Clinical-radiomics predictors to identify the suitability of transarterial chemoembolization treatment in intermediate-stage hepatocellular carcinoma: A multicenter study
Dan-Dan Wang ,Jin-Fng Zhang ,Lin-Han Zhang ,Mng Niu ,Hui-Ji Jiang ,Fu-Cang Jia ,,Shi-Ting Fng f,
a Department of Radiology,the Second Affiliated Hospital of Harbin Medical University,Harbin 150086,China
b Shenzhen Institute of Advanced Technology,Chinese Academy of Sciences,Shenzhen 518055,China
c Department of Breast Surgery,Harbin Medical University Cancer Hospital,Harbin 150081,China
d Department of PET/CT,the First Affiliated Hospital of Harbin Medical University,Harbin 150 0 07,China
e Department of Interventional Therapy,the First Affiliated Hospital of China Medical University,Shenyang 110 0 01,China
f Department of Radiology,the First Affiliated Hospital of Sun Yat-sen University,Guangzhou 510080,China
Keywords: Transarterial chemoembolization Hepatocellular carcinoma Radiomics Machine learning Prediction
ABSTRACT Background: Although transarterial chemoembolization (TACE) is the first-line therapy for intermediatestage hepatocellular carcinoma (HCC),it is not suitable for all patients.This study aimed to determine how to select patients who are not suitable for TACE as the first treatment choice.Methods: A total of 243 intermediate-stage HCC patients treated with TACE at three centers were retrospectively enrolled,of which 171 were used for model training and 72 for testing.Radiomics features were screened using the Spearman correlation analysis and the least absolute shrinkage and selection operator (LASSO) algorithm.Subsequently,a radiomics model was established using extreme gradient boosting (XGBoost) with 5-fold cross-validation.The Shapley additive explanations (SHAP) method was used to visualize the radiomics model.A clinical model was constructed using univariate and multivariate logistic regression.The combined model comprising the radiomics signature and clinical factors was then established.This model’s performance was evaluated by discrimination,calibration,and clinical application.Generalization ability was evaluated by the testing cohort.Finally,the model was used to analyze overall and progression-free survival of different groups.Results: A third of the patients (81/243) were unsuitable for TACE treatment.The combined model had a high degree of accuracy as it identified TACE-unsuitable cases,at a sensitivity,specificity,and area under the receiver operating characteristic curve (AUC) of 0.759,0.885,0.906 [95% confidence interval(CI): 0.859-0.953]in the training cohort and 0.826,0.776,and 0.894 (95% CI: 0.815-0.972) in the testing cohort,respectively.Conclusions: The high degree of accuracy of our clinical-radiomics model makes it clinically useful in identifying intermediate-stage HCC patients who are unsuitable for TACE treatment.
Introduction
Hepatocellular carcinoma (HCC) and concomitant mortalities are on the rise and this is a current public health challenge [1].Although HCC is mainly treated through surgery,local and systemic therapies,it is nonetheless associated with poor clinical outcomes,as most cases are detected at advanced stage [2].
Transarterial chemoembolization (TACE) is a minimally invasive,first-line treatment for intermediate-stage HCC without extrahepatic metastasis or macrovascular invasion [3–7].The Asia-Pacific Primary Liver Cancer Expert (APPLE) characterizes intermediatestage HCC as I,a single tumor of maximum size ≥ 5 cm of Barcelona Clinic Liver Cancer (BCLC) A stage,and II,at BCLC B stage– both types are qualified for TACE therapy [8].Intermediatestage HCCs are highly heterogeneous in tumor size,number,and liver function,and this results in patients at the same stage having different outcomes [9,10].Although several consecutive rounds of TACE are used to suppress tumor growth,they are still ineffective in some cases [11–13].Furthermore,continuous use of TACE until refractory not only impairs liver function but also eliminates the possibility of switching to systemic therapies like sorafenib [14].Thus,it is important to determine whether a patient is suitable for TACE therapy,and for those who are unsuitable,other therapies like lenvatinib should be applied first,followed by TACE (LEN-TACE sequential therapy) [15].
Medical imaging techniques,like contrast-enhanced computed tomography (CT),are crucial in the diagnosis and treatment of HCC [16,17].Unlike traditional imaging techniques that only have a limited observation of the biological characteristics of tumors,the nascent technique of radiomics is currently used to extract and analyze numerous high-dimensional features from medical images,for a deeper understanding of diseases [18].Indeed,the diagnosis,evaluation of treatment response,prognosis prediction and the understanding of molecular and immunological features of HCC have been advanced through radiomics [19–23].
Here,we developed and validated a novel model for accurately identifying patients who may not benefit from initial TACE treatment,via analysis of radiomics-based liver tumor imaging informatics and clinical characteristics.
Patients and methods
Patients
Patients for training and testing cohorts were enrolled from the Second Affiliated Hospital of Harbin Medical University,the First Affiliated Hospital of China Medical University,and the First Affiliated Hospital of Sun Yat-sen University respectively after the grant of ethical approval for the study from respective ethical committees.Due to the nature of this study,the patients’ written informed consent was waived.Records for 506 consecutive HCC patients who underwent TACE monotherapy between January 2012 and December 2019 were obtained,and those that met the following criteria were included in the study: (i) intermediate-stage HCC based on APPLE consensus,(ii) patients aged 18–80 years,(iii)TACE as initial treatment (both conventional and drug-eluting bead TACE),and iv) Child-Pugh A or B before treatment.Exclusion criteria: (i) diffuse distributed HCC (n=75),(ii) incomplete follow-up data for evaluation of consecutive treatment response (n=126),and (iii) unsatisfactory image quality due to artifacts,or slice thickness >5 mm (n=62).Thus,171 and 72 patients for the training and testing cohorts were enrolled (Fig.1).The TACE procedure was performed by experienced clinicians and is depicted in Supplementary materials.The first follow-up was 4-6 weeks after TACE,then every 1-3 months.
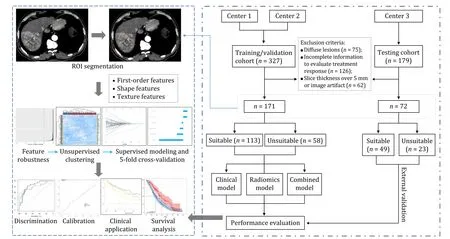
Fig.1.Workflow of the study.The ROI for each transverse section was manually segmented on contrast-enhanced computed tomography.Spearman correlation test and feature reduction algorithms were used to determine radiomics features.Models were constructed using supervised machine learning methods with 5-fold cross-validations.Univariate and multivariate logistic regression analyses were used to identify clinical features.The performance of models was evaluated via receiver operating characteristic,calibration,and decision curves.ROI: region of interest.
For each procedure,a consensus-based evaluation of TACE response was done by two radiologists using the modified response evaluation criteria in solid tumors (mRECIST) criteria [24].Based on contrast-enhanced CT images,objective response (OR) was defined as either complete response (CR,disappearance of arterial enhancement in all target lesions),or partial response (PR,a reduction in the diameter of arterial enhancement lesions by >30%).In this study,HCC patients were determined as unsuitable for TACE as the first-line treatment if: ≥2 consecutive treatments failed to achieve an OR after selective TACE;the number of tumors increased (as detected via imaging within 1-3 months) after changing the chemotherapeutic agents;extrahepatic spread or vascular invasion was detected;liver function damage occurred (like the occurrence of severe ascites or hepatic encephalopathy);there was a continuous elevation in the tumor marker (alpha-fetoprotein,AFP)after TACE (Fig.2).

Fig.2.Images of patients unsuitable for TACE.A: The images showing liver function damage after TACE,resulting in ascites (arrows indicating the lesions diameter increased);B: two consecutive treatments failed to achieve objective response (arrows indicating continuously enhanced lesions after TACE);C: produced a portal venous embolus after TACE (arrows showing the filling defect of portal vein);D: extrahepatic invasion occurred after TACE (arrows showing primary lesion in the liver,normal adrenal gland before TACE,and metastases in the adrenal gland after TACE,respectively).TACE: transarterial chemoembolization.
Lesion segmentation and feature extraction
Images were anonymized via conversion into NIfTI format.All segmentations of targeted lesions,based on the largest diameter in each patient,were done by a single radiologist using the Medical Imaging Interaction Toolkit (MITK,version 2016.11.0;https://www.mitk.org/).Regions of interest (ROIs) were manually outlined on consecutive slices of the largest lesion in the arterial and portal venous phase images.
PyRadiomics (version 2.1.0;https://pyradiomics.readthedocs.io/)was used to extract radiomics features from labeled ROIs.Image standardization was done via B-spline interpolation sampling technology for resampling.The resampled pixel spacing was (1,1,1).Original CT images were transformed using Laplacian of Gaussian and wavelet filters.Feature categories comprised first-order statistics,shape,and texture features.
Segmentation was done by two physicians,both with over 5-year experience in abdominal radiological diagnosis,who were blinded to treatment responses.To test feature robustness,the intra-class correlation coefficient (ICC) was determined.After an interval of 14 days for the first segmentation,30 patients were randomly selected and subjected to another segmentation.The intraand inter-observer ICCs were calculated,and ICC features less than 0.75 were regarded as unreliable and thus excluded.All remaining features were then standardized by calculating their z-scores(mean,0;variance,1).
Harmonization
Although individual CT scanning and reconstruction parameters uniquely influence radiomics features,standardizing CT scans done at the three different centers were difficult-CT scan protocols used by the three centers are described in Supplementary materials.Yet combat harmonization [25],a procedure that retains biological variations unrelated to the center protocol effect,is not only widely used in genomic studies but has also proved reliable for radiomics,and was thus used to harmonize radiomics features after ICC.The probability density distribution map of features for the three centers,before and after harmonization,is shown in Fig.S1.
Feature engineering and prediction model construction
The most predictive features were identified from the training cohort.Spearman correlation analysis was done and features with correlation coefficients >0.85 were excluded.The least absolute shrinkage and selection operator (LASSO) was used to identify candidate features.This process of feature identification was repeated for the arterial and portal venous phases.Finally,selected features from the two phases were combined in a set,and using seven different machine learning classifiers — support vector machine (SVM),decision tree (DT),random forest (RF),extreme gradient boosting (XGBoost),K-nearest neighbors (KNN),linear discriminant analysis (LDA),and Adaboost — we trained radiomics models with 5-fold cross-validation.The model with the highest area under the receiver operating characteristic (ROC) curve (AUC) value in external validation was selected as the final radiomics model,and its predicted probability was taken as the radiomics signature.Moreover,due to the inherent “black box” nature of the machine learning model,Shapley additive explanations (SHAP) method was adopted to make visualizations — visualizations make easy applying to the model in clinical practice.Shapley values originate from game theory and were used to determine the contribution of each feature in the model to predictions,using scikit-learn and SHAP package of Python 3.7 (https://www.python.org/downloads/release/python-370/).Briefly,for a variable of interest,outputs of each possible combination of other variables were first collected;then the difference between the average of all these possible outputs without this interesting variable and the output of the model when the interesting variable was included was calculated,yielding the Shapley value of the variable.Thus,through Shapley values,the level of contribution of each feature to the prediction was determined [26].
Clinical risk factors were selected by univariate and multivariate logistic regression analyses via a backward stepwise process.Factors withP<0.1 in univariate logistic regression were included in multivariate analysis and those withP<0.05 were identified as independent risk factors.Through multivariate logistic regression,radiomics and clinical features were integrated into a combined model.
Model evaluation
The performance of diagnostic model was evaluated based on the confusion matrix predicted by the model and the calculated AUC,sensitivity,specificity,and accuracy.The fitting effect and clinical usefulness of models were demonstrated by calibration and decision curves.
Survival analysis
Overall survival (OS) was defined as the period between the first TACE and the date of death (in month).Progression-free survival (PFS) was defined as the duration between the first TACE and disease progression or death from any cause (in month).The patient who did not have an event or lost to follow up was defined as censored.
Statistical analysis
Statistical analyses and model development were done in R software version 3.6.3 (https://www.R-project.org/).The statistical significance of differences between continuous variables was assessed using either independent samplet-test or Mann-WhitneyUtest whereas for categorical variables,Chi-square test and Fisher’s exact probability method were used.DeLong’s test was used to compare the AUCs of various models.A nomogram for predicting the probability of TACE unsuitability was constructed based on the multivariate logistic regression.Survival curves were made using Kaplan-Meier analysis and their statistical significance was evaluated using the log-rank test.An alpha level ofP<0.05 (twotailed) was used in all analyses.Specific R packages used in the study are listed in Table S1.
Results
Patient characteristics
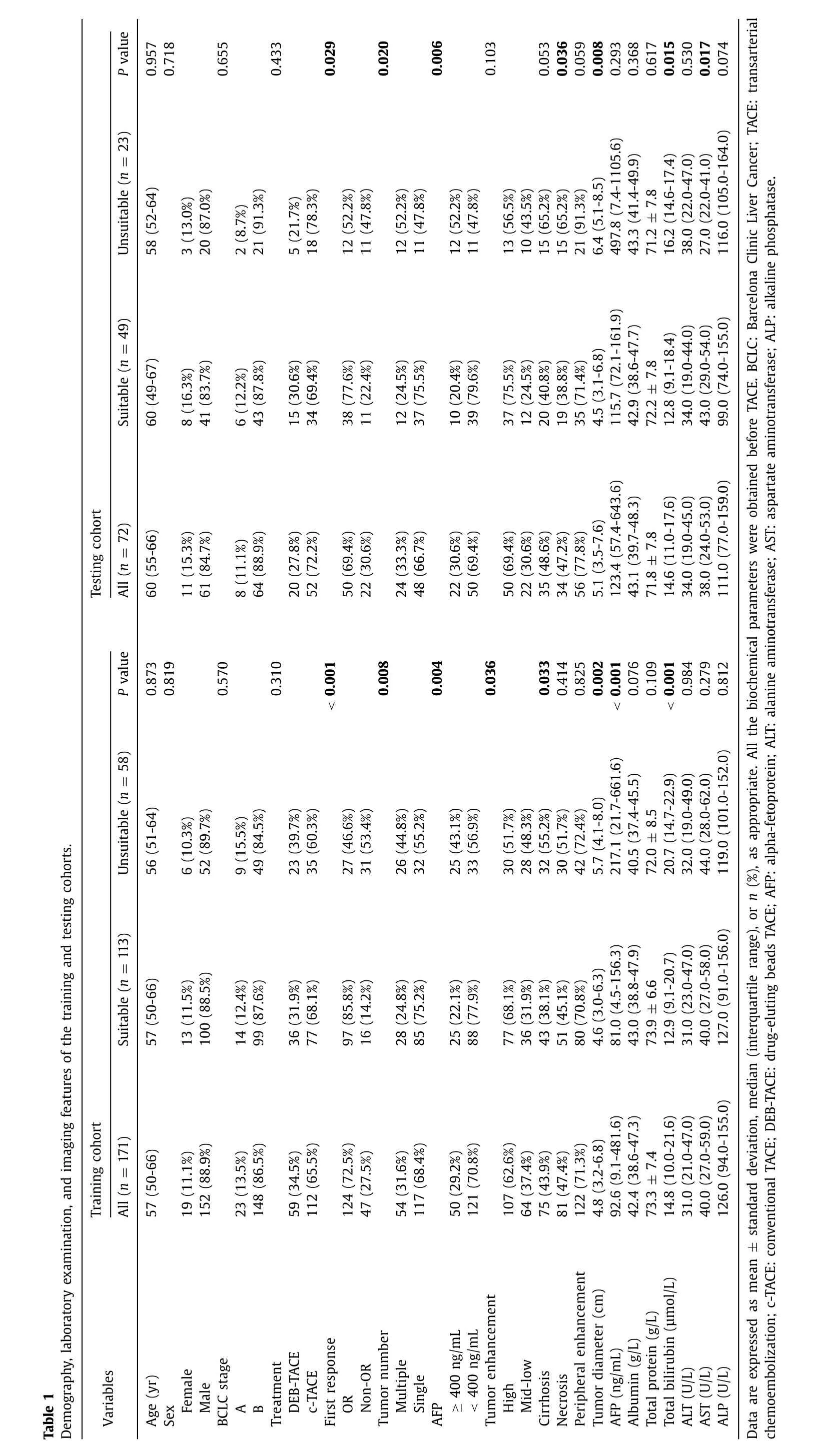
The median age of the entire cohort was 58 years old;213(87.7%) were male and 110 (45.3%) had liver cirrhosis.Median tumor diameter was 4.9 cm.A total of 81 (33.3%) patients were confirmed as TACE unsuitable,of which 32 (13.2%) had no OR after two consecutive procedures,4 (1.6%) had tumor number increases after consecutive TACE,11 (4.5%) had liver function deterioration,20 (8.2%) had vascular invasion,9 (3.7%) had extrahepatic spread,and 5 (2.1%) had elevated AFP level.A total of 124 (72.5%) patients in the training cohort showed OR to the first TACE treatment (86 CR and 38 PR),whereas 50 (69.4%) patients in the testing cohort showed OR (31 CR and 19 PR).More detailed demographic and baseline information is listed in Table 1.
Radiomics analyses
From the entire cohort,we extracted 1302 radiomics features from the arterial phase and 1302 from the portal venous phase of CT images respectively,of which 78 features with ICC <0.75 were excluded (Fig.S2).From the training cohort,six and four radiomics features from the arterial phase and portal venous phase respectively were identified after feature screening (Fig.3),which were all used to train the radiomics model.For the seven machine learning models,AUC values ranged from 0.844 to 0.875 and 0.647 to 0.838 in the internal and external validations respectively (Figs.S3 and S4).The XGBoost model had the highest AUC in the external validation and the best performance when six features were used.Thus,the final radiomics signature was constructed using the XGBoost method with six radiomics features: wavelet-LHH_gldm_Small Dependence Low Gray Level Emphasis_V,wavelet-HLL_gldm_Dependence NonUniformity Normalized_A,wavelet-LLH_glszm_Gray Level NonUniformity Normalized_A,log-sigma-2-0-mm-3D_glcm_Maximum Probability_A,original_firstorder_Median_V,original_firstorder_Median_A (“A” means arterial phase features and “V” means portal venous phase features).Parameters of the classifier were set as follows: objective,binary logistic;learning_rate,0.3;max_depth,8;min_child_weight,2;reg_lambda,0.5.
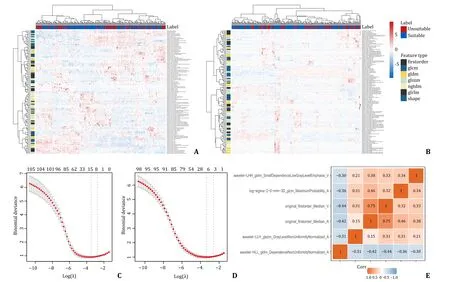
Fig.3.Identification of features and dimensionality reduction.A,B: The heatmap of unsupervised clustering analysis in the training cohort of arterial phase and portal venous phase,respectively.Red represented positive correlation and blue represented negative correlation;C,D: LASSO algorithm for feature selection of arterial phase and portal venous phase,with the optimal λ values of 0.065 and 0.060 for the corresponding number of variables six and four respectively;E: correlation matrix of six selected radiomics features.LASSO: least absolute shrinkage and selection operator.
In the SHAP summary plot (Fig.4 A),each dot represented a sample whereas its color represented the cumulative Shapley value(red-highest value,blue-lowest value) — the higher the Shapley value the more the feature influenced the final prediction.For specific patients,we also quantified the impact of each feature on a prediction,as shown in Fig.4 B and C — arrow size indicates the Shapley value (degree of a feature’s contribution to the prediction),arrow direction indicates whether a feature either raises (red) or lowers (blue) a prediction result.
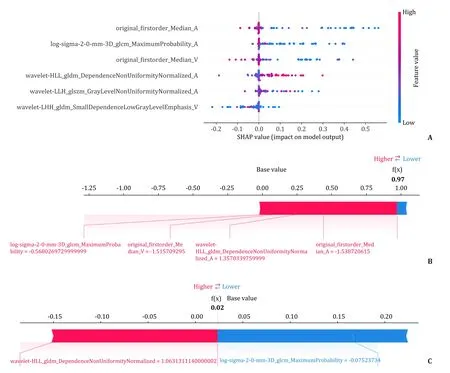
Fig.4.Visualization of XGBoost radiomics model.A: SHAP summary plot: ranking of the selected radiomics features according to the Shapley values;B: a patient who is TACE-unsuitable and predicted TACE-unsuitable (the probability of TACE-unsuitability was 97%);C: a patient who is TACE-suitable and predicted TACE-suitable (the probability of TACE-unsuitability is 2%).XGBoost: extreme gradient boosting;SHAP: Shapley additive explanations;TACE: transarterial chemoembolization.
Clinical-radiomic nomogram and model performance evaluation
In the training cohort,tumor diameter,tumor number,cirrhosis,AFP,first response,and tumor enhancement were all significant factors (P<0.1) as per univariate analysis.From multivariate regression analysis,four independent factors (tumor diameter,first response,AFP,and tumor enhancement;P<0.05) were selected and subsequently incorporated into the clinical model (Table 2).Resultantly,a clinical-radiomic model based on the XGBoost signature and four clinical factors was developed that determined patient unsuitability of TACE therapy (Fig.5).
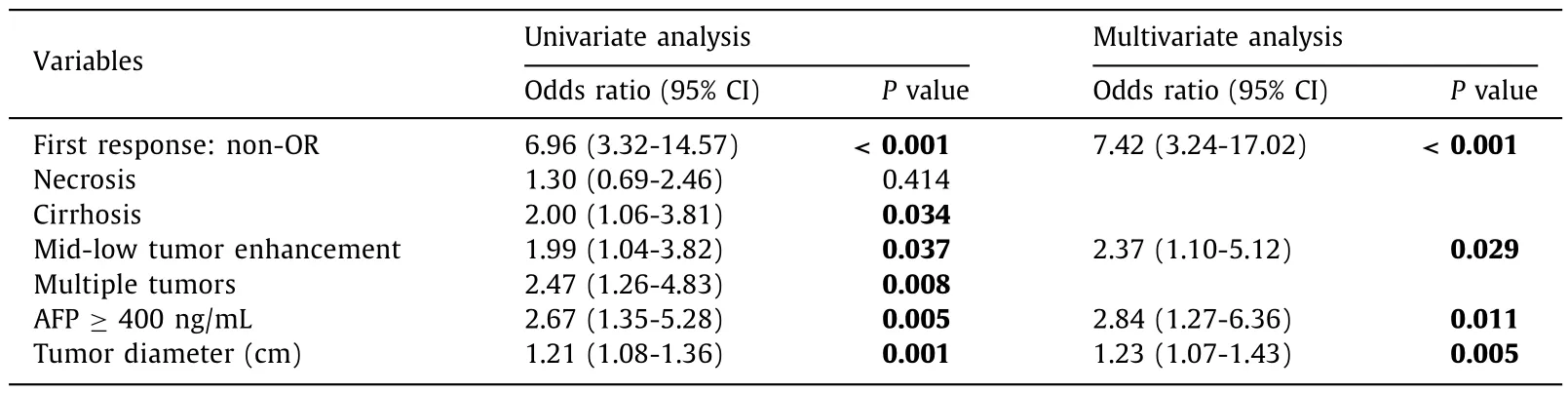
Table 2Univariate and multivariate analyses of the clinical factors.

Fig.5.The evaluation of the constructed models.A: A forest plot of the established combined model composed of the XGBoost signature and four clinical factors.B: A nomogram for predicting the probability of TACE unsuitability.The nomogram was constructed by summing up the points identified on a points scale for each variable.The nomogram was used to determine the likelihood of TACE unsuitability for each patient.XGBoost: extreme gradient boosting;TACE: transarterial chemoembolization;AFP:alpha-fetoprotein;OR: objective response.
The AUC values of the combined model (clinical and radiomics)were 0.906 and 0.894 in the training and testing cohorts,respectively (Fig.6 A and B).The efficiency of different models is shown in Table 3.The combined model had a high degree of accuracy as it identified TACE-unsuitable cases,at a sensitivity,specificity,and AUC of 0.759,0.885,0.906 [95% confidence interval (CI): 0.859-0.953]in the training and 0.826,0.776,and 0.894 (95% CI: 0.815-0.972) in the testing cohort,respectively.The calibration curves(Fig.6 C and D) revealed optimal agreement between predicted probabilities computed by the combined model and actual observations from the two cohorts.A threshold probability of the decision curves (Fig.6 E and F) ranging from 6% to 85% indicated superior net benefit in predicting TACE suitability using the combined model.Delong’s test was used to evaluate models’ robustness in the training and testing cohorts (Table S2).No statistically significant differences were found between the two cohorts (P>0.05).Fig.S5 showed four typical cases of model predictions in the test cohort.
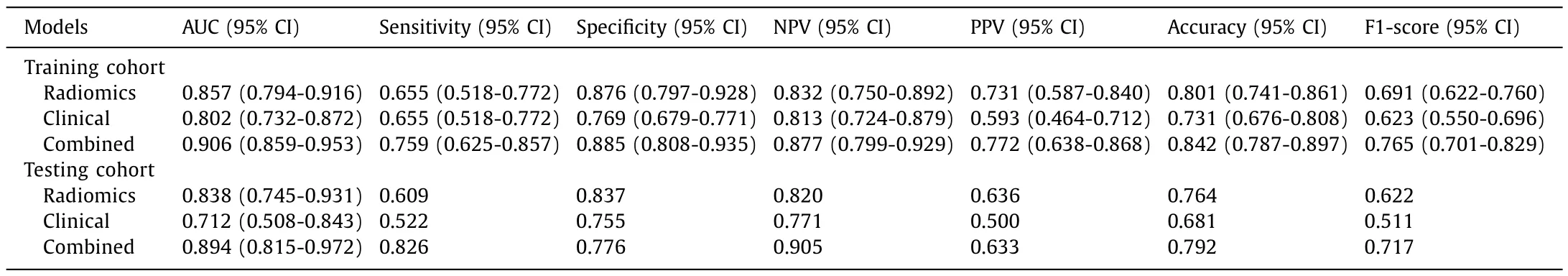
Table 3The efficiency of different models in the training and testing cohorts.

Fig.6.Performance of the constructed model.ROC,calibration,and decision curves for the models in the training cohort (A,C,E) and testing cohort (B,D,F).ROC: receiver operating characteristic;AUC: area under the ROC curve.
The median OS in the training cohort for patients who were unsuitable for TACE as initial treatment was 23.9 months compared to 57.1 months for those who were suitable (P<0.001).Significant differences in median OS were also seen in the testing cohort (26.4 months for TACE unsuitable vs.49.8 months for TACE suitable,P<0.001) (Fig.7 A and C).The prediction model grouped patients into high and low risk,and these two groups significantly differed in OS for both training (P=0.004) (Fig.7 B) and testing cohorts(P=0.005) (Fig.7 D).Median PFS for patients who were unsuitable for TACE as initial treatment in the training and testing cohorts was 17.5 and 17.2 months,compared with 35.3 and 31.7 months for TACE suitable patients respectively (Fig.7 E and G).Similarly,high and low risk groups had significantly different PFS in both training(P<0.001) (Fig.7 F) and testing cohorts (P=0.028) (Fig.7 H).
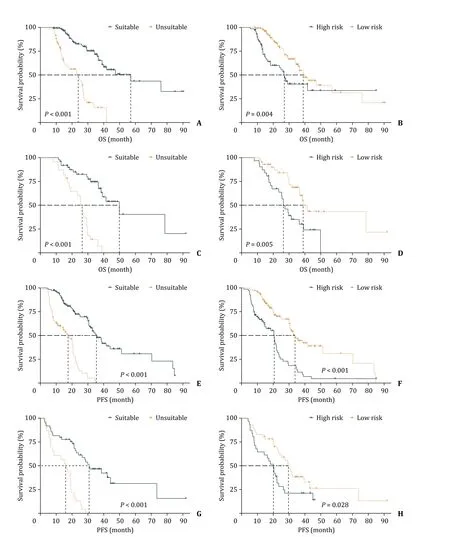
Fig.7.Survival curves.True OS (A,C) and PFS (E,G) of different TACE suitability groups in the training and testing cohorts,respectively;the predicted OS (B,D) and PFS(F,H) using the constructed combined model in two cohorts,respectively.OS: overall survival;PFS: progression-free survival.
Discussion
In this multicenter study,we developed and validated a model that identified intermediate-stage HCC patients who were suitable for TACE as initial treatment.The model utilized both a radiomics signature and clinical factors to give predictions that had a high degree of accuracy.
Although TACE is recommended as first-line treatment for intermediate-stage HCC [27,28],few have investigated the possibility of identifying patients unlikely to benefit from it.This is crucial because repeated TACE treatment is likely to damage liver function and even affect survival — a condition initially known as TACE refractoriness that was first proposed by the Liver Cancer Study Group of Japan (JSH-LCSGJ).Explicitly TACE refractoriness occurs when there are 1) more than two incomplete tumor responses;2) an increased number of tumors in 1-3 months after fully selective TACE;3) a rise in tumor marker levels after TACE and 4)vascular or extrahepatic invasion [29].An international,prospective,non-interventionist study showed that switching to sorafenib due to TACE refractoriness may lead to longer OS [30].In the current era of multimolecular targeting agents,this concept is gradually replaced by another more important concept,“TACE unsuitability”,which is defined by one of these three aspects: unlikely to respond to TACE,likely to develop TACE refractoriness,or deterioration of liver function after TACE [8].Patients who are unsuitable for TACE as initial treatment are thus recommended for systematic therapy using agents with a high response rate,such as sorafenib or lenvatinib,followed by selective TACE [31].Kawamura et al.[32]found the PFS (16.0 months) and OR rate (73.3%)in a group treated with lenvatinib were significantly better than in the TACE group (PFS 3.0 months,OR rate 33.3%),with patients also experiencing better curative effects and faster recoveries of liver function.Jin et al [33]evaluated portal venous phase CT-based radiomics and clinic-radiological factors to predict either extrahepatic spread or vascular invasion after initial TACE monotherapy(EVIT),and the model had an AUC of 0.847.However,patients who developed EVIT were only a small proportion of those treated with TACE,and not all non-EVIT patients benefited from TACE.Thus,other important factors like liver function deterioration and treatment response should also be considered when making decisions on TACE therapy.A radiomics-based model for predicting TACE refractoriness before treatment exhibited a C-index of 0.831 and was notably good in predicting OS [34].Wang et al.[35]constructed a model for early prediction of TACE refractoriness using clinical indicators like bilobar tumor distribution,>3 tumors,and observed an AUC of 0.706 in the validation set.In our study,we considered multiple factors that influenced the benefit of TACE to patients,including the treatment response,changes in liver function,tumor marker status,and disease outcomes.
Clinical factors that indicate a poor response to TACE treatment may also predict poor TACE suitability.In our study,the clinical model was thus composed of four clinical predictors,including AFP level,tumor diameter,tumor enhancement and first TACE response status.In recent years,more and more biomarkers have been recommended for early screening and prognosis prediction of HCC [36].Elevated level of AFP is a common,clinically recognized biomarker for poor prognosis and tumor recurrence [37]and in our study,AFP >400 ng/mL identified patients who were unsuitable for TACE.Tumor size (usually more than 5 cm in diameter) significantly affects therapeutic response [38].Shim et al.[39]found that patients with sizable tumors had response rates of only 30%.In our clinical model,tumor diameter was an independent risk factor that predicted TACE unsuitability as per multivariate logistic regression (P=0.005).Because TACE mainly embolizes the hepatic artery,and lesions without obvious arterial phase enhancement (mid-low enhancement) are likely to be TACEresistant.Zhang et al.[40]believed that lesions of arterial hyperenhancement were more susceptible to TACE than iso-or hypoenhancement.Relatedly,in a model that predicted the OR after the first TACE treatment,the arterial hyper-enhancement was an independent risk factor (P=0.050) [12]corroborating our findings that arterial blood supply affects the efficacy of TACE.Although a single TACE response is not a strong predictor of whether a patient is suitable for TACE,because for some larger tumors,multiple TACE treatments are usually required to completely embolize the tumor [11],it is still an important consideration,because patients who respond to the first TACE treatment usually have a better prognosis [41,42].
Currently,radiomics is widely applied in medicine,and its workflow entails images preparation,ROI delineation,highdimensional features extraction,feature screening and model fitting [19].LASSO is the most commonly used dimension reduction method and is useful for creating refined models by both constructing a penalty function and compressing unimportant feature coefficients to zero.Moreover,it not only has the advantage of subset contraction but is also a biased estimator in dealing with complex collinear data [43].Thus,it has often been used in radiomics prediction studies for feature identification,and this effectively reduced overfitting of models [44–46].In our study,six and four features were identified in the arterial and the portal venous phase respectively by LASSO.In machine learning classification algorithms,ensemble learning constructs several weak classifiers,and then integrates the prediction results into the final prediction.Based on whether there exists dependency between each weak classifier,ensemble learning is divided into boosting and bagging [47].As a boosting classifier,XGBoost performed well in various medical classification tasks [48–50].When constructing the radiomics model,we tested seven machine learning classification methods,of which the XGBoost model had a good fitting effect and the best performance in external validations.The XGBoost model had four arterial phase and two portal venous phase fusion features;features with the two largest proportions come from the arterial phase: original_firstorder_Median and log-sigma-2-0-mm-3D_glcm_MaximumProbability.Finally,we took the prediction probability of each patient as a radiomics signature and combined it with clinical features to construct a combined model— this model performed best in terms of differentiation,calibration,and clinical usefulness.This suggests that doctors should fully consider the heterogeneous data of patients so that they can make the best clinical decision.
Our study had some limitations.First,the retrospective nature inherited selection bias.Second,there was a class imbalance in the number of positive (TACE unsuitable) and negative (TACE suitable)cases.Third,due to the lack of standardized follow-up data,a large number of patients were excluded from this study,reducing its sample size and thus the robustness of analyses.
In summary,we developed and validated a model that combines clinical and radiomics factors to predict the unsuitability of TACE as first-line treatment in intermediate-stage HCC patients,which will help clinicians make better decisions and thus improve the treatment outcomes.
Acknowledgments
None.CRediTauthorshipcontributionstatement
Dan-DanWang:Conceptualization,Data curation,Formal analysis,Investigation,Methodology,Writing -original draft.Jin-FengZhang:Formal analysis,Funding acquisition,Investigation,Methodology,Writing -review &editing.Lin-HanZhang:Conceptualization,Investigation,Methodology,Writing -review &editing.MengNiu:Methodology,Writing -review &editing.Hui-JieJiang:Conceptualization,Funding acquisition,Project administration,Resources,Supervision,Writing -review &editing.Fu-CangJia:Conceptualization,Funding acquisition,Project administration,Writing-original draft,Writing -review &editing.Shi-TingFeng:Conceptualization,Project administration,Resources,Supervision,Writing-review &editing.
Funding
This study was supported in part by grants from the National Key Research and Development Program (2019YFC0118100 and 2017YFC0110903),the National Natural Science Foundation of China (12026602 and 81802649),the Guangdong Key Area Research and Development Program (2020B010165004),the Shenzhen Key Basic Science Program (JCYJ20180507182437217),and the Shenzhen Key Laboratory Program (ZDSYS201707271637577).
Ethicalapproval
This study was approved by relevant institutional review boards or ethics committees.All study procedures were carried out in accordance with theDeclarationofHelsinki.
Competinginterest
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
Supplementarymaterials
Supplementary material associated with this article can be found,in the online version,at doi: 10.1016/j.hbpd.2022.11.005.
 Hepatobiliary & Pancreatic Diseases International2023年6期
Hepatobiliary & Pancreatic Diseases International2023年6期
- Hepatobiliary & Pancreatic Diseases International的其它文章
- A new prognostic model for drug-induced liver injury especially suitable for Chinese population
- Post-hepatectomy liver failure: A timeline centered review
- INSTRUCTIONS FOR AUTHORS
- Value and prognostic factors of repeat hepatectomy for recurrent colorectal liver metastasis
- Older liver grafts from donation after circulatory death are associated with impaired survival and higher incidence of biliarynon-anastomotic structure
- Development and validation of a novel model to predict liver-related mortality in patients with idiosyncratic drug-induced liver injury
