In-depth analysis of de novo lipogenesis in non-alcoholic fatty liver disease: Mechanism and pharmacological interventions☆
Zhixian Zhu ,Xiaoxun Zhang ,Qiong Pan ,Liangjun Zhang ,Jin Chai ,*
a Department of Gastroenterology, The First Affiliated Hospital (Southwest Hospital), Third Military Medical University (Army Medical University),Chongqing, China
b Institute of Digestive Diseases of PLA, Third Military Medical University (Army Medical University), Chongqing, China
c Center for Metabolic Associated Fatty Liver Disease and Cholestatic Liver Diseases Center,The First Affiliated Hospital(Southwest Hospital),Third Military Medical University (Army Medical University), Chongqing, China
Keywords: Non-alcoholic fatty liver disease (NAFLD)De novo lipogenesis (DNL)Therapeutics Molecular mechanism
ABSTRACT Non-alcoholic fatty liver disease (NAFLD) is characterized by the abnormal buildup of lipids in the liver tissue.Non-alcoholic fatty liver(NAFL)may progress to non-alcoholic steatohepatitis.Triglycerides in the liver can originate from various sources,including de novo lipogenesis(DNL).Research indicates that DNL significantly escalates in NAFLD,worsening steatosis.However,the precise regulatory mechanism of DNL in the development of this disease is not fully understood.Therefore,the targeted reduction of DNL could be a crucial therapeutic strategy.Currently,numerous pharmaceutical agents targeting DNL have been developed,attracting significant attention.This review examines the mechanism of DNL upregulation in NAFLD,assessing its potential as a therapeutic target for hepatic steatosis.Furthermore,we thoroughly examine hepatocellular lipotoxicity and provide an extensive review of the application and limitations of relevant therapeutic drugs,with a focus on key enzymes involved in DNL.The implementation of these pharmacological strategies is expected to significantly improve the management and overall outcomes for patients with NAFLD.
1.Introduction
Non-alcoholic fatty liver disease(NAFLD),a condition affecting a quarter of the global population,has become the most prevalent liver disorder.1Currently,there are limited pharmaceutical treatments available to effectively combat steatosis.Consequently,the optimal treatment for NAFLD remains the adoption of a healthy lifestyle and weight reduction,as suggested by studies.1,2Understanding the mechanism behind the accumulation of triglycerides(TGs)in the liver is crucial for understanding the pathogenesis and treatment of NAFLD.Fatty acids (FA),crucial substrates in TG synthesis,originate from three primary sources under physiological conditions.First,dietary TGs are transported to liver cells,known as hepatocytes,for decomposition.This process is aided by chylomicron remnants(CMR)after partial absorption.The second source of FA stems fromde novolipogenesis (DNL),a process that converts carbohydrates into FA.Additionally,a portion of FA is derived from the mobilization of tissues,resulting in the release of circulating free fatty acids(FFA).These FA can either oxidize or be synthesized into TGs within the liver.3-5Subsequently,the hepatic TGs can be excreted via very low-density lipoprotein (VLDL) or stored in the liver.In the context of NAFLD,dysregulation of endogenous lipogenesis can disrupt the balance between lipid synthesis and oxidation,leading to the accumulation of TGs in the liver.The excessive accumulation of TG deposits could contribute to NAFLD and progression to non-alcoholic steatohepatitis (NASH) (Fig.1).
Early research into NAFLD overlooked the crucial role of DNL in its pathogenesis.This may be because DNL contributes to only 5%of FA in healthy individuals.6-8However,in patients with NAFLD,DNL accounts for up to 26% of liver TGs (Fig.1).5Currently,there is substantial evidence highlighting the significant role of DNL upregulation in the development of NAFLD.9-12The combined effect of excessive tissue fat mobilization and upregulation of DNL further exacerbates the accumulation of liver TGs,leading to further changes in liver TG content.13Thus,DNL may play a crucial role in the development of NAFLD.Therefore,DNL is believed to play a vital role in the development of NAFLD.However,the mechanism responsible for the upregulation of DNL is multifaceted and not fully understood.
This review highlights the pathogenesis and treatment of NAFLD,specifically focusing on DNL.We offer a thorough review of the current knowledge on DNL in NAFLD,aiming to uncover the mechanisms that trigger its upregulation at the onset of the disease.We also present a comprehensive summary of the potential applications and limitations of therapeutic drugs targeting key enzymes involved in DNL.We anticipate that these pharmacological approaches will lead to more effective management and improved outcomes for patients.
2.DNL in the liver
DNL is a biological process that enables the production of endogenous TGs from dietary substrates.8A high intake of carbohydrates has been shown to significantly enhance DNL in the liver.14This complex process can be divided into three sequential steps:fatty acid synthesis,elongation/desaturation,and assembly into TGs.8
2.1. Basic raw materials and enzymes for DNL
The synthesis of TGs requires two primary components:glycerol and FA.These building blocks are primarily obtained via carbohydrate metabolism.Glycerol-3 phosphate,specifically,serves as the initial material esterified with FA to form TGs.15The liver uses carbohydrate metabolism to generate endogenous FA.The enzyme acetyl-CoA carboxylase (ACC),which catalyzes the conversion of acetyl-CoA into malonyl-CoA,is central to lipogenesis.Fatty acid synthase (FAS or FASN),an enzyme responsible for producing palmitic acid,is another crucial component.Fatty acid elongases and desaturases catalyze the transformation of palmitic acid into various FA.
2.2. DNL pathway
The first stage of DNL involves the production of endogenous FA.Carbohydrates are crucial for producing substantial amounts of acetyl-CoA within the mitochondria.16Citrate-isocitrate carrier(CIC)is essential for facilitating the transport of acetyl-CoA into the cytoplasm by converting it into citric acid.ATP-citrate lyase(ACLY)subsequently cleaves citric acid to produce acetyl-CoA.17This acetyl-CoA is then transformed into malonyl-CoA by ACC,which acts as the precursor for FA synthesis.FA is synthesized in a stepwise process,incorporating two-carbon units from malonyl-CoA,ultimately producing palmitic acid.15This palmitic acid can then be further elongated and modified in the endoplasmic reticulum and mitochondria.Ultimately,the resulting FA and glycerol 3-phosphate are converted into TG.TG synthesis relies on the coordinated action of various enzymes.Modulating the activity or expression of these enzymes can significantly affect DNL (Fig.2).
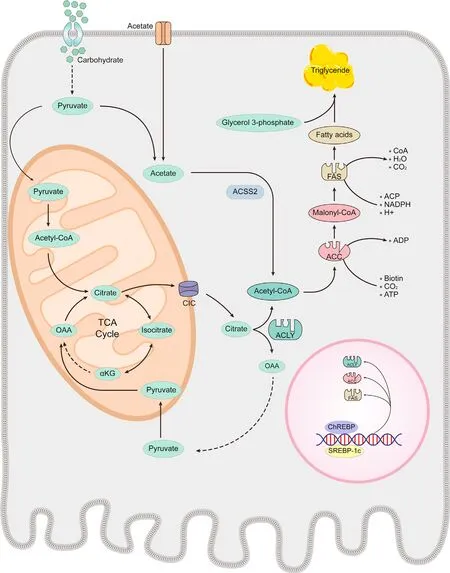
Fig.2.De novo lipogenesis (DNL). DNL relies on the orchestration of numerous enzymatic reactions.The regulation of enzyme catalytic activity and expression may play a significant role in controlling intrahepatic lipogenesis.Abbreviations: ACC,acetyl-CoA carboxylase;ACLY,ATP-citrate lyase;ACSS,acetyl-CoA synthetase 2;ChREBP,carbohydrate response element-binding protein;CIC,citrate-isocitrate carrier;FAS,fatty acid synthase;αKG,alpha-ketoglutarate;OAA,oxaloacetic acid;SREBP-1c sterol regulatory elementbinding protein-1c;TCA,tricarboxylic acid.
3.Molecular mechanisms of DNL upregulation in NAFLD
DNL regulation primarily occurs at the transcriptional level.18,19This is especially noticeable during postprandial periods,characterized by a significant increase in glucose uptake and insulin secretion,which induces the upregulation of lipogenic genes.20,21Following this,glucose and insulin activate two key transcription factors: carbohydrate response element-binding protein (ChREBP)and sterol regulatory element-binding protein-1c (SREBP-1c).These proteins are crucial in regulating lipogenic gene expression.Several genes,including FAS,ACC,and stearoyl-CoA desaturase 1(SCD1),responsible for lipogenesis,are upregulated.18,22Modifications to these factors could potentially serve as key determinants of DNL upregulation.
3.1. Overconsumption of fructose
Excessive fructose intake significantly contributes to the development of NAFLD.23Recent research indicates a positive correlation between increased consumption of fructose-based beverages and the onset of fatty liver.24A specific study revealed that NAFLD patients consumed 2-3 times more fructose than controls.25Fructose can stimulate DNL in two ways: through long-term induction effects and by directly providing a carbon source for DNL.
Fructose primarily activates ChREBP.High fructose intake can activate low-activity ChREBP-α,subsequently inducing highactivity ChREBP-β.26Experimental studies have shown that such dietary interventions in animal models increase the expression of ChREBP target genes.Fructose is associated with the activation of SREBP-1c and can induce its expression in both insulin-dependent and insulin-independent manners.27,28Additionally,fructose can activate other transcription factors associated with TG metabolism,such as liver X receptor (LXR),29and peroxisome proliferatoractivated receptor (PPAR)-gamma coactivator-1 beta (PGC-1β),30which can further enhance DNL reactions.
Fructose is a significant carbohydrate that provides a substantial carbon source.In the conventional DNL pathway,ACLY converts citric acid to acetyl-CoA.When fructose intake exceeds the small intestine's capacity,gut microbiota metabolizes fructose into acetate.31Recent studies have revealed that acyl-CoA synthetase shortchain family member 2(ACSS2) can convert acetate to acetyl-CoA,establishing an alternative pathway for TG production independent of ACLY (Fig.2).32
In conclusion,the increased intake of fructose stimulates the transcriptional activation of DNL and provides essential substrates,both of which significantly contribute to the development of hepatic steatosis.This emphasizes the complex interaction between dietary factors,gut microbiota,and host organs in regulating liver metabolism.Controlling fructose intake long-term through dietary intervention is challenging.Therefore,directly targeting fructose metabolism or DNL could potentially improve the efficacy of pharmacological treatments for NAFLD.
3.2. Insulin resistance
Insulin resistance,a key feature of NAFLD,23promotes DNL in a high insulin environment.33Recent studies have found a positive correlation between hepatic DNL and 24-h plasma glucose and insulin levels,and a negative correlation with the hepatic insulin sensitivity index.33NAFLD is typically associated with selective insulin resistance,15,23which maintains the sensitivity of the SREBP-1c pathway,leading to increased SREBP-1c levels and enhanced DNL.34SREBP-1c can also be activated by noninsulindependent mechanisms such as the mechanistic target of rapamycin complex 1(mTORC1),35,36and endoplasmic reticulum stress in insulin-resistant conditions.37This state is often marked by blood glucose levels exceeding normal physiological levels.The surge in glucose levels stimulates liver DNL by enhancing ChREBP and SREBP-1c,activating nearly all associated genes.33DNL may produce toxic metabolites like diacylglycerol and ceramides,potentially leading to insulin resistance.38,39This creates a positive feedback loop with insulin resistance,worsening disease progression.33,34Both NAFLD and type 2 diabetes mellitus (T2DM) are characterized by insulin resistance,with a significant epidemiological and pathological link between them.40,41Therefore,drugs targeting insulin resistance for T2DM could be highly effective in NAFLD treatment.
3.3. MicroRNA (miRNA)
Recently,there has been a surge in interest regarding the implications of dysregulated miRNA expression in the context of NAFLD.Certain miRNA markers,namely miRNA-34a,miRNA-122,and miRNA-192,42have been identified as potential diagnostic indicators for NAFLD.These miRNAs act as vital epigenetic mechanisms,reprogramming the entire liver metabolism.They primarily suppress gene expression and bind to complementary mRNA sequences to inhibit their translation,demonstrating a form of posttranscriptional regulation.Some miRNAs can enhance gene expression,adding to their functional complexity.For example,miRNA-122,which makes up 70%of the total hepatic miRNA pool,is one of the most abundant miRNAs in the liver.43Research indicates a significant elevation in serum miRNA-122 content in NAFLD patients.43-45Furthermore,a study by Longet al.,44revealed that miRNA-122 directly targets silent information regulator 1(SIRT1).MiRNA-122 binds to the mRNA of SIRT1,inhibiting its function and reducing its expression.This triggers an upregulation of the downstream SIRT1/liver kinase B1 (LKB1)/AMP-activated protein kinase (AMPK) pathway,increasing the expression of lipogenic enzymes such as SREBP-1,FASN,SCD1,ACC1,and apolipoprotein A5 (ApoA5).Specific miRNAs,such as miRNA-24,significantly enhance hepatic TG accumulation by effectively suppressing insulin-induced gene 1 (INSIG1) expression.46Recent studies also indicate that miRNAs such as miRNA-17,miRNA-20a,and miRNA-20b are closely associated with NAFLD development and progression.47While further investigation of the relevant pathways is needed,these findings underscore the crucial role of miRNAs in hepatic TG metabolism.Currently,clinical research on miRNArelated transformations is receiving significant attention.Besides serving as biomarkers,miRNA drugs also hold substantial potential for therapeutic effects.Despite the significant challenges in this field,advancements in related preclinical work indicate that solutions are imminent.48-50
3.4. Inflammation
Inflammation plays a significant role in the progression of NAFLD.This process involves various cells and molecules,with the immune system playing a pivotal role.Evidence suggests that inflammation is associated with DNL.For instance,the antiinflammatory factor interleukin (IL)-22 has been demonstrated to alleviate fatty liver degeneration induced by a high-fat diet.51However,the molecular mechanism linking inflammation and DNL remains largely unexplored.52During an inflammatory response,inflammatory factors may promote DNL through various pathways.53Nuclear factor-kappaB(NF-kB)is a significant signaling pathway that can be activated by numerous inflammatory factors,such as tumor necrosis factor-alpha (TNF-α) and IL-1β.NF-kB activation may stimulate DNL through a rare post-translational regulatory mechanism.54DNL upregulation could be due to increased protein stability of ACLY and FAS,55and altered phosphorylation of ACC1 protein,54leading to increased FA production.Other inflammation-related pathways,like mTORC1 and PDE3,52,56may also contribute to DNL upregulation.
3.5. Oxidative stress
Patients with NAFLD frequently experience oxidative stress.Reactive oxygen species (ROS),such as superoxide anion radicals and hydrogen peroxide(H2O2),consistently accumulate within the body.57Extensive research indicates that ROS regularly influence intracellular nuclear receptors,altering downstream metabolism.58H2O2can increase the expression of SREBP-1c and promote DNL.59Hyperuricemia,closely linked to NAFLD,60can also promote DNL through ROS-dependent c-Jun N-terminal kinase (JNK)/activator protein-1 (AP-1) signaling pathways.Certain antioxidants can reduce SREBP-1c expression and mitigate hepatic steatosis.61Therefore,ROS signaling plays a crucial role in regulating liver lipid homeostasis.
4.Excessive lipids damage hepatocytes
Overactivation of DNL can result in excessive fat accumulation in liver cells,potentially leading to NAFLD.62This process is governed by intricate mechanisms involving various cellular signaling pathways and molecular regulatory systems.We summarize the toxicity of excessive lipids to hepatocytes,focusing on aspects of epigenetic regulation,oxidative stress,and cell death.
4.1. Epigenetics regulation
Initially,dysfunctions in lipid metabolism can lead to changes in the epigenetic state,including DNA methylation and histone modification.These changes may affect various cellular physiological processes,leading to accelerated liver cell deterioration and death.63Numerous studies have highlighted the impact of high-fat diet on the transcriptional profiles of liver mRNA,including ApoB and dipeptidyl peptidase-4(DPP-4).Research findings suggest that the suppression of essential genes is triggered by an increase in DNA methylation frequency.64,65These epigenetic mechanisms may contribute to the development of NAFLD by modifying the cellular cycle,apoptosis,and inflammation signaling pathways.66Furthermore,the accumulation of ROS will intensify TG accumulation,leading to a vicious cycle.
4.2. Oxidative stress
Excess lipids lead to heightened mitochondrial stress and ROS production,initiating an oxidative stress response that disrupts the structure and function of hepatocytes.A study found that treatment with palmitic acid,a key fat metabolite,significantly increased ROS levels in cells,leading to mitophagy and endothelial cell damage.67Scientists have long recognized various forms of cell death,including apoptosis,autophagy,and necrosis,in the development of liver disease.Notably,oxidative stress critically regulates these processes.68,69
4.3. Cell death
Liver diseases primarily originate from the death of numerous hepatocytes,often triggered by a high-fat diet or other lipid metabolic disorders,through various mechanisms.In the NASH model,hepatocytes showed increased expression of apoptosisrelated proteins,such as caspase and JNK.70Simultaneously,there was activation of phosphorylation of necrosis-associated receptorinteracting protein(RIP)1,RIP3,and mixed lineage kinase domainlike (MLKL) proteins.71,72Lipid accumulation concurrently promotes necrosis and apoptosis in hepatocytes.70
5.DNL-targeted NAFLD therapy
5.1. Traditional NAFLD treatment
National guidelines for diagnosing and treating NAFLD recommend lifestyle-based interventions,primarily exercise and dietary modifications,as the most effective approach to manage the disease.1,73Reducing total caloric intake and increasing the consumption of soy protein,74,75omega-3 FA,76,77and monounsaturated fatty acids (MUFA) are recognized for their preventive and therapeutic benefits.Sport is equally important,with a 5%-10% body mass reduction significantly improving histological alterations in NAFLD.78Research shows that daily 60-min treadmill walks,along with improved sedentary habits could improve NAFLD cell apoptosis markers and insulin sensitivity.79Weight loss is also crucial in NAFLD treatment.Bariatric surgery,in addition to lifestyle changes,is the most effective treatment for obesity and NAFLD.80,81A study showed that after one year,85% of the 109 recipients did not develop NASH,and 33% experienced remission of fibrosis(Fig.3).82However,achieving sustained weight loss through lifestyle modifications alone can be challenging for most people with obesity,including those with NAFLD.Moreover,bariatric surgery may not be feasible for all NASH patients and should only be considered for those with concurrent obesity-related comorbidities.The 2018 American Association for the Study of Liver Diseases(AASLD) guidelines assert,“It is premature to consider foregut bariatric surgery as an established option to specifically treat NASH.”83As a result,patients frequently need medication for additional treatment.

Fig.3.NAFLD treatment.Current treatment options for NAFLD include lifestyle modifications,bariatric surgery,and drug interventions.Certain drugs that inhibit DNL show promise in managing NAFLD,with some even reaching clinical trials and gaining regulatory approval for treating other diseases.Abbreviations:ACC,acetyl-CoA carboxylase;ACLY,ATP-citrate lyase;BTC,benzenetricarboxylate;CIC,citrate-isocitrate carrier;DNL, de novo lipogenesis;FAS,fatty acid synthase;NAFLD,non-alcoholic fatty liver disease.
Recent scientific advancements in understanding the pathogenesis of NAFLD have led to the identification of numerous therapeutic drug targets.These drugs can be classified into four categories.84(i) Primarily focusing on metabolic-related factors to target the metabolic stress induced by hepatic lipid deposition.(ii)Mitigating oxidative stress,inflammation,and injuries due to metabolic stress.(iii) Regulating the gut-liver axis interactions in NAFLD.(iv) Liver fibrosis therapeutics could potentially mitigate the progression of fibrosis and its associated complications(Fig.3).
5.2. Drugs that inhibit DNL
As previously mentioned,several factors in NAFLD can collectively stimulate the upregulation of DNL,subsequently triggering lipotoxicity.Single-factor therapy may not effectively reverse the progression of NAFLD.Pharmacological inhibition of DNL may present a promising therapeutic intervention.Recently,numerous plant-derived natural products have been identified as potential DNL inhibitors,85setting the stage for the development of synthetic equivalents.Some DNL inhibitors are currently under clinical trial,offering potential strategies for NAFLD treatment.86This review evaluates inhibitors targeting the primary enzymes of DNL,namely CIC inhibitors,ACLY inhibitors,ACC inhibitors,and FAS inhibitors(Fig.3 and Table 1).

Table 1 DNL inhibitors have entered clinical trials.
5.2.1.CIC inhibitor
The CIC,located within the inner mitochondrial membrane,facilitates the transport of citrate from the mitochondria into the cytoplasm.87Benzenetricarboxylate(BTC)has been used as a firstgeneration inhibitor of CIC.87However,its ability to bind to other proteins presents challenges for therapeutic development.The second-generation inhibitor,CTPI-1,was discovered in yeast CIC.By enhancing CTPI-1,CTPI-2 demonstrated a 20 times higher affinity than its predecessor.88In patients with NAFLD,there was an increase in CIC expression in the liver.Remarkably,the administration of CTPI-2 in high-fat diet-fed mice resulted in the reversal of steatohepatitis and liver injury,along with reductions in cholesterol and TG levels.89CTPI-2 may also influence gluconeogenesis,leading to improved glucose tolerance and insulin sensitivity.Inhibitors of CIC hold potential for use in weight loss,anti-inflammation,90and anti-cancer therapies.91,92For instance,inhibiting CIC could hinder the proliferation of acute myeloid leukemia cells,while simultaneously increasing the rate of apoptosis.93CIC is widely expressed across various physiological areas,including the liver,reproductive organs,gastrointestinal tract,and adipose tissue.86Despite its promise for treating NAFLD,regulatory requirements necessitate that it remains in preclinical trial stages.
5.2.2.ACLY inhibitor
ACLY,exhibiting the highest expression levels in fat and liver tissue and the lowest in skeletal muscle,is a compelling drug target.Several ACLY inhibitors have been identified that effectively inhibit the synthesis of both FA and cholesterol,while simultaneously promoting the vital process of FA β-oxidation.The initial ACLY inhibitors discovered and studied were natural compounds:(-)-hydroxycitric acid (HCA),94purpurone,95radicicol,96and cucurbitacin b.97Despite their potent activity in inhibiting ACLY,their action mechanisms and specificity remain uninvestigated.The USA Food and Drug Administration (FDA) recently authorized the use of bempedoic acid for patients with heterozygous familial hypercholesterolemia and arteriosclerotic cardiovascular disease(ASCVD).The drug,being the first oral lipid-lowering medication approved in the USA in two decades,specifically targets ACLY.Bempedoic acid has shown its competitive potential in producing a CoA conjugate.The conversion of this substance to CoA conjugates involves the long-chain acyl-CoA synthetase (ACSVL1/FATP2/SCL27A2).This enzyme is predominantly expressed in liver tissue and rarely in other areas.Bempedoic acid's characteristics make it liver-specific.98Research has shown that administering bempedoic acid to NASH model mice increases FA oxidation.This intervention concurrently suppressed lipogenesis,alleviated steatosis,and reduced serum TG.Interestingly,bempedoic acid participates in the collagen production pathway,lessening liver fibrosis and encouraging hepatic stellate cells to adopt an anti-fibrotic role.99While it is currently only approved as a prescription drug for hypercholesterolemia and ASCVD,the therapeutic potential and liver-specific characteristics of bempedoic acid indicate a possible future use in NAFLD treatment.
5.2.3.ACC inhibitor
In mammals,ACC comprises two subtypes,termed ACC1 (also known as ACC α) and ACC2 (also known as ACC β).ACC1 is ubiquitously expressed,while ACC2 is primarily found in skeletal muscle,breast,fat,and liver.86As a potential therapeutic target,ACC inhibition offers a dual benefit: it both suppresses DNL and enhances FA oxidation.Significant advancements in developing ACC-targeting inhibitors have led to numerous such inhibitors entering clinical trials.PF-05221304,developed by Pfizer,is a notable inhibitor with excellent liver selectivity.100This drug avoids potential toxicity by not inhibiting platelet formation and preventing developmental defects.101,102Phase II studies of PF-05221304,used alone or combined with diacylglycerol acyltransferase(DGAT)inhibitors(PF-06865571)for NAFLD,have been completed.103The drug independently facilitated a 50%-65%dosedependent reduction in liver fat for dosages ≥10 mg once daily(QD).However,complications like increased TG production arose.In contrast,when combined with PF-06865571,patients did not discontinue their medication despite some adverse effects,suggesting that co-administration of both drugs could potentially address the shortcomings of ACC inhibitors.Nimbus Therapeutics has developed a variety of ACC inhibitors,including ND-630 that targets a distinct domain.The inhibitors are designated as ND-646,ND-654,etc.ND-630 (firsocostat),currently in phase II trials for NASH,showed promising results in guinea pig models,reducing liver DNL by 22% and liver steatosis by 21%.104This indicates significant potential benefits for NASH patients.105
5.2.4.FAS inhibitor
FAS is highly expressed in human adipose tissue and reproductive organs.Eukaryotic FAS typically falls into two categories.Type 1 FAS multifunctional complexes,expressed in the cytoplasm,106exclusively synthesize palmitic acid.Impressively,type 2 FAS operates independently of its counterpart,functioning exclusively within mitochondria to produce a variety of FA.107Successful inhibitors of FAS,such as orlistat,GSK2194069,Bi-99179,TVB-3166,and TVB-2640,have been developed.Among them,orlistat is an FDA-approved drug currently used for weight management in obese individuals.These drugs,some of which have demonstrated significant cytotoxicity against cancer cellsin vitro,have advanced to clinical trials.This suggests potential therapeutic benefits in tumor treatment.
5.3. DNL inhibitor challenges
5.3.1.Drug safety
The safety of any drug remains a paramount concern.Various safety challenges exist regarding DNL inhibitors.One such challenge involves their potential detrimental effects on embryonic development.Mice deficient in ACLY,108ACC1,109or FAS died at different stages of embryonic development,110while those lacking CIC developed inflammatory neurodevelopmental disorders.Genetic defects are not the sole culprits,as pharmacological inhibition of DNL also leads to toxic effects.The ACC inhibitor PF-05175157 significantly induced developmental toxicity in both rat and rabbit models.101These findings suggest that DNL likely plays a vital role in embryonic neural development.It is essential to avoid systemic suppression of DNL to prevent negative effects on developing embryos.Further research is necessary to evaluate the safety of DNL inhibitors in pregnant women,and caution should be exercised until this data is available.
The differences between Glires and humans significantly influence the use of DNL inhibitors.Consider the clinical trial for the ACC inhibitor PF05175157,where no toxic effects were observed in rodents,but it led to a reduction in human platelet numbers.102Indeed,ACC inhibition in bone marrow can halt megakaryocyte maturation.102Recent studies have suggested the feasibility of selectively inhibiting key enzymes such as FAS,ACLY,and ACC within the liver.86However,it remains uncertain whether novel studies on other organs and cells will determine the long-term safety of inhibiting DNL.Therefore,DNL inhibitors must be as liver-selective as possible.
5.3.2.Metabolic imbalance
The use of DNL inhibitors represents a potentially innovative factor that could influence metabolic balance.For instance,their use can redistribute lipids,elevate serum TG levels,and induce hyperlipidemia.111These adverse effects were noted in studies on MK-4074 and ND-630.105It is now clear that ACC inhibition triggers FA feedback,leading to the suppression of the PPAR pathway and clinical signs of increased TG.ACC inhibitors are currently in development,and it is expected that they will be used in combination with PPAR or farnesoid X receptor (FXR) agonists to minimize potential adverse reactions.Moreover,inhibition of DNL is known to cause the accumulation of metabolic intermediates,leading to significant biological effects.112Optimal treatment outcomes may necessitate the integration of lifestyle modifications with the use of these inhibitors.Furthermore,ACLY inhibitors were initially anticipated to be effective due to their ability to inhibit TG production.However,excessive fructose consumption can lead to TG production independently of ACLY,potentially limiting the efficacy of these inhibitors.The activation of this compensatory pathway could potentially affect the body's metabolic profile.
6.Conclusions and future perspectives
In recent years,significant progress has been made in the investigation of the pathogenesis and pharmacotherapy of NAFLD.Nevertheless,achieving histological improvement in NASH treatment,particularly surpassing a 50%threshold,remains a significant challenge.Hepatic steatosis is the foundation of conditions associated with NAFLD.Therefore,understanding the main factors contributing to TG accumulation is of utmost importance.Among the various TG sources,DNL warrants our attention.There is substantial evidence suggesting significant alterations in DNL occur in NAFLD.Though DNL-derived TG may not form a significant part of liver fat,its production notably increases in NAFLD and is subject to complex regulation.Thus,targeting DNL is a critical research focus for the treatment of NAFLD.
Our literature review has analyzed the mechanisms of DNL upregulation,including high fructose intake,insulin resistance,microRNA dysregulation,and other factors.Despite their distinct characteristics,all these regulatory mechanisms enhance DNL gene expression at the transcriptional level.Excessive TG accumulation can lead to liver tissue damage and further disease progression.Targeting downstream DNL could effectively reduce TG accumulation,considering the complex nature of the upstream regulatory network.Some drugs have proven effective in both preclinical and clinical trials,with a few even gaining FDA approval for treating other diseases.
Nevertheless,it is crucial to acknowledge the limitations of this therapeutic strategy,including concerns about drug safety and potential metabolic imbalances.Increasing evidence suggests that the pathways implicated in NAFLD are interconnected,influencing each other as both causes and effects.23Therefore,targeting a single pathway or specific target may unintentionally trigger compensatory mechanisms or side effects through other pathways.To address these limitations,future strategies could explore the implementation of multi-target combination therapy.This approach aims to achieve a more comprehensive and effective intervention by simultaneously targeting multiple pathways.Clinical trials(NCT02891408,NCT03987074)are currently underway to investigate the combination of ACC inhibitors with PPAR or FXR agonists.This combination therapy has the potential to reduce side effects and potentially improve histological outcomes.
The target population for drug use needs to be further clarified.We need to improve the early detection of liver steatosis in individuals and promptly encourage them to enhance their lifestyle.The initial phases of NAFLD are typically reversible,but early drug intervention is generally not recommended.Nonetheless,it is crucial to acknowledge the potential risk of rapid progression.Once the disease enters an irreversible phase,drug interventions can effectively manage the progression of liver disease.We anticipate that implementing drug interventions for patients will yield significant benefits for a larger number of individuals.
This review emphasizes the significance of upregulating and treating DNL in NAFLD.We anticipate this will inspire further research in this area.
Authors’ contributions
Jin Chai and Zhixian Zhu conceptualized the manuscript.Zhixian Zhu and Xiaoxun Zhang wrote this manuscript.Qiong Pan and Liangjun Zhang provided constructive comments and editing to the manuscript.All authors have read and approved the final version of this manuscript.
Declaration of competing interest
The authors declare that there is no conflicts of interest.
Acknowledgements
This work was supported by grant from the National Natural Science Foundation of China (82325008).
- Liver Research的其它文章
- Dietary pattern and hepatic lipid metabolism☆
- The contributions of bacteria metabolites to the development of hepatic encephalopathy☆
- Autophagy modulates physiologic and adaptive response in the liver☆
- Hepatocellular carcinoma recurrence: Predictors and management☆
- Unveiling the effect of estrogen receptors in alcoholic liver disease:A novel outlook☆
- Sequential ultrasound molecular imaging for noninvasive identification and assessment of non-alcoholic steatohepatitis in mouse models☆

