtained,and the concentrations of kynurenic acid and creatinine were determined.
2.13.Statistical analysis
Pharmacokinetic data were analyzed by Drug and Statistics of Windows 2.0 (DAS 2.0) statistical software.Data are expressed as mean ± standard deviation (SD),and statistical analyses were carried out by using one-way analysis of variance (ANOVA) and Student'st-test.AP<0.05 was considered to be statistically significant.
3.Results
3.1.Screening UTs from database and literature
Twenty-six UTs found at the micromole level in humans,including symmetric dimethylarginine (SDMA),S-adenosyl-L-homocysteine,pseudouridine,orotic acid,1-methyl-inosine,N,N-dimethylguanosine,N-acetyl-L-arginine,N-acetylcytidine,creatinine,1-methyl-5-carboxylamide-2-pyridone (MCAP),indole-3-acetic acid,3-indoxyl sulfate,3-indoxyl-β-D-glucopyranoside,D-kynurenine,kynurenic acid,CMPF,3-(3,4-dihydroxyphenyl)-L-alanine,p-cresol glucuronide,p-cresol sulfate,phenyl-β-D-glucuronide,4-ethylphenyl sulfate,3-deoxyglucosone,hippuric acid,DL-homocysteine,N-(1-carboxymethyl)-L-lysine,andN-(cinnamoyl)glycine,were selected from the Uremic Solutes Database (https://database.uremic-toxins.org/home.php)and literatures[26,28,32,33].
3.2.Evaluation of protein-binding ratio of UTs
The protein-binding ratio of UTs in vivo and in vitro is shown in Table S4.The protein-binding ratios of SDMA,S-adenosyl-L-homocysteine,pseudouridine,orotic acid,1-methyl-inosine,N,Ndimethylguanosine,N-acetyl-L-arginine,N-acetylcytidine,and creatinine in rats were less than 24% and belonged to the lowmolecular-weight UTs class (free UTs),where the protein-binding ratio with HSA in vitro was below 50%.In rats,protein-binding ratios of indole-3-acetic acid,3-(3,4-dihydroxyphenyl)-L-alanine,p-cresol glucuronide,phenyl-β-D-glucuronide,3-deoxyglucosone,hippuric acid,DL-homocysteine,andN-(1-carboxymethyl)-L-lysine were <70%,and 3-indoxyl sulfate,3-indoxyl-β-D-glucopyranoside,D-kynurenine,kynurenic acid,CMPF,p-cresol sulfate,and 4-ethylphenyl sulfate were >90%,which were consistent with our previous studies in human [31].The protein-binding ratios of UTs,not including D-kynurenine,were similar in vivo and in vitro.
3.3.Characterization of OATs and MRP4 substrates from UTs in vitro
Uptake results of free UTs in OAT1-,OAT3-,and MRP4-overexpressing cells are shown in Fig.S2.The uptake of 1-methyl-inosine in HEK293T-OAT3 cells was significantly higher than that in control cells (P<0.05),indicating that 1-methyl-inosine is a substrate of OAT3.The uptake ofN-acetylcytidine was significantly increased in OAT1-and OAT3-overexpressing cells(P<0.05),indicating thatN-acetylcytidine is a common substrate of OAT1 and OAT3.The uptake ofN-acetyl-L-arginine was significantly decreased in MRP4-overexpressing cells,indicating thatNacetyl-L-arginine is a substrate of MRP4.
The uptake results of protein-bound UTs in OAT1-,OAT3-,and MRP4-overexpressing cells are shown in Fig.2.The uptake of free and HSA-bound kynurenic acid,CMPF,andN-(cinnamoyl)glycine was dramatically increased in OAT1-and OAT3-overexpressing cell lines (P<0.01) compared to that in control cells,and their intracellular accumulation was significantly decreased in MRP4-overexpressing cell lines (P<0.05).The uptake of free and HSAbound indole-3-acetic acid was significantly increased in OAT1-overexpressing cells,and indole-3-acetic acid was significantly decreased in MRP4-overexpressing cells (P<0.05).The uptake of free 3-indoxyl sulfate and 4-ethylphenyl sulfate was substantially elevated in OAT1-and OAT3-overexpressing cells,but there was no significant change in the uptake of HSA binding form between OAT1-and OAT3-overexpressing cells and control cells.Uptake of free and HSA-bound 3-indoxyl-β-D-glucopyranoside andp-cresol glucuronide was markedly increased in OAT3-overexpressing cells,and D-kynurenine was markedly increased in OAT1-overexpressing cells.The uptake of free and HSA-boundp-cresol sulfate was significantly increased in OAT1-overexpressing cells,and freepcresol sulfate was significantly increased in OAT3-overexpressing cells.The uptake of free and HSA-bound phenyl-β-D-glucuronide was significantly increased in OAT3-overexpressing cells.The uptake of free and HSA-bound hippuric acid was dramatically increased in OAT1-and OAT3-overexpressing cells (P<0.01) and slightly decreased in MRP4-overexpressing cells (P>0.05).3-indoxyl-β-D-glucopyranoside,p-cresol glucuronide,and phenyl-β-D-glucuronide were not common substrates of OAT1 and OAT3.Free 3-indoxyl sulfate and 4-ethylphenyl sulfate,but not HSA binding,were common substrates of OAT1 and OAT3.Although hippuric acid transport was mediated by OATs and MRP4,the proteinbinding ratio in humans was less than 50%.Therefore,the amount of hippuric acid in urine was both filtered and secreted,which could not specifically reflect the function of OATs-MRP4.Consistently,when rOATs were inhibited,there was no significant change in serum levels of hippuric acid due to the compensatory increase in glomerular filtration (Fig.S3).Collectively,CMPF,kynurenic acid,andN-(cinnamoyl)glycine are substrates of OATs and MRP4,and exist a high degree of albumin binding,suggesting that CMPF,kynurenic acid,andN-(cinnamoyl)glycine are potential biomarkers for evaluating the function of OATs-MRP4 channel.
3.4.Validation of potential biomarkers of OATs and MRP4 in vitro

Fig.2.The uptake of human serum albumin(HSA)-bound uremic toxins(UTs)in human embryonic kidney 293T(HEK293T)-(A)organic anion transporter 1(OAT1),(B)-OAT3,and(C)-multidrug resistance-associated protein 4(MRP4)cells.Data shown are the mean±standard deviation(SD)(n =4).*P <0.05 and **P <0.01 vs.control;#P <0.05 and ##P <0.01 vs.without HSA.MCAP: 1-methyl-5-carboxylamide-2-pyridone;CMPF: 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid.
There was no significant difference in the uptake of kynurenic acid at 4°C between control and OAT1-,OAT3-,and MRP4-overexpressing cells (P>0.05).However,at 37°C,the uptake of kynurenic acid was significantly higher in OAT1-and OAT3-overexpressing cells than that in control cells (P<0.01),and intracellular accumulation in MRP4-overexpressing cells was markedly lower(P<0.01;Fig.3A).The uptake of kynurenic acid at 37°C in OAT3-MRP4 cells,instead of OAT1-MRP4 cells,was significantly increased compared with double control cells(P<0.01;Fig.S4),which could be attributed to the amount of OAT1≈MRP4 transport in OAT1-MRP4 cells,and OAT3 >MRP4 in OAT3-MRP4 cells.OAT1-and OAT3-mediated uptake of kynurenic acid was inhibited by PROB,a strong OATs inhibitor [34],with an IC50of 71.45 μM and 7.91 μM,respectively (Fig.3A).MRP4-mediated efflux of kynurenic acid was decreased by MK-571,an inhibitor of MRP4[35],in MRP4-overexpressing cells(P<0.01).In addition,MK-571 dose-dependently increased the uptake of kynurenic acid in OAT1/3 and MRP4 double-transfected cells(P<0.01;Fig.3A).
CMPF uptake at 4°C was not significantly different between control and OAT1-,OAT3-,and MRP4-overexpressing cells(P>0.05),but at 37°C,the uptake of CMPF in OAT1-and OAT3-overexpressing cells was markedly higher than that in control cells (P<0.01),and intracellular accumulation was significantly decreased in MRP4-overexpressing cells (Fig.3B).There was no significant difference in CMPF uptake in OAT1/3 and MRP4 doubletransfected cells at 4°C (P>0.05),whereas CMPF uptake was significantly increased in OAT1/3-MRP4 cells at 37°C (P<0.01;Fig.S5).PROB significantly inhibited CMPF uptake mediated by OAT1 and OAT3 with an IC50of 64.03 μM and 23.89 μM,respectively.MK-571 was able to inhibit the efflux of CMPF mediated by MRP4 (P<0.01;Fig.3B).MK-571 dose-dependently increased CMPF uptake in OAT1/3 and MRP4 double-transfected cells(P<0.01;Fig.3B).
At 4°C,the uptake ofN-(cinnamoyl)glycine was not significantly different between control and OAT1-,OAT3-,and MRP4-overexpressing cells,while at 37°C,the uptake ofN-(cinnamoyl)glycine was significantly elevated in OAT1-and OAT3-overexpressing cells compared with control cells (P<0.01),and intracellular accumulation in MRP4-overexpressing cells was markedly reduced compared with control cells (P<0.01;Fig.3C).PROB significantly inhibited the uptake ofN-(cinnamoyl)glycine mediated by OAT1 and OAT3 with an IC50of 36.98 μM and 30.78 μM,respectively.MK-571 inhibited the efflux ofN-(cinnamoyl)glycine mediated by MRP4 (P<0.01),and increased the intracellular accumulation in OAT1/3 and MRP4 double-transfected cells (P<0.01;Fig.3C).
These results demonstrate that kynurenic acid,CMPF,andN-(cinnamoyl)glycine uptake is mediated by OAT1 and OAT3,and efflux by MRP4 in vitro.
3.5.Validation of potential biomarkers of OATs and MRP4 in vivo
To further verify whether these potential biomarkers were eliminated by renal tubular OATs and MRP4 in vivo,the serum levels and renal excretions were determined in rats treated with the rOATs inhibitor PROB and the rMRP4 inhibitor MK-571,and in rOAT1/3-/-and rMRP4-/-rats.
The glomerular filtration index cystatin C,creatinine,and urea nitrogen were unaffected by PROB (P>0.05) and the histological structure of kidney was unchanged (Figs.S6A and B).PROB increased the serum levels and decreased the renal uptake ratios of kynurenic acid and CMPF in a dose-dependent manner (Figs.4A and B),suggesting that renal tubular rOATs expressed in basolateral membranes mediated the uptake of kynurenic acid and CMPF.The serum level ofN-(cinnamoyl)glycine was below the detection limit in rats.However,PROB had no significant effects on the cumulative urinary excretion of kynurenic acid,CMPF,andN-(cinnamoyl)glycine (P>0.05;Fig.4C).
MK-571 at a dose of 20 mg/kg significantly increased serum creatinine and urea nitrogen levels (P<0.05;Fig.S6C).No significant pathological changes occurred in the kidneys after administration of different doses of MK-571 (Fig.S6D).The levels of kynurenic acid and CMPF in renal tissues,but not in serum,were significantly increased by MK-571(Figs.4D and E),suggesting that rMRP4 expressed in apical membranes mediated the efflux of kynurenic acid and CMPF from renal tubular epithelial cells into the urine.The cumulative urinary excretion of CMPF was significantly decreased by 20 mg/kg MK-571(P<0.05),and kynurenic acid andN-(cinnamoyl)glycine excretions were not affected by MK-571(Fig.4F).
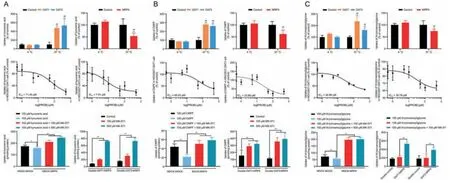
Fig.3.Validation of organic anion transporter 1 (OAT1)-,OAT3-,and multidrug resistance-associated protein 4 (MRP4)-mediated uptake of (A) kynurenic acid,(B) 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF),and (C) N-(cinnamoyl)glycine in vitro.(Upper) Effect of temperature on the uptake of kynurenic acid,CMPF,and N-(cinnamoyl)glycine in human embryonic kidney 293T(HEK293T)-OAT1,-OAT3,and-MRP4 cells.(Middle)Inhibitory effect of probenecid(PROB)on the uptake of kynurenic acid,CMPF,and N-(cinnamoyl)glycine in HEK293T-OAT1 and-OAT3 cells.(Lower)Inhibitory effect of MK-571 on the efflux of kynurenic acid,CMPF,and N-(cinnamoyl)glycine in Madin-Darby canine kidney(MDCK)-MRP4 and MDCK-double-OAT1/3-MRP4 cells.Data shown are the mean ±standard deviation (SD)(n =4).*P <0.05 and **P <0.01 vs.control;##P <0.01 vs.4 °C.

Fig.4.Validation of rat organic anion transporters(rOATs)-and rat multidrug resistance-associated protein 4(rMRP4)-mediated renal disposition of the potential biomarker in rats.Effect of rOATs inhibitor probenecid(PROB)on the(A)serum concentration,(B)renal uptake ratio,and(C)accumulative urinary excretion of kynurenic acid,3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid(CMPF),and N-(cinnamoyl)glycine.Data shown are the mean± standard deviation (SD) (n =7).*P <0.01 and **P <0.01 vs.control.Effect of rMRP4 inhibitor MK-571 on the(D)serum concentration,(E)renal concentration,and (F)accumulative urinary excretion of kynurenic acid,CMPF,and N-(cinnamoyl)glycine.Data shown are the mean ± SD (n =6).*P <0.05 and **P <0.01 vs.control.(G) Serum concentration,(H) renal uptake ratio,and (I) renal clearance rate of kynurenic acid,CMPF,and N-(cinnamoyl)glycine in rOAT1 and rOAT3 double knockout(rOAT1/3-/-)rats.Data shown are the mean±SD(n =7).**P <0.01 vs.wild-type(WT).(J)Serum concentration,(K)renal concentration,and(L)renal clearance rate of kynurenic acid,CMPF,and N-(cinnamoyl)glycine in rMRP4 knockout(rMRP4-/-)rats.Data shown are the mean±SD(n =6).**P <0.01 vs.WT./: below detection limit or no data.
DNA and mRNA analysis for rOAT1 and rOAT3 showed that rOAT1/3-/-rats were successfully constructed (Figs.S7A-C).After rOAT1/3-/-,the serum biochemical indexes related to the liver and kidney function did not change significantly,and the kidney did not show obvious pathological changes(Figs.S7D and E).Hippuric acid,a classical substrate of rOAT1 and rOAT3 [36],was used to elevate the function of OATs.In rOAT1/3-/-rats,renal elimination of hippuric acid was substantially inhibited,and the OCT2 substrate of creatinine was unaffected(Fig.S7F).The serum levels of kynurenic acid and CMPF were significantly increased,and the renal tissue uptake ratio and renal clearance rate were significantly decreased(P<0.01;Figs.4G-I).In addition,the cumulative excretion ofN-(cinnamoyl)glycine was significantly decreased (16.16 ± 5.27 vs.8.56 ±3.43;P<0.01).
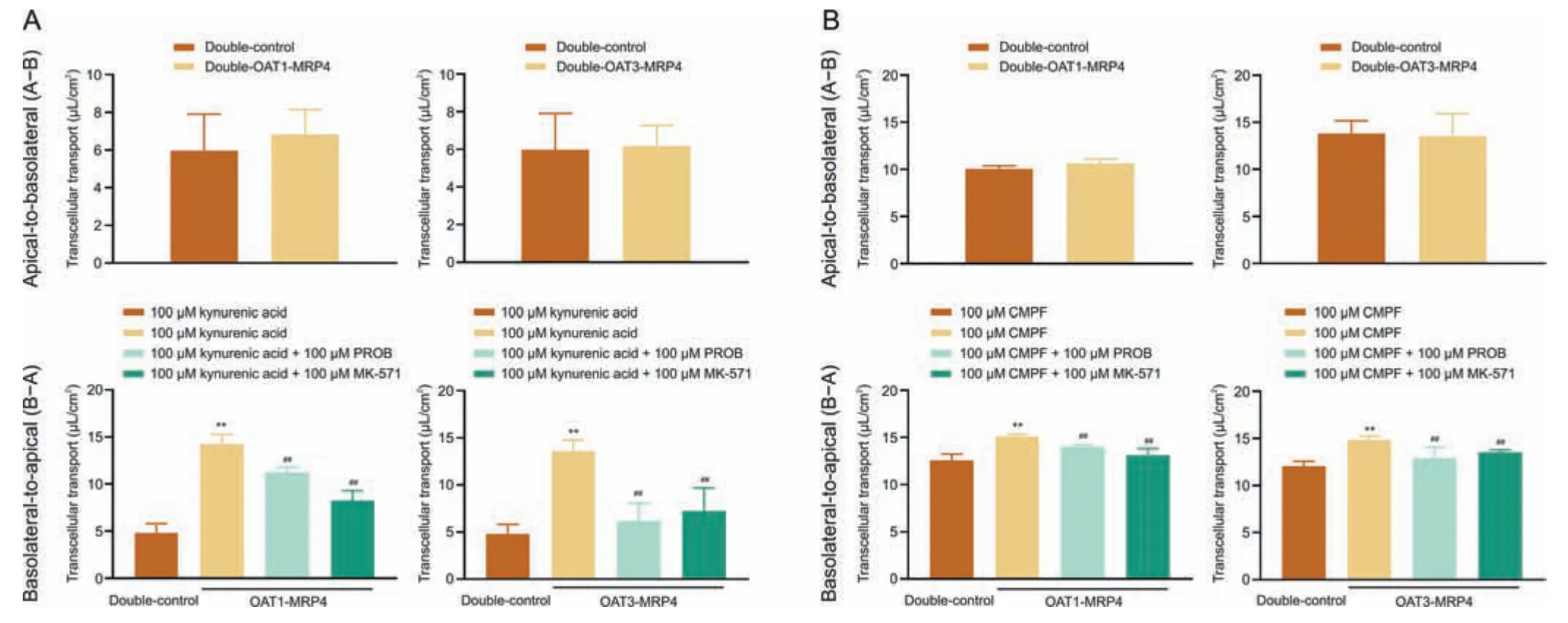
Fig.5.Transcellular transport of (A) kynurenic acid and (B) 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF) from the apical-to-basolateral direction (A-B) and from the basolateral-to-apical (B-A) direction and in double-control Madin-Darby canine kidney (MDCK) cells and organic anion transporter 1/3 (OAT1/3)-multidrug resistanceassociated protein 4 (MRP4) double-transfected MDCK cells.Data shown are the mean ± standard deviation (SD) (n =4).**P <0.01 vs.double-control;##P <0.01 vs.without inhibitor probenecid (PROB) or MK-571.
The successful knockout of the rMRP4 gene in rats was confirmed by DNA and mRNA analysis (Figs.S8A-C).In rMRP4-/-rats,the kidney and liver functions were not obviously dysfunctional(Figs.S8D and E).Serum levels,the renal uptake ratio,and clearance of creatinine were not significantly altered after rMRP4-/-(Fig.S8F).However,the serum levels and renal clearance rate of kynurenic acid and CMPF did not change significantly(P>0.05),and the renal tissue concentration of CMPF,but not kynurenic acid,was significantly elevated(P<0.01;Figs.4J-L).The kidney expresses not only rMRP4 but also rMRP2,and due to the extensive overlap of rMRP2 and rMRP4 substrates,rMRP2 function may be compensatively increased after rMRP4-/-[37].In addition,the circulating level of kynurenic acid in the body is determined by the activities of several tryptophan-metabolizing pathways and efflux from metabolic organs [38,39].After rMRP4-/-,the efflux of kynurenic acid from hepatocytes into the blood was hindered(Fig.S9).
Above all,kynurenic acid and CMPF as endogenous substrates of rOAT1/3 and rMRP4 can be used to evaluate the OATs-MRP4 channel.However,the serum level ofN-(cinnamoyl)glycine was below the lowest detection limit of LC-MS/MS,and thus is not suitable as a biomarker.
3.6.Vectorial transport of potential biomarkers mediated OATs-MRP4 channel in vitro
To determine whether the OATs-MRP4 channel mediated the flux across of monolayers of potential biomarkers,a transmembrane transport assay using double-control cells and OAT1/3-MRP4 double-transfected cells was carried out.The transport of kynurenic acid from the apical membrane (A) to the basolateral membrane (B) was not significantly different between the double control cells and double transfected OAT1/3-MRP4 cells(Fig.5A).In contrast,the transport of kynurenic acid from the basolateral membrane (B) to the apical membrane (A) was significantly increased in double-transfected OAT1/3-MRP4 cell lines(P<0.01),and PROB and MK-571 significantly reduced B-A transport(P<0.01;Fig.5A).Consistent with the transmembrane transport of kynurenic acid,CMPF was only transported from B to A in doubletransfected OAT1/3-MRP4 cells,but not from A to B,and this transmembrane transport was substantially inhibited by PROB and MK-571 (Fig.5B).The flux ofN-(cinnamoyl)glycine was mediated by OATs-MRP4 channel from B-A (Fig.S10).These results suggest that kynurenic acid and CMPF transport are mediated by a vector transport channel composed of uptake transporters OATs and efflux transporter MRP4.
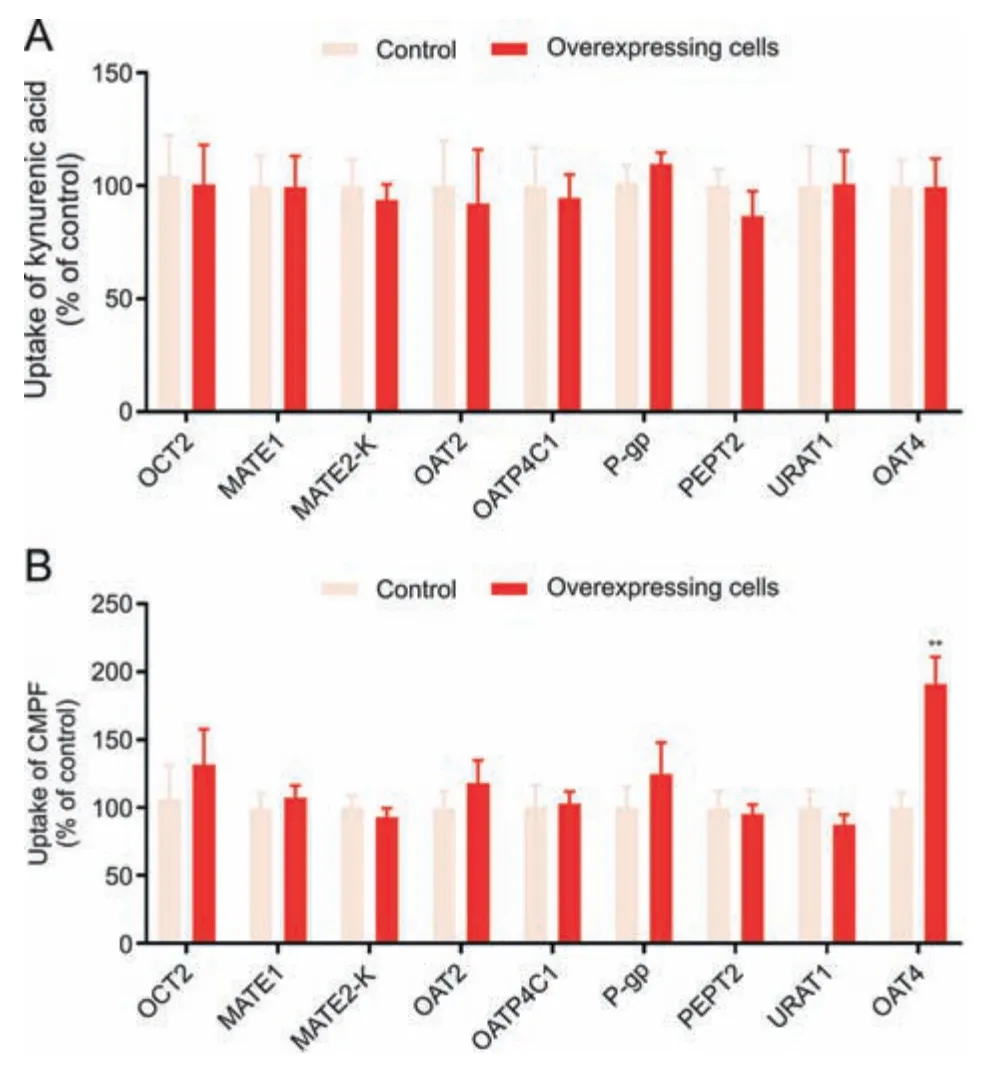
Fig.6.The uptake of (A) kynurenic acid and (B) 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid(CMPF)in renal tubular transporter-overexpressing cells.Data shown are the mean ± standard deviation (SD) (n =6).**P <0.01 vs.control.OCT2: organic cation transporter 2;MATE:multidrug and toxin extrusion protein;OAT:organic anion transporter;OATP4C1: organic anion transporting polypeptide 4C1;P-gp: P-glycoprotein;PEPT2: peptide transporter 2;URAT1: urate transporter 1.

Fig.7.Kinetics and metabolism analysis of kynurenic acid.(A)Uptake-time profile of kynurenic acid and(B and C)Eadie-Hofstee plot of kynurenic acid in MOCK and organic anion transporter 1/3 (OAT1/3)-overexpressing cells.Data shown are the mean ±standard deviation (SD) (n =4).Effect of probenecid(PROB) or MK-571 on the urinary excretion of(D)d5-kynurenic acid(2 mg/kg),(E)d4-xanthurenic,and(F)kynurenic acid in rats.Data shown are the mean±SD(n =6).**P <0.01 vs.control.(G)Kynurenic acid biosynthesis in vivo.(H)Xanthurenic and(I)3-hyrdroxykynurenine uptake in MOCK and OAT1-,OAT3-,and multidrug resistance-associated protein 4(MRP4)-overexpressing cells.Data shown are the mean ± SD (n =4).**P <0.01 vs.MOCK;##P <0.01 vs.without human serum albumin (HSA). v: uptake rate;[S]: substrate concentration.
3.7.Specificity evaluation of potential biomarkers
The biomarkers of the OATs-MRP4 channel can neither be filtered by the glomerulus nor be secreted or reabsorbed by other transporters.The HSA-binding ratios of kynurenic acid and CMPF were approximately 100%,and thus they are not filtered.Therefore,the transport specificity of kynurenic acid and CMPF was evaluated using cells overexpressing different tubular transporters.The transport of kynurenic acid(Fig.6A)andN-(cinnamoyl)glycine(Fig.S11)was not mediated by OCT2,MATE1,MATE2-K,OAT2,OATP4C1,P-gp,PEPT2,URAT1,or OAT4.These results show that kynurenic acid is a specific substrate of the OAT1/3-MRP4 channel.The uptake of CMPF in OAT4-overexpressing cells was significantly higher than that in control cells (Fig.6B),suggesting that CMPF may exhibit renal tubular reabsorption mediated by OAT4.
3.8.Sensitivity and suitability assessment of potential biomarkers
Sensitivity evaluation is another key aspect of identifying biomarkers of the transporter,which can reflect genetic polymorphisms as well as the functional activity of transporters in vivo[40].The sensitivity of biomarkers is closely related to the affinity for transporters and eliminationt1/2.The linear-uptake time of kynurenic acid was 0-10 min,and the concentration-dependent uptake time was set to 5 min.OAT1 and OAT3 mediated the uptake of kynurenic acid withKmvalues of 496.7 and 382.2 μM,respectively,andvmaxvalues of 197.1 pmol/mg protein/min and 120.1 pmol/mg protein/min,respectively (Figs.7A-C),suggesting that OATs have a high affinity for kynurenic acid.
Kynurenic acid is an endogenous compound,and it is difficult to evaluate clearancet1/2in vivo.In this study,we used exogenous d5-kynurenic acid to measure its clearancet1/2.Results showed that the renal clearancet1/2of d5-kynurenic acid was 3.7 ± 0.7 h(Table S5).PROB and MK-571 were able to reduce the renal excretion of d5-kynurenic acid,d4-xanthurenic acid,and kynurenic acid(Figs.7D-F).A previous study showed that kynurenic acid was an end-product of the irreversible transamination of kynurenine[38].However,our study showed that d5-kynurenic acid was metabolized to d4-xanthurenic acid with a rate of 5.2%-11.8%in vivo (Fig.7G).These results indicate that kynurenic acid has a suitablet1/2(0.5-8 h)and a lower metabolic ratio.In addition,the serum levels of kynurenic acid determined by LC-MS/MS had the advantages of short detection time and simple pretreatment method,which is suitable for clinical use (Fig.S12).
To estimate whether the kynurenine metabolites 3-hyrdroxykynurenine and xanthurenic acid also had potential as biomarkers of the OATs-MRP4 channel,the uptake of 3-hyrdroxykynurenine and xanthurenic in HEK293T-OAT1,OAT3 and MRP4 cells was measured.The uptake of xanthurenic was mediated by OAT1 and OAT3,and efflux by MRP4 (Fig.7H),but serum levels in vivo were below the lowest detection limit.3-hyrdroxykynurenine uptake was mediated by OAT1 and efflux by MRP4 (Fig.7I).These results demonstrated that 3-hyrdroxykynurenine and xanthurenic could not be used as biomarker of the OATs-MRP4 channel.
The linearuptake time of CMPF was 0-10 min,and theKmandvmaxof OAT1 for CMPF uptake were 1,483 μM and 323 pmol/mg protein/min,respectively,and for OAT3 were 447.1 μM and 133.2 pmol/mg protein/min,respectively (Figs.8A-C).PROB and MK-571 did not affect the renal excretion of d3-CMPF and CMPF(Figs.8D and E),and the renal clearancet1/2of d3-CMPF in vivo was 1.9±0.3 h(Table S5).These results indicate that OAT1 has a low affinity for CMPF uptake.
In addition,OAT1 and OAT3 mediated the uptake ofN-(cinnamoyl)glycine with low affinity of 4,265 and 2,984 μM (Fig.S13).
3.9.Estimation of influencing factors of biomarkers
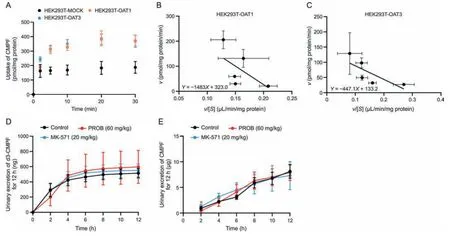
Fig.8.Kinetics and urinary excretion of 3-carboxy-4-methyl-5-propyl-2-furanpropanoic acid (CMPF).(A) Uptake-time profile and (B and C) Eadie-Hofstee plot of CMPF in MOCK and organic anion transporter 1/3(OAT1/3)-overexpressing cells.Data shown are the mean±standard deviation(SD)(n =4).Effect of probenecid(PROB)or MK-571 on the urinary excretion of (D) d3-CMPF (2 mg/kg) and (E) CMPF in rats.Data shown are the mean ± SD (n =6).*P <0.05 vs.control. v: uptake rate;[S]: substrate concentration.
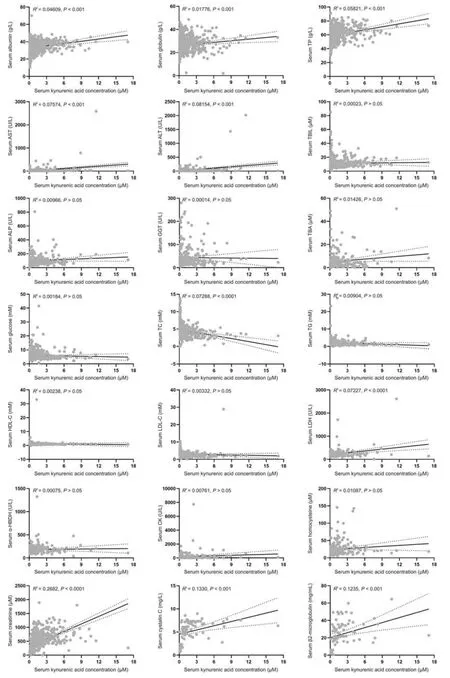
Fig.9.Correlation analysis between biochemical markers and kynurenic acid in humans: albumin,globulin,and total protein (TP) (n =600);aspartate aminotransferase (AST),alanine aminotransferase(ALT),and total bilirubin(TBIL)(n =601);alkaline phosphatase(ALP)and γ-glutamyl transferase(GGT)(n =267);total bile acid(TBA)(n =265);glucose(n =472);total cholesterol(TC),triglyceride(TG),and high-density lipoprotein(HDL-C)(n =382);low-density lipoprotein(LDL-C)(n =381);lactate dehydrogenase(LDH)and αhydroxybutyrate dehydrogenase(α-HBDH)(n =216);creatine kinase(CK)(n =219);homocysteine(n =205);creatinine(n =678);and cystatin C and β2-microglobulin(n =77).
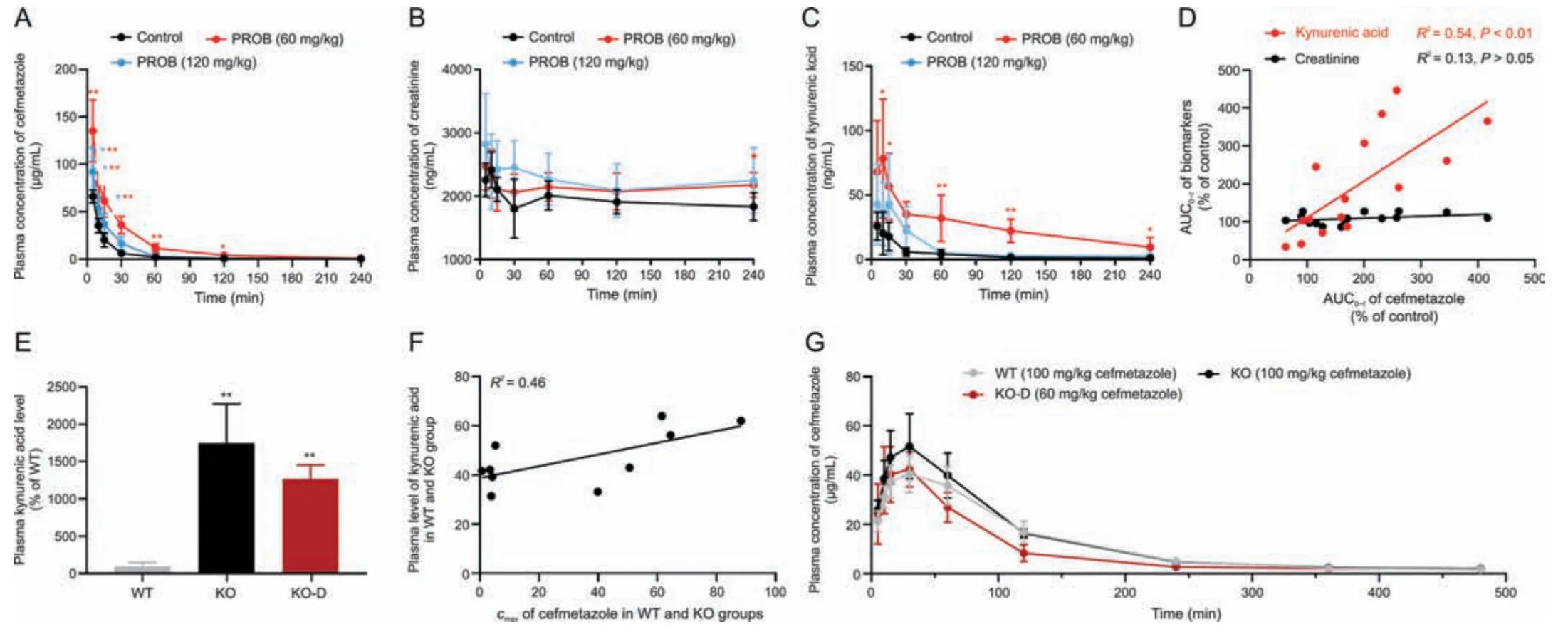
Fig.10.Correlation analysis of cefmetazole and kynurenic acid in rats treated with probenecid (PROB)and in rat organic anion transporter 1(rOAT1) and rOAT3 double knockout(rOAT1/3-/-) rats.Plasma concentration-time curves of (A) cefmetazole (100 mg/kg),(B) creatinine,and (C) kynurenic acid,and (D) correlation of area under the plasma drug concentration-time curve(AUC0-t)between cefmetazole and kynurenic acid/creatinine in rats treated with 60 and 120 mg/kg PROB.(E)Plasma concentration of kynurenic acid,(F)correlation of maximum plasma concentration(cmax)between cefmetazole and kynurenic acid in wild-type(WT)and rOAT1/3-/-rats,and(G)plasma concentration-time curves of cefmetazole in WT and knockout(KO)rats given doses of 100 mg/kg and KO-dose adjustment(KO-D)rats given adjusted doses of 60 mg/kg.Data shown are the mean±standard deviation (SD) (n =5).*P <0.05 and **P <0.01 vs.control or WT. cmax: maximum plasma concentration.
By studying the correlation between different pathophysiological indicators and biomarkers,the influencing factors on biomarkers in vivo were explored,which will be important for clinical application.The serum level of kynurenic acid was significantly correlated with albumin,globulin,TP,AST,ALT,TC,LDH,creatinine,cystatin C,and β2-microglobulin (P<0.01),but the correlation coefficientR2was less than 0.3 to be a weak correlation(Fig.9).Although the renal injury indexes,creatinine,cystatin C,and β2-microglobulin had higher correlation coefficients with kynurenic acid than other biochemical indexes,they were still weakly correlated because these markers were not directly related with the changes in renal tubular OATs-MRP4 channel.There were no correlations in serum levels between kynurenic acid and TBIL,ALP,GGT,TBA,glucose,TG,HDL-C,LDL-C,α-HBDH,CK,and homocysteine.Thus,serum levels of kynurenic acid were not affected by serum protein level,liver function,heart function,and glomerular filtration function in vivo,suggesting that kynurenic acid is suitable to be used as an endogenous biomarker to evaluate the function of the OATs-MRP4 channel.The serum CMPF level was significantly correlated with the levels of albumin,TP,AST,ALT,TBA,glucose,TC,LDL-C,creatinine,and cystatin C (P<0.05),but the correlation coefficientsR2were all less than 0.3.There was no significant correlation between CMPF and globulin,TBIL,ALP,GGT,TG,HDL-C,LDH,α-HBDH,CK,homocysteine,and β2-microglobulin(Fig.S14).
3.10.Application of biomarker
After transport validation,specificity evaluation,and assessment of the sensitivity and suitability of potential biomarkers kynurenic acid,CMPF,andN-(cinnamoyl)glycine,we found that kynurenic acid was the most suitable biomarker for assessing the function of OATs-MRP4 channel function.CMPF exhibited renal tubular reabsorption by OAT4 and was a low affinity substrate for OAT1,N-(cinnamoyl)glycine in serum was below the detection limit,and OATs had a low affinity for this compound.
To verify the ability of kynurenic acid to measure the function of the OATs-MRP4 channel,we exploited the pharmacokinetic relationship between cefmetazole and kynurenic acid in rats treated with PROB.Plasma concentrations of kynurenic acid and cefmetazole were increased by PROB in a dose-dependent manner,while plasma concentrations of creatinine did not change significantly(Figs.10A-C).Intriguingly,there was a consistent correlation in AUC0-tbetween cefmetazole with kynurenic acid,but not with creatinine(Fig.10D).Plasma kynurenic acid level in rOAT1/3-/-rats was markedly increased compared to WT rats,and the correlation of maximum plasma concentration (cmax) between kynurenic acid and cefmetazole was moderate(Figs.10E and F).The proportion of renal rOATs-MRP4 channel was completely abolished after rOAT1/3-/-,and thus the renal tubular secretion of cefmetazole (about 40%[41,42])should be deducted when adjusting the drug dose.The result showed that thecmaxof cefmetazole in WT rats given doses of 100 mg/kg was close to that of KO rats given doses of 60 mg/kg(Fig.10G).These results indicate that kynurenic acid can be used as a biomarker of OATs-MRP4 and may be useful to evaluate the excretion of substrate drugs.
In acute renal tubular injury rats induced by cisplatin,serum levels of kynurenic acid were increased in a time-dependent manner,and were significantly elevated on day 3,as were cystatin C on day 5 (Fig.11A).Consistently,the period of significantly increased kynurenic acid levels occurred earlier than that of creatinine in ICU patients(1-4)who eventually progressed to acute renal injury(Fig.11B).To evaluate the profiling of the biomarker in healthy people and patients with different types of kidney diseases,serum levels of kynurenic acid were measured.Compared with healthy people,serum kynurenic acid levels in patients with acute kidney injury,chronic renal failure,uremia,glomerulonephritis,diabetic nephropathy,and hypertensive nephropathy were significantly increased (P<0.01),while kynurenic acid levels were significantly decreased in patients with primary nephrotic syndrome(P<0.01;Fig.11C),which may indicate changes in the renal tubular OATs-MRP4 channel vary in different types of renal injury.
4.Discussion
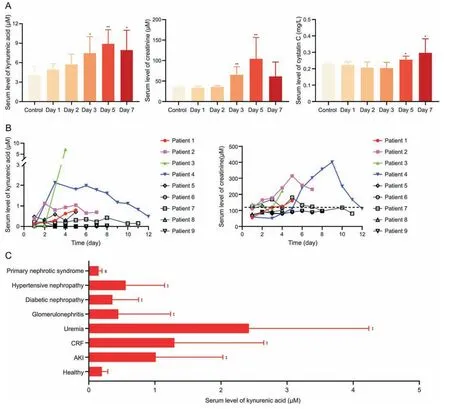
Fig.11.The role of kynurenic acid in evaluating renal injury.(A) Serum levels of kynurenic acid,creatinine and cystatin C in rats treated with cisplatin (1 mg/kg intraperitoneal injection).Data shown are the mean±standard deviation(SD)(n =6).*P <0.05 and **P <0.01 vs.control.(B)Serum levels of kynurenic acid and creatinine in intensive care unit(ICU)patients(1-4)with acute kidney injury and patients(5-9)without acute kidney injury during treatment.(C)Serum levels of kynurenic acid in healthy people and in patients with various kidney diseases.Healthy people(n =50);acute kidney injury(AKI)(n =5);chronic renal failure(CRF)(n =73);uremia(n =62);glomerulonephritis(n =9);diabetic nephropathy(n =23);hypertensive nephropathy(n =10);and primary nephrotic syndrome(n =30).Data shown are the mean±SD.**P <0.01 vs.healthy,significantly increased;##P <0.01 vs.healthy,significantly decreased.
Glomerular filtration and renal tubular secretion constitute the renal excretion process of drugs together.However,renal function is evaluated unilaterally by the indexes of glomerular filtration in clinical settings.The renal tubular OATs-MRPs channel plays an important role in the renal excretion of organic anion drugs,but the identification of biomarkers for the OATs-MRPs channel in vitro and in vivo has not been accomplished.Biomarker that could be used for evaluation of renal tubular OATs-MRP4 channel function should have little or no glomerular filtration(>50 kDa)and should be specifically transported through this channel.Given that HSA-bound compounds (>66 kDa) cannot be filtered by glomerulus and can be secreted by transporters,it is expected that endogenous biomarker forevaluating the function of renal tubular transport channels will be identified by screening high HSA-bound substances.
Previous studies have shown that many UTs undergo renal elimination by OATs[26,27,43].Therefore,we screened this class of compound for OATs-MRP4 channel biomarkers.Twenty-six candidate UTs were selected from the Uremic Solutes Database and related literature by screening for the substrates of OAT1,OAT3,and MRP4.The results showed that the protein-binding ratios of MCAP,indole-3-acetic acid,D-kynurenine,phenyl-β-D-glucuronide,3-indoxyl sulfate,3-indoxyl-β-D-glucopyranoside,kynurenic acid,CMPF,p-cresol sulfate,4-ethylphenyl sulfate,andN-(cinnamoyl)glycine were more than 70%.During the uptake experiments by OAT1 and OAT3,these UTs were incubated with HSA to form conjugates to simulate OATs-mediated uptake,and kynurenic acid,CMPF,hippuric acid,andN-(cinnamoyl)glycine were shown to be taken up by both OAT1 and OAT3 in the free and protein-bound forms,with the unbound form secreted MRP4.Currently,hippuric acid is considered to be an endogenous biomarker of OATs.However,the protein-binding ratio of hippuric acid in humans is about 50%,which exhibited poor specificity due to massive filtration via the glomerulus.Therefore,kynurenic acid,CMPF,andN-(cinnamoyl)glycine were selected as potential biomarkers for evaluating the function of OATs-MRP4 channel.
Potential biomarkers were further validated in vitro and in vivo.Results demonstrate that kynurenic acid,CMPF,andN-(cinnamoyl)glycine are substrates of OAT1,OAT3,and MRP4,and were parallel transported by OATs-MRP4 channel in vitro.PROB,a rOATs inhibitor,dose-dependently increased the serum levels of kynurenic acid and CMPF and diminished the renal uptake ratio in rats.Consistently,in rOAT1/3-/-rats,serum kynurenic acid and CMPF levels were obviously increased,and the renal uptake ratio and renal clearance rate were strikingly reduced.After administration of the rMRP4 inhibitor MK-571,renal tissue concentrations of kynurenic acid and CMPF were significantly increased in rats.The renal concentration of CMPF was significantly increased with depletion of rMRP4,but kynurenic acid was not affected,which could indicate that the changes in the serum level mainly depended on uptake transporters,and the changes in tissue concentrations were essentially governed by efflux transporters[44,45].However,after rMRP4-/-,the level of kynurenic acid in renal tissue was unaffected,which could be related to the blocked efflux of kynurenic acid from metabolic organs into the blood after rMRP4-/-,or a compensatory increase of renal rMRP2.Although kynurenic acid is also a potential substrate of MRP2 (Fig.S15),it can be excreted by the rOAT1/3-MRP4 channel in renal tubules,and the contribution of rMRP2 appears to be minor due to the strong differences between rMRP4 and rMRP2 in the kidney of rats [46].These results in vitro and in vivo demonstrate that kynurenic acid and CMPF are potential biomarkers of the OATs-MRP4 channel,while the serum level ofN-(cinnamoyl)glycine is below the detection limit in vivo.
Previous studies identified substrates of transporters that could not assign biomarkers because they did not systematically assess the specificity,sensitivity,and suitability of the substrates[15,16,47-51].In terms of the specificity of renal tubular secretory transporters,we firstly proposed to screen biomarkers of renal tubular transporters that can form HSA conjugates,and evaluate the uptake and efflux by other transporters-overexpressing cells.We found that kynurenic acid was neither filtered by the glomerulus (99% protein binding) nor transported by OCT2,MATE1,MATE2-K,OAT2,OATP4C1,P-gp,PEPT2,URAT1,and OAT4.However,CMPF uptake was mediated by OAT4,a renal tubular reabsorption transporter,suggesting that CMPF has a poor specificity in the renal tubular transport process.In addition,kynurenic acid had a suitable affinity for OAT1 and OAT3 and renal eliminationt1/2,and could be metabolized to xanthurenic with a metabolic ratio of <12%in vivo.OAT1 and OAT3 had a low affinity forN-(cinnamoyl)glycine,making it unsuitable as a functional biomarker.Our studies in humans show that kynurenic acid is minimally affected by serum protein levels,liver function,cardiac function,and glomerular filtration function.Encouragingly,the magnitude of effect size in people completed by Lai and co-workers[52]showed that kynurenic acid is a superior biomarker for assessing OAT1 and OAT3.These results support our proposition that kynurenic acid is an endogenous OATs-MRP4 biomarker with good specificity,sensitivity,and suitability.
Finally,preliminary clinical applications of kynurenic acid were evaluated in this study.The rOATs inhibitor PROB dosedependently increased the plasma levels of kynurenic acid and cefmetazole,and there was a significant correlation in AUC0-tbetween cefmetazole and kynurenic acid,but not with creatinine,a GFR index for adjusting drug dosage[53].Thus,when OATs function is inhibited or abnormal,only kynurenic acid can be used to accurately adjust the dosage of substrate drugs.In addition,kynurenic acid as the biomarker was better than creatinine in evaluating acute renal injury in rats and patients.The profile of kynurenic acid was obviously different in patients with acute kidney injury,chronic renal failure,uremia,glomerulonephritis,diabetic nephropathy,hypertensive nephropathy,and primary nephrotic syndrome.As the effect of renal injury on glomerular filtration and renal tubular secretion can differ [20],multiple biomarkers are needed to adjust the dosage of drugs eliminated by the glomerulus and the various renal tubular channels.
5.Conclusion
Twenty-six candidate UTs were identified by screening databases and the literature,and three UTs were selected as potential biomarkers for the OATs-MRP4 channel through evaluation of proteinbinding ratio and uptake in OAT1-,OAT3-,and MRP4-overexpressing cell lines.Three potential biomarkers were validated in vitro and in vivo,and after determination of specificity,sensitivity,and suitability,we selected kynurenic acid as the suitable biomarker for evaluating the function of the OATs-MRP4 channel.Importantly,kynurenic acid has a significant correlation with AUC0-tof cefmetazole in vivo,and the period of increased kynurenic acid level is earlier than that of creatinine in the acute kidney injury process.These results clearly demonstrate that HSA-kynurenic acid is an appropriate endogenous biomarker for adjusting the dosage of drugs secreted by this channel or predicting renal injury.
CRediT author statement
Yanrong Ma:Methodology,Formal analysis,Data curation,Writing-Reviewing and Editing,Funding acquisition;Fenglin Ran:Methodology,Validation,Writing -Original draft preparation;Mingyan Xin:Methodology,Validation,Investigation;Xueyan Gou:Methodology,Investigation,Formal analysis;Xinyi Wang:Writing -Original draft preparation;Xinan Wu:Writing -Reviewing and Editing,Supervision,Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant Nos.: 81803611,82160705,and U21A20424) and the Natural Science Foundation of Gansu Province,China (Grant No.: 21ZD4FA014).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.05.007.
 Journal of Pharmaceutical Analysis2023年10期
Journal of Pharmaceutical Analysis2023年10期
- Journal of Pharmaceutical Analysis的其它文章
- Protective effects of catalpol on cardio-cerebrovascular diseases: A comprehensive review
- Recent advancements in single-cell metabolic analysis for pharmacological research
- SPME as a green sample-preparation technique for the monitoring of phytocannabinoids and endocannabinoids in complex matrices
- Monoclonal antibody targeting mu-opioid receptor attenuates morphine tolerance via enhancing morphine-induced receptor endocytosis
- Protective effects of dioscin against Parkinson's disease via regulating bile acid metabolism through remodeling gut microbiome/GLP-1 signaling
- Histone deacetylase inhibitor pracinostat suppresses colorectal cancer by inducing CDK5-Drp1 signaling-mediated peripheral mitofission
