Should they wait?Two children under 3 years old infected by HCV 1b successfully treated by ledipasvir/sofosbuvir: A report of two cases☆
Mingn Li ,Kuernnis Wulyin ,Shuto Lin ,Cho Wu ,Luio Chen ,*
a Department of Infectious Diseases, The Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, Guangdong, China
b Department of Infectious Diseases, The First People’s Hospital of Kashi, Kashi, Xinjiang, China
Keywords: Hepatitis C virus (HCV)Ledipasvir (LDV)Sofosbuvir (SOF)Direct-acting antivirals (DAAs)Genotype 1b Children
ABSTRACT Although direct-acting antivirals(DAAs)have notably increased the sustained virological response(SVR)rates in hepatitis C virus (HCV)-infected adolescent patients,the efficacy and safety for young children under 3 years old remain unclear.Currently,no guidelines recommend DAA therapy for this situation worldwide.Furthermore,the China National Medical Products Administration has not approved any DAA for treating children below 12 years old.Here,we described the characteristics of two children approximately 2 years old,who were infected by HCV genotype 1b and had significant clinical symptoms.Both received 12 weeks of ledipasvir/sofosbuvir(Case 1:45.00 mg/200 mg per day,weight 17 kg;Case 2:33.75 mg/150 mg per day,weight 12 kg).They achieved SVR at 12 weeks after treatment completion without obvious treatment-related adverse effects.Therefore,the safety and benefits of ledipasvir/sofosbuvir treatment in children under 3 years old seem to be confirmed.Our findings require further evaluation.
1.Introduction
The global disease burden of the pediatric population under 18 years old with hepatitis C virus(HCV)infection was estimated to be 0.13% (3.26 million children) in 2018.1Although HCV infection is usually asymptomatic and progresses slowly during childhood,it can lead to liver cirrhosis,end-stage liver disease,and hepatocellular carcinoma in adulthood without efficient antiviral treatment.2Therefore,antiviral treatment of HCV infection is crucial.
Antiviral therapy for HCV infection has achieved good clinical outcomes,progressing from a poorly tolerated interferon-based therapy that required subcutaneous injections and was associated with major side effects to an all-oral well-tolerated regimen with virological cures for over 90% of patients.3Direct-acting antiviral(DAA)drugs,considered highly effective curative therapies for HCV infection,have been approved by the United States Food and Drug Administration(FDA)for pediatric patients.4For children aged over 3 years,the FDA approved ledipasvir/sofosbuvir (LDV/SOF) in August 2019,SOF plus ribavirin(RBV)in September 2019,4and two pan-genotypic antiviral regimens (SOF/velpatasvir (VEL) and glecaprevir/pibrentasvir (GLE/PIB)) in June 2021.5In 2019,the American Association for the Study of Liver Diseases (AASLD)recommended that all HCV-infected children and adolescents over 3 years old can be treated with approved DAA drugs,including SOF plus RBV and LDV/SOF.4Furthermore,SOF/VEL and GLE/PIB were recommended for patients over 3 years old by the European Association for the Study of the Liver (EASL) in 2020.6In 2019,the Chinese Medical Association (CMA) had not recommended any DAA treatment for children under 12 years old.7Nonetheless,in 2022,the CMA recommended SOF/VEL,LDV/SOF,and SOF plus RBV for treating children over 3 years old.8However,no DAAs have been approved for treating children less than 12 years old by the China National Medical Products Administration (CNMPA).8Although LDV/SOF is not recommended by EASL or approved by CNMPA for treating children over 3 years old,three clinical trials have shown that sustained virological response (SVR) of LDV/SOF at 12 weeks after the end of treatment(SVR12)is comparable to those observed in adults,with rates of 97% (33/34) in those aged 3-5 years,99%(91/92) in those aged 6-11 years,and 98% (98/100) in those aged 12-17 years.9-11Nevertheless,the efficacy and safety profile of DAA therapy for children under 3 years old have rarely been reported.Hence,we report the cases of two 2-year-old children infected by HCV genotype 1b who were treated with LDV/SOF and achieved SVR12.
2.Case report
2.1. Ethical approval
The study complied with the Declaration of Helsinki and was approved by the Ethics Committee of The First People’s Hospital of Kashi (approval No.2022-03).LDV/SOF antiviral therapy was initiated with the informed consent of the patients’ parents.
2.2. Case 1
A 30-month-old Han male child was admitted to the First People’s Hospital of Kashi on September 11,2021,because of poor appetite,intermittent diarrhea (3-5 times/day),and no weight gain for several weeks.Clinical examination showed fluctuations in alanine aminotransferase (ALT) and aspartate aminotransferase(AST) levels,with the highest values of 320 U/L (reference range:5-40 U/L) and 117 U/L (reference range: 8-40 U/L),respectively.Serum bilirubin,gamma-glutamyl transferase (GGT),and prothrombin time activity (PTA) were within normal limits.Serologic testing revealed the presence of anti-HCV antibody,and hepatitis C was confirmed by HCV-RNA detection with the polymerase chain reaction (PCR) method.We excluded hepatitis A virus (HAV),hepatitis B virus(HBV),hepatitis D virus(HDV),hepatitis E virus(HEV),cytomegalovirus(CMV),human immunodeficiency virus(HIV),and treponema pallidum (TP) infections,as well as autoimmune hepatitis.The Epstein-Barr virus(EBV)viral load showed 10,000 copies/mL of deoxyribonucleic acid,so we could not exclude the possibility that it contributed to the diarrhea.Considering the child’s young age,the patient’s parents did not consent to antiviral treatment for the EBV infection.Apart from hematological diagnostics,we looked for other diarrhea etiologies and did not find norovirus or rotavirus in stool samples.The bacteriological culture of stool was also negative.
2.3. Case 2
A 29-month-old Han boy was found to be anti-HCV antibody positive before a preoperative examination for inguinal hernia in a local county hospital.The HCV-RNA result was also positive by PCR.His parents complained that he had a poor appetite and had been underweight for a long time.Laboratory tests at the First People's Hospital of Kashi showed that aminotransferases were elevated(ALT 226 U/L,AST 161 U/L)on December 24,2021.Serum bilirubin,GGT,and PTA were within normal limits.He was fully inoculated with the HBV vaccine,and the hepatitis B surface antibody was positive.HAV,HBV,HDV,HEV,EBV,CMV,HIV,and TP infections were excluded.
The baseline and follow-up data of these two patients are shown in Table 1.They were admitted to the First People’s Hospital of Kashi with elevated transaminase levels and obvious symptoms such as poor appetite,diarrhea,and underweight.Abdominal ultrasonography showed normal parenchymal echogenicity and no evidence of liver cirrhosis or ascites.Based on the laboratory tests and ultrasonography,both were found to be infected by HCV genotype 1b,and cirrhosis was excluded.Vertical transmissions from their mothers were ruled out.The two children had never been transfused.Due to the high prevalence of hepatitis C in the Kashi region of Xinjiang,China,where genotype 1b is predominant,we could not rule out that the two patients may have been exposed to nontransfusion healthcare-related transmission.12For instance,the second patient had undergone bronchoscopy at a local hospital because of a foreign body in the airway.
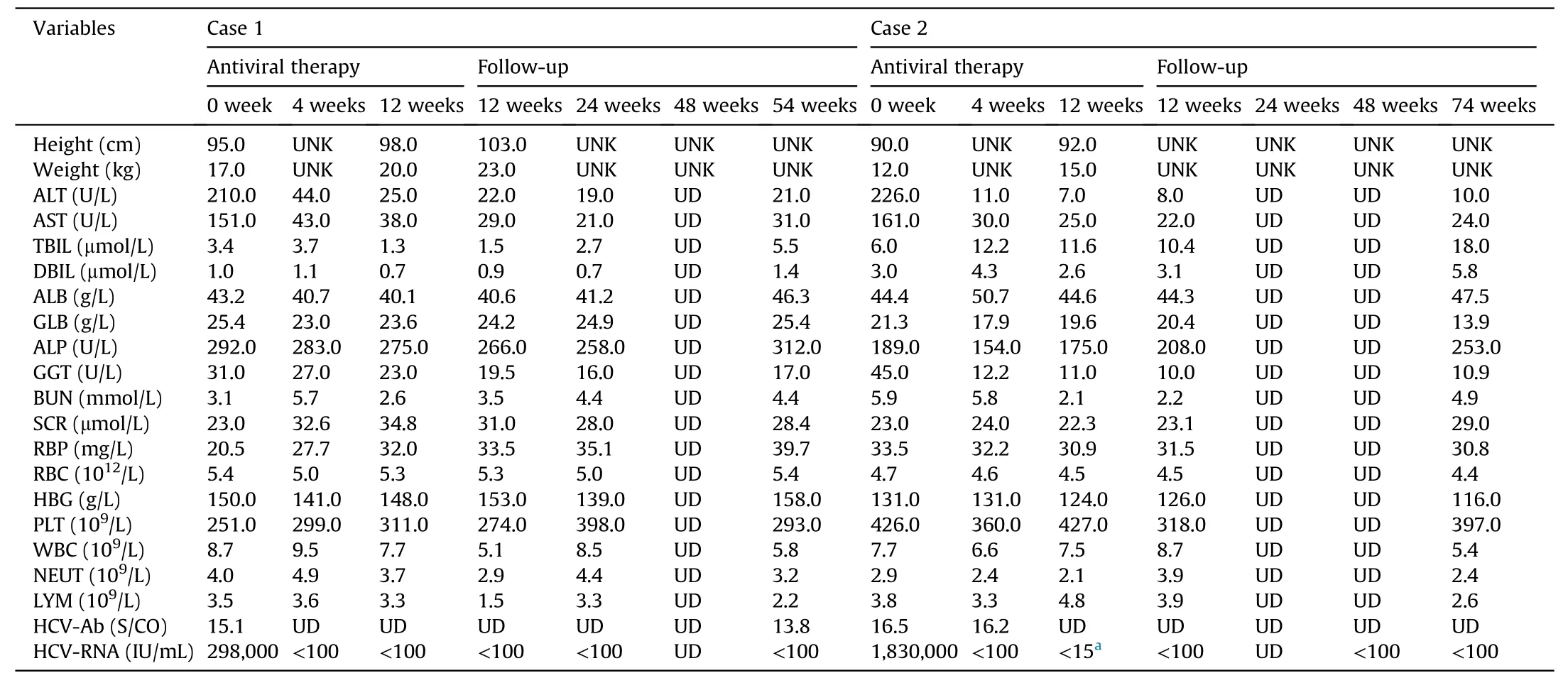
Table 1 Baseline and follow-up data of two male children with HCV genotype 1b infection.
2.4. Antiviral treatment and follow-up
In the two cases,we could not pinpoint the specific timing of the infections.Consequently,we could not distinguish acute fromchronic infection.These two children had active hepatitis,apparent clinical symptoms,and poor weight gain.During the hospitalization of two patients,the First People’s Hospital of Kashi only had the DAA drugs (LDV/SOF).After providing information to the patients’ families according to relevant guidelines,they requested LDV/SOF therapy.Thus,Case 1 and Case 2 began antiviral treatment on September 20,2021,and January 6,2022,respectively;the dosing was weight-based.Case 1 was treated with LDV/SOF(45.00 mg/200 mg per day)for 12 weeks,and Case 2 received 12 weeks of LDV/SOF at a lower dose (33.75 mg/150 mg per day).
Both of the cases achieved early complete viral response(HCVRNA undetectable,<100 IU/mL) 4 weeks after the initiation of therapy;the therapy ended in the 12thweek (Case 2 was tested according to the COBAS AmpliPrep/COBAS TaqMan HCV test),and SVR12 was achieved.Case 1’s parents refused the HCV-RNA test by the COBAS method for financial reasons.Thus,the more precise approach for HCV testing was performed once in Case 2.In addition,Case 1 achieved undetectable HCV-RNA 24 weeks after the end of treatment (SVR24),while Case 2 was lost to follow-up at the 24thweek after treatment completion owing to the impact of the COVID-19 pandemic.Fifty-four weeks after treatment completion for Case 1 (December 26,2022) and 74 weeks after treatment completion for Case 2 (September 3,2023),HCV-RNA remained cured.Both completed their antiviral therapy without dosage adjustment during their antiviral therapy.Throughout treatment and follow-up,neither experienced obvious adverse effects,including fever,jaundice,rash,headache,fatigue,nausea,and vomiting.Case 1 stopped having diarrhea and gradually regained appetite after 2 weeks of antiviral treatment,gaining 3 kg by the end of the treatment.Case 2 reported increased appetite during the follow-up visit after 4 weeks of treatment and gained 3 kg by the end of the treatment.
3.Discussion
We briefly reported the cases of two pediatric patients approximately 2 years old with HCV genotype 1b infection.DAA therapy for children under 3 years old has rarely been reported,and its safety profile has rarely been documented.To our knowledge,no guidelines have recommended DAA therapy for children under 3 years old,and no DAA has been approved for treating children under 12 years old by the NMPA of China.
A recent study showed that approximately 224,000 children under 2 years old were infected with HCV globally,of whom children in China accounted for 17,800.1However,the situation may be more serious because of China's relatively high birth rate and vast population.Hepatitis C may affect more children in western areas due to its high prevalence in western China.12The spontaneous resolution rate in untreated children varies from 9%to 20%.13Once infection occurs,it often persists into adulthood.13Despite the widespread belief that HCV in children progresses slowly and benignly,some cases experience accelerated progression to cirrhosis and end-stage liver disease.14-16Chronic HCV infection is also associated with impaired health-related quality of life in pediatric patients.17Highly effective and well-tolerated DAA therapy for children over 3 years old has been validated and recommended in guidelines,4-6,8so an expansion of pediatric indications for DAAs may benefit children below 3 years old.
Schwarzet al.9conducted a clinical trial investigating weightbased LDV/SOF treatment in children aged 3-5 years with chronic HCV genotype 1 (33 patients) or genotype 4 (1 patient).Although they did not include any patients with genotype 5 or 6,the FDA approved the expansion of LDV/SOF indications in August 2019 to include patients aged 3-11 years with HCV genotypes 1,4,5,and 6 who do not develop cirrhosis or progress to compensated cirrhosis.4This decision was based on similar pharmacokinetic exposures,favorable safety profiles,and similar data compared to previous adolescent and adult studies.Limited real-world data further confirm the efficacy of LDV/SOF.18,19Although the CNMPA has not approved any DAA for treating children under 12 years old with chronic HCV infection,the patients in the current report had elevated transaminase levels with obvious symptoms,including poor appetite,diarrhea,and poor weight.Considering that the FDA has already approved LDV/SOF for children over 3 years old,we decided to initiate antiviral therapy with care after obtaining written informed consent from the patients’parents.The two cases in the report demonstrated that LDV/SOF therapy in children under 3 years old was feasible and achieved SVR12.Moreover,LDV/SOF was well tolerated without obvious treatment-related adverse events.
HCV eradication in children is associated with health benefits,such as the prevention of transmission and elimination of adverse effects on cognitive and psychosocial health.LDV/SOF for HCV eradication enhances patients’ quality of life.20Moreover,Greenawayet al.21reported that early intervention for children was economically beneficial.Thus,early antiviral therapy appears favorable for HCV-infected children based on these advantages.
In conclusion,this report suggests that LDV/SOF therapy is safe and feasible for HCV-infected children with high hepatitis activity,even if they are below 3 years old.Clinical trials using DAAs to treat children with HCV infection have yielded encouraging outcomes.However,the clinical policy for young children has not been standardized.Further investigations are needed to determine the optimal antiviral therapy for children with HCV infection,including the appropriate initiation time for antiviral agents,medications and dosing,and treatment duration.
Authors’contributions
Mingna Li and Shutao Lin drafted the manuscript.Kuerbannisa Wulayin and Chao Wu followed up the patients and collected clinical data.Lubiao Chen critically revised the manuscript and provided funding support.All authors have read and agreed to the final version of the manuscript.
Declaration of competing interest
The authors declare that there is no conflicts of interest.
Acknowledgements
The study was funded by the Special Project of Guangdong Rural Science and Technology Commissioner of China (KTPYJ2021014).
- Liver Research的其它文章
- Dietary pattern and hepatic lipid metabolism☆
- In-depth analysis of de novo lipogenesis in non-alcoholic fatty liver disease: Mechanism and pharmacological interventions☆
- The contributions of bacteria metabolites to the development of hepatic encephalopathy☆
- Autophagy modulates physiologic and adaptive response in the liver☆
- Hepatocellular carcinoma recurrence: Predictors and management☆
- Unveiling the effect of estrogen receptors in alcoholic liver disease:A novel outlook☆

