Nanostructure enabled extracellular vesicles separation and detection
Xinyuan He, Wei Wei, and Xuexin Duan,2,a)
AFFILIATIONS
1 State Key Laboratory of Precision Measuring Technology and Instruments,Tianjin University,Tianjin 300072,China
2Department of Chemistry,School of Science,Tianjin University,Tianjin 300072,China
ABSTRACT Extracellular vesicles (EVs) have recently attracted significan research attention owing to their important biological functions,including cell-to-cell communication.EVs are a type of membrane vesicles that are secreted into the extracellular space by most types of cells.Several biological biomolecules found in EVs,such as proteins,microRNA,and DNA,are closely related to the pathogenesis of human malignancies,making EVs valuable biomarkers for disease diagnosis,treatment,and prognosis.Therefore,EV separation and detection are prerequisites for providing important information for clinical research.Conventional separation methods suffer from low levels of purity,as well as the need for cumbersome and prolonged operations.Moreover,detection methods require trained operators and present challenges such as high operational expenses and low sensitivity and specificity In the past decade,platforms for EV separation and detection based on nanostructures have emerged.This article reviews recent advances in nanostructure-based EV separation and detection techniques.First,nanostructures based on membranes,nanowires,nanoscale deterministic lateral displacement,and surface modificatio are presented.Second,high-throughput separation of EVs based on nanostructures combined with acoustic and electric field is described.Third,techniques combining nanostructures with immunofluorescence surface plasmon resonance,surface-enhanced Raman scattering,electrochemical detection,or piezoelectric sensors for high-precision EV analysis are summarized.Finally,the potential of nanostructures to detect individual EVs is explored,with the aim of providing insights into the further development of nanostructure-based EV separation and detection techniques.
KEYWORDS Nanostructure,Extracellular vesicle,Separation,Detection,Individual
I.INTRODUCTION
A.Introduction to extracellular vesicles
Cells from diverse domains of life secrete membrane-enclosed vesicles known as extracellular vesicles (EVs).1EVs carry specifi protocellular components,including DNA,RNA,lipids,proteins,and metabolites.2,3These components mediate intercellular communication,especially in oncology.4EVs participate in cell–microenvironment interactions and influenc cell proliferation,migration,immune regulation,pregnancy,and cardiovascular diseases,among other conditions.5EVs are ubiquitous in various human body fluid such as blood,saliva,semen,sputum,urine,cerebrospinal fluid amniotic fluid and breast milk.6EV formation involves a complex process in which cells endocytose cell surface proteins and extracellular soluble proteins to form early endosomes(ESEs).ESEs mature into late endosomes (LSEs),which eventually become multivesicular bodies(MVBs).MVBs fuse with the plasma membrane and release their intraluminal vesicles(ILVs)as EVs by exocytosis.7EVs are classifie into exosomes,microvesicles(MVs),and apoptotic bodies based on their origin and size.8Exosomes are small lipid bilayer vesicles with an average thickness of about 5 nm,derived from MVBs with diameters of 30–150 nm and densities of 1.08–1.22 g/ml.9MVs have diameters of 50–1000 nm and densities of 1.12–1.16 g/ml,and they bud directly from the outer plasma membrane.Apoptotic bodies have diameters of 500–5000 nm and are secreted by apoptotic cells during the late stages of programmed cell death.10
In recent years,EVs have attracted much research interest.EVs carry specifi biomarkers,such as tetraspanins (CD9,CD63,and CD81),integrins,immunomodulatory proteins,and nucleic acids[DNA,mRNA,and microRNA (miRNA)].11These biomarkers act as a biological fingerprin of the source cell,reflectin its biological information.12EVs are intimately involved in tumor initiation,proliferation,and metastasis.7,13,14Studies have shown that tumor cells secrete more EVs than normal tissue cells.15,16The proteins and nucleic acids in EVs differ not only between cancer patients and healthy individuals,but also at different stages of cancer or other disease.17,18Therefore,EVs are potential biomarkers for early diagnosis and prognosis of cancer and other diseases and have great clinical diagnostic value.19,20Dashet al.21explored the potential of plasma EV membrane proteins from tumor cells as biomarkers for early colorectal cancer(CRC)detection.They found that the expression levels of ADAM10,CD59,and TSPAN9 were 2.19–5.26-fold higher in plasma EVs of CRC patients.Liet al.22examined circular RNA (circRNA) in EVs secreted by high-grade astrocytoma.They constructed a circRNA panel of serum EVs and discovered that circRNA could serve as a liquid biopsy target for monitoring and treating high-grade astrocytoma (HGA).Moreover,EVs can be used not only for diagnosis but also for therapy.In addition,EVs are an excellent natural delivery vehicle,which can be used to load biomolecules for targeted therapy.Lianget al.23designed EVs derived from HEK293T cells as vectors targeting CRC cells.Anticancer miR-21 inhibitors were loaded onto these EVs,mediated by electroporation.It was observed that tumors were reduced in mice with colon cancer by intravenous injection of these EVsin vivo.
B.Existing separation and detection techniques
Traditional techniques for separating EVs include ultracentrifugation,precipitation,immunoaffinity ultrafiltration and size exclusion chromatography.24Ultracentrifugation (UC) is considered the gold standard for separating EVs.19This method extracts EVs by centrifuging at low speeds (300g–2000g) and gradually increasing to high speeds(100 000g–200 000g)to separate cells,cell debris,vesicles,and proteins.25However,UC also has clear drawbacks.First,the requirement for a large sample volume (>10 ml)makes it unsuitable for processing trace biological samples.26Second,the long centrifugation time and high rotational speeds damage the integrity of EVs,potentially affecting the feasibility of their downstream analysis.This method also causes co-precipitation of proteins,leading to poor recovery.19Third,UC requires expensive equipment and trained operators,which limits its clinical application.19,26
Size exclusion chromatography (SEC) is a method that separates EVs based on their size.20,27SEC utilizes a stationary phase column consisting of spherical polymer porous beads with varying pore sizes to separate EVs.10,28This technique enables smaller EVs to enter the pores and be eluted with phosphate-buffered saline(PBS),while larger EVs cannot diffuse into the pores and move between the porous beads.Compared with UC,SEC does not require expensive equipment,it is easy to operate,and it separates EVs more completely.29However,contaminants such as lipoprotein may still remain in the EVs after separation.30Furthermore,the SEC stationary phase may interact nonspecificall with the sample,which can alter the selectivity of separation.SEC is also limited by low concentration and low size resolution.27,31
Another approach to separate EVs using solubility or dispersibility is a precipitation-based technique.Polymer or saline solution,such as polyethylene glycol (PEG),is usually used as a precipitating agent.32The polymer binds water molecules and forces poorly soluble EVs out of solution.33Precipitants and samples are incubated overnight under certain conditions,and then the precipitate containing EVs is separated by low-speed centrifugation(1500g)or filtration34,35Finally,the residue is washed with PBS solution for further downstream analysis.Reports in the literature mention that the pH value of the EV separation environment is one of the key parameters affecting the separation quality and yield.Although this method does not require specialized equipment,is easy to use,and can handle large sample volumes,the precipitating agent interferes with downstream analysis and the precipitation process damages the membrane structure of EVs.Moreover,the co-precipitation of non-EV particles (e.g.,proteins) can reduce separation efficiency36
One of the more popular methods for separating EVs using physical properties is ultrafiltratio (UF).37The working principle of UF is similar to that of traditional filtration which is based on the size or molecular weight of particles for classification During the filtratio process,particles larger than the pore size are trapped on the membrane,while particles smaller than the pore size pass through the membrane.38,39The operational steps of UF are simple and easy,and it is time-saving and low-cost,but it also has prominent disadvantages.During the passage of EVs through the membrane pores,they will be subjected to high pressures,leading to extrusion deformation,which can result in morphological changes or damage,thus interfering with downstream analysis.40Also,the membrane will become clogged after filtration both by large particles on the membrane surface and by small particles inside the membrane pores,resulting in a decrease in flu and a decrease in recovery.41
The immunoaffinit method relies on interaction between antigen and antibody or between receptor and ligand to separate EVs.10,27This technique is supported by abundant antigen markers on the membrane surface of EVs,such as CD9,CD63,CD81,and others.25,42A common approach is to immobilize specifi antibodies on magnetic beads,chromatographic matrices,multiwell plates,and microfluidic to capture EVs.43,44Immunoaffinity based chromatography methods resemble traditional chromatographic separations by immobilizing antibodies in the stationary phase and EVs in the mobile phase.Different components in the sample can be separated by exploiting their different elution rates.45Some literature reports indicate that the immunoaffin ity method has much higher capture efficienc and yield of EVs than UC.40,46
In addition,traditional detection technologies are divided into many categories,which are only listed here and not described in detail.Methods for characterizing the size and concentration of EVs include nanoparticle tracking analysis (NTA),47dynamic light scattering (DLS),48and flo cytometry (FCM).49Methods for characterizing EV morphology include scanning electron microscopy (SEM),transmission electron microscopy (TEM),and atomic force microscopy(AFM).37Enzyme-linked immunosorbent assay (ELISA)50and immunoblotting are used to characterize the types of proteins contained in EVs.51Methods for detecting RNA in EVs include quantitative reverse transcription polymerase chain reaction(qRT-PCR).In addition,surface plasmon resonance(SPR)imaging,52tunable resistive pulse sensing (TRPS),53laser tweezers Raman spectroscopy(LTRS),54and other methods are used for EV detection.
In the past decade,researchers have developed a variety of commercial instruments or kits to separate and detect EVs from cell cultures or body fluids55However,owing to the complex composition of biological samples and where EV components intersect in density and size range,most available methods cannot accurately isolate or analyze specifi constituent parts of EVs—particularly proteins and nucleic acids.56,57Consequently,most of the EV-related research literature currently available is typically based on mixed subsets of EVs secreted by different cells,including additional EV components and biologically active acellular components.Therefore,the true nature of EVs with their specifi functions and structural dimensions has been challenging to elucidate clearly,thus hindering the investigation of their biological and clinical applications.24The limitations of the methods mentioned above have brought signifi cant challenges to the development of EV separation and detection techniques.
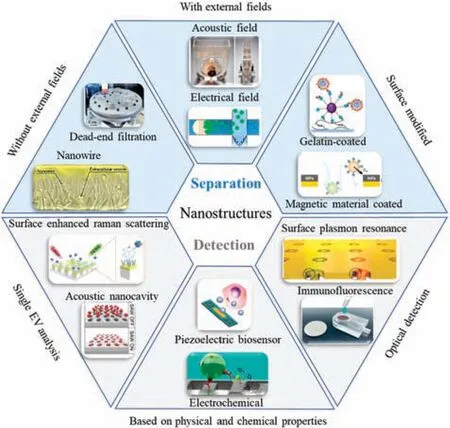
SCHEME 1.Nanostructure-based EV separation and detection methods.
Advances in nanotechnology and micro-and nanoprocessing techniques have enabled the production of nanostructures that are precisely sized to fi EVs.Incorporating these structures into microfluidi chips increases the EV exposure probability by expanding the surface area available.Nanostructures not only provide higher separation efficiency purity,and throughput for EV separation,but also enable enhanced accuracy,specificity and sensitivity through nanoscale detection.58–60Through nanotechnology,researchers have designed a variety of functional nanostructures with the advantages of low sample consumption,low cost,and portable operation.The combined advantages of nanotechnology and microfluidi technology provide precious resources for obtaining suitable EV samples for further research and clinical application.Although microfluidi systems for separating and detecting EVs are still in their infancy,the fusion of nanotechnology and microfluidic has achieved outstanding results.In this review,we firs divide the nanostructures used to separate EVs into three categories,namely,nanostructures without external field nanostructures with an external field and surface-modifie nanostructures,and we review the development of nanostructure-based separation methods in typical examples of biomedical and clinical analysis.Second,we introduce the nanostructure-based EV detection methods and review the innovative applications of nanostructures in the detection field Third,we review the capture and detection of individual EVs based on nanostructures,fully demonstrating the advantages of nanostructures in the analysis of individual EVs (Scheme 1).In each section,we will introduce the principles and mechanisms in detail and provide an in-depth analysis of their advantages and challenges.Finally,we discuss the future development trends of nanostructures and their potential applications in biomedical fields
II.NANOSTRUCTURE-BASED EV SEPARATION METHODS
The separation of EVs based on nanostructures is an emerging technique that is compatible with microfluidi chips to create nanoscale lab-on-a-chip devices.Nanostructures provide a high area-to-volume ratio,increasing the contact probability with EVs,and requiring smaller sample volumes with fewer reagents.This approach also eliminates the need for the bulky equipment required by conventional methods,since it combines multiple separation methods and components on a single chip.Techniques for separating EVs based on nanostructures are classifie according to whether they use physical properties or biological characteristics.Physical property-based mechanisms use nanostructures with or without external fields taking advantage of EV density,size,and electrical properties.Such microfluidi platforms rely on filtra tion,nanowires,acoustics,deterministic lateral displacement,and electricity.Meanwhile,biological property-based separation relies on specifi interactions of EV surface biomarkers with capture agents.These approaches employ immunoaffinity-base antibodies or aptamers,as well as polymeric reagents.
A.Nanostructure without external fields
Generally speaking,the platforms based on microfluidic combined with nanostructures without external field use pressure or centrifugal force to achieve the separation of EVs.These platforms rely on differences in physical properties to separate different kinds of EVs.24,58According to the current literature,exosomes typically range in diameter from 30 to 150 nm and have densities ranging from 1.08 to 1.22 g/ml.9Nanostructures without external field separate EVs with the same physical properties,sacrificin purity while improving size resolution,and thus the requiring further downstream analysis.61In this subsection,we will introduce platforms to achieve separation based on the above EV physical properties,divided into (1) the dead-end filtratio method,(2) the tangential flo filtratio method,(3)the nanowire filtratio method,and(4) the nanoscale deterministic lateral displacement (nano-DLD)method(Table I).
1. Dead-end filtration method
Dead-end filtratio is a classic filterin mode often used in a variety of application scenarios.Particles and solutions smaller than the membrane pore diameter pass through the membrane,while particles larger than the pore diameter are trapped on the membrane.62There are two ways to create the required pressure difference:the firs is to add positive pressure upstream of the membrane(i.e.,at the inlet),and the second is to add negative pressure downstream of the membrane (i.e.,at the outlet).Combining two or more membranes can be used for sequential filtratio to remove large particles and proteins,thereby retaining EVs in a specifi size range.63
On a microfluidic-base filtratio platform,Lianget al.14developed an integrated dual-filte microfluidi device to separate EVs from the urine of bladder cancer patients [Fig.1(a)].The device contained polymethyl methacrylate (PMMA) layers with two fixe polycarbonate (PC) membranes of sizes 200 and 30 nm,respectively.Subsequent to positive pressure filtration microbeads and particles exceeding 200 nm were trapped on the larger membrane,while nucleic acids and proteins below 30 nm passed through the smaller one,enabling EV retention.Anti-CD63 antibody labeled and formed an immune complex with EVs,which was followed by interaction with streptavidin–horseradish peroxidase(SA-HRP),leading to on-chip separation,enrichment,and quantificatio of EVs in urine.The nonspecifi adsorption of EVs on the membrane surface in such as device may reduce the recovery rate.As a result,the membrane surface becomes clogged,decreasing filtratio effi ciency.Also using a dual-filtratio system,Donget al.64devised an integrated microfluidi chip named ExoIDChip for efficien separation and high-sensitivity detection of EVs [Fig.1(b)].The chip had a slidable mechanical structure connecting two flui channels.After completion of filtratio in the left channel,the upper channel was moved to the right,and the excess AptCD63 aptamer passed through.Enrichment of unbound aptamers was performed by photonic crystal nanostructures,which enhanced fluorescenc when bound to nitrocellulose membranes.In the fina detection stage,infused SA-HRP quantifie EVs by a competitive immunoassay,enabling sensitive EV detection.The photonic crystal nanostructure had a fluorescenc enhancement effect.ExoID-Chip was used to differentiate serum samples from breast cancer patients and healthy individuals.
On the basis of the dead-end filtratio method,Liuet al.63developed a new type of filte chip called ExoTIC [Fig.1(c)].This used PC membranes with different pore sizes as filter to separate EVs from different clinical samples of blood,lungs bronchoalveolar lavage fluid cell culture medium,saliva,and urine.On this platform,the membranes were modularized to allow easy connection to syringes and syringe pumps.After filtratio for some time,the device was rotated through 180○to prevent clogging from causing a drop in flux ExoTIC gave a four times higher yield than UC,and a three to four times higher yield than a PEG precipitation kit,with a separation efficienc exceeding 90%.Furthermore,it was possible to separate EV subpopulations in different size ranges by cascading together filter of different sizes(e.g.,30,50,80,100,and 200 nm).
Sederet al.65fabricated an automated non-microfluidi platform[Fig.1(d)].The working principle of this platform was similar to that of ExoTIC,cascading membranes with different pore sizes(30,50,80,100,and 200 nm) and using positive pressure to filteEVs.The main structure of this automatic device was composed of a vertically movable piston and a rotatable disc.Seven open chambers were distributed on the disk,with each chamber being connected by a microchannel and a one-way valve,which also contained PC membranes with different pore sizes.Experiments with this device showed that when the piston was pressurized at a speed of 5 μm/s,EVs did not deform significantly Compared with UC,the purity of the isolate was ten times higher,and the recovery rate was as high as 89%.The results of EV characterization showed that the greater the number of EVs secreted by the cells,the higher were the expressions of CD63 protein and TSG101 protein.It was found that the device could separate EVs with different sizes of 30,50,80,100,and 200 nm at high resolution and could separate EV subpopulations according to size.
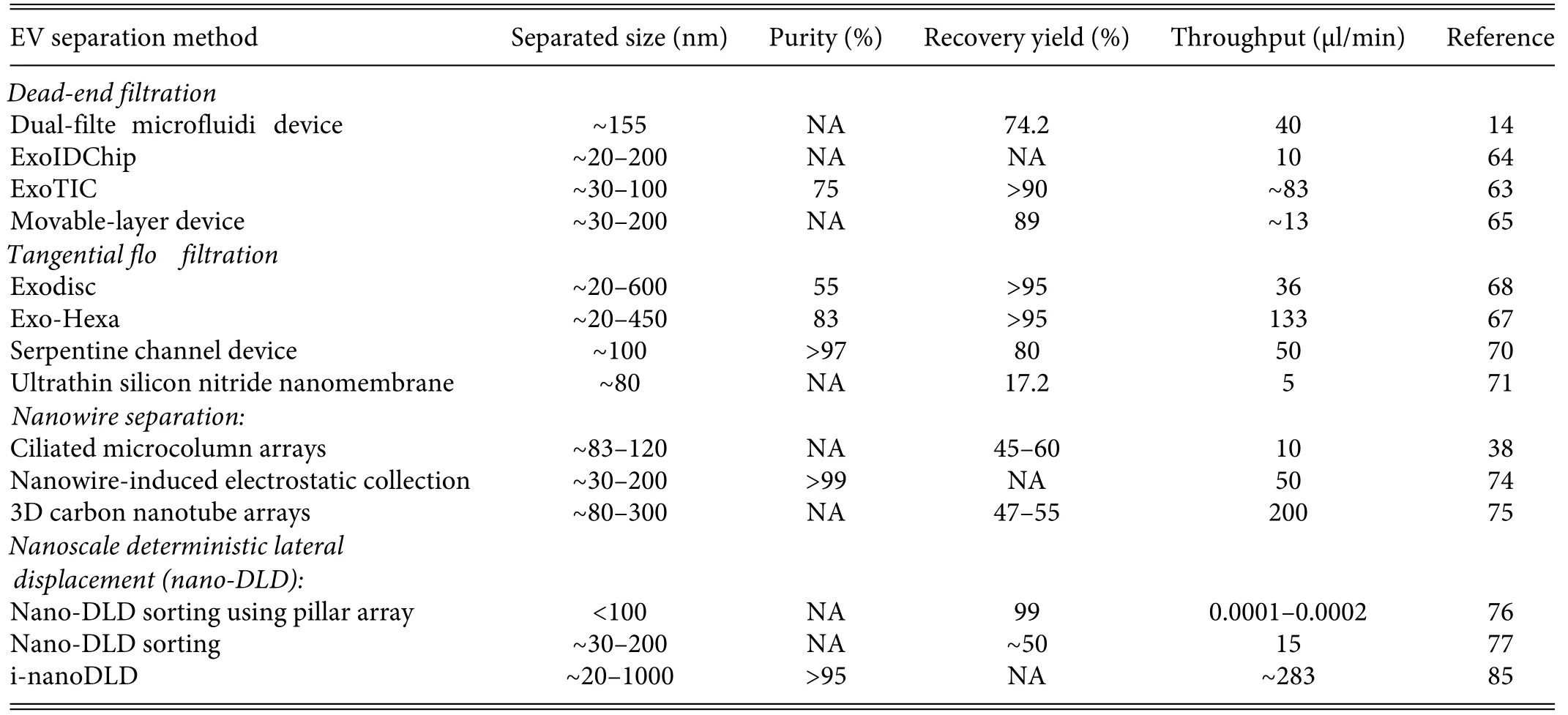
TABLE I.Nanostructure-based EV separation method without external fields
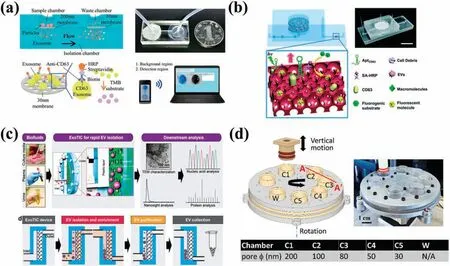
FIG.1.Platforms for EV separation using dead-end filtration (a)Microfluidi dual-filtratio platform for EV separation.14 Reproduced from Liang et al.,Sci.Rep.7,46224(2017).(b) Schematic of the ExoIDchip.64 Reproduced from Dong et al.,Lab Chip 19,2897–2904 (2019).(c) Schematic and workflo of ExoTIC.63 Reproduced from Liu et al.,ACS Nano 11,0712–10723 (2017).(d) EV separation device with a vertically moving piston and a rotating chip to separate EVs according to their sizes.65 Reproduced from Seder et al.,Lab Chip 22,3699–3707(2022).
Although dead-end filtratio has a simple structure,easy operation,and low cost,as the filtratio time becomes longer,large particles will accumulate on the membrane,forming a cake structure and causing membrane pollution.The cake will cause the filtra tion resistance to increase continuously,the permeability of the membrane will decrease,and this will eventually lead to failure of separation.In addition,higher pressures may damage the integrity of EVs.
2. Tangential flow filtration method
Tangential flo filtratio (TFF) is a filtratio method where the flo direction of the sample is perpendicular to the filtratio direction,so that the shear force of the tangential flui reduces the accumulation of large particles on the membrane surface.66TFF equipment has been developed,such as LabSpinner’s lab-on-a-disc,which uses centrifugal force to achieve TFF.67Wooet al.68developed Exodisc for label-free,rapid,and sensitive EV separation and detection.This platform enabled fully automated EV separation of 1 ml samples within 30 min from cell culture supernatant(CCS)or cancer patient urine,with the sample being under a centrifugal force(about 500g) much less than that in UC.The sample passed successively through two membranes,with the combination of a 20 nm anodic aluminum oxide (AAO) membrane and a 600 nm PC membrane proving most suitable.Microvalves automatically controlled different chambers,and the 20 nm AAO membrane was able to filte out protein.Although EV recovery from CCS was found to be greater than 95%,the purity of the EVs was affected by co-segregation of larger EVs (size range 200–600 nm) [Fig.2(a)].Subsequently,the same group made adjustments to Exodisc and developed Exodisc-B.69With this,they were able to enrich EVs from whole blood samples with a volume of 30–600μl within 40 min.The device had a capture efficienc of 75%at a lower centrifugal force(66g).Furthermore,Exodisc extracted mRNA from EVs with a 100-fold increase compared with UC.68Finally,on-chip ELISA was used to detect EVs from the urine of bladder cancer patients and showed high levels of CD9 and CD81 expression.This platform is clinically significan for detecting EVs in urine for cancer diagnosis.In 2019,Wooet al.67developed Exo-Hexa,which had an improved chamber structure compared with Exodisc,increasing the purity of the isolate from 55%to 83%.Throughput,was also improved with this device,with one disc being able to process six samples simultaneously[Fig.2(b)].
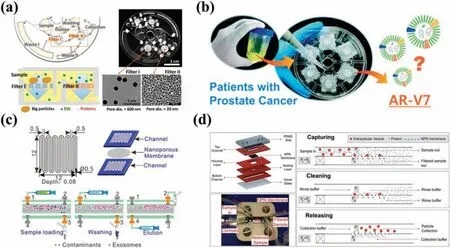
FIG.2.Platforms for EV separation using tangential flo filtration (a)Schematic of Exodisc.68 Reproduced from Woo et al.,ACS Nano.11,1360–1370(2017).(b)Schematic of Exo-Hexa.67 Reproduced from Woo et al.,Lab Chip 19,87–97(2019).(c)Tangential flo filte with serpentine microchannels.70 Reproduced from Han et al.,Sensors Act.B Chem.333,129563(2021).(d)Ultrathin silicon nitride membrane filtratio system.71 Reproduced from Dehghani et al.,Adv.Mater.Technol.4,1900539(2019).
Apart from centrifugal force,the driving force of tangential flo can also be provided by a syringe pump or a vacuum pump.Hanet al.70successfully separated and purifie EVs from human blood using tangential flo via a symmetrical two-layer PMMA serpentine flo channel and a 100 nm PC membrane,which separated over 97% of protein and other impurities.The collected EVs were analyzed using matrix-assisted laser desorption/ionization time-offligh (MALDI-TOF) mass spectrometry (MS),proving effective clearance of plasma proteins,with only the protein spectrum of EVs present[Fig.2(c)].Dehghaniet al.71developed a tangential flo analyte capture (TFAC) EV separation and purificatio method using an ultrathin silicon nitride nanomembrane of 10μm thickness and 80 nm pore size to increase capture efficienc and avoid cake formation,reducing pressure drop.Compared with PC membranes,ultrathin silicon nitride membranes demonstrate low clogging and transmembrane pressure drop,sustaining continuous filtratio for a longer period while also enhancing the separation and release efficienc of EVs.The device also supportedin situEV analysis,outperforming dead-end filtratio [Fig.2(d)].
3. Nanowire separation method
Nanowires represent another size-based technology that leverages the physical properties of EVs for separation by integrating mechanical structures into microfluidi platforms.A prime example of nanowire implementation is an EV filtratio system comprising a structural array of ciliated microcolumns,developed by Wanget al.38This configuratio possessed hierarchical filtratio capability [Fig.3(a)].Traditional micromachining techniques were used to fabricate a microcolumn array on a silicon substrate,permitting the use of an electrodeposited silver nanoparticle catalyst in electroless etching on the sidewall of the microcolumn,finall causing cilia formation.Since the spacing of the nanowires was adjustable,ranging from 30 to 200 nm,this enabled the capture of EVs within the corresponding size range(40–100 nm).Furthermore,EVs could be retrieved without damaging the nanowires,by soaking them in PBS solution.38However,owing to its low throughput,this platform had limited prospects for practical application.Rahonget al.72fabricated a 3D nanowire structure embedded in a PDMS microchannel via a bottom-up approach,allowing them to grow tin oxide nanowires with an average diameter of 18.9 nm on a quartz substrate.This method was able to quickly separate biomolecules,such as proteins,DNA,and RNA.The concentration of EVs in urine is extremely low,posing specifi challenges to EV extraction.73To address this,Yasuiet al.74used a device composed of zinc oxide nanowires and herringbone substrates immobilized in a microchannel to efficientl separate low-concentration EVs from 1 ml urine [Fig.3(b)].They verifie that urine EVs carried a negative charge,while nanowires displayed a positive charge at pH 6–8,thus increasing EV capture efficiency Consequently,within 40 min ofin situextraction of miRNAs,in an improvement compared with UC and commercially available kits,this platform was able to harvest ∼1000 types of miRNAs.
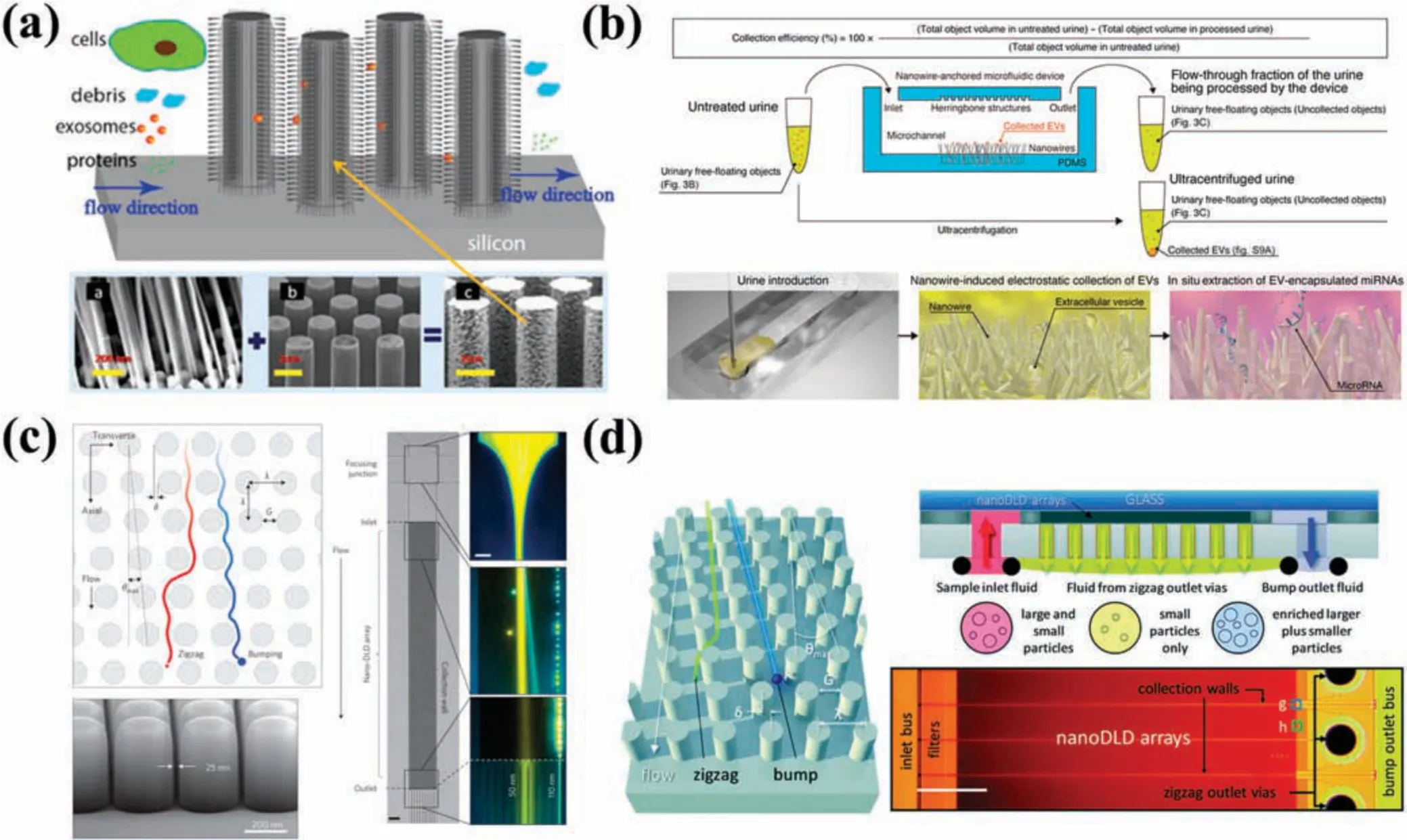
FIG.3.Platforms for separating EVs based on size.(a)EV filtratio system composed of ciliated microcolumn arrays that is capable of hierarchical filtration38 Reproduced from Wang et al.,Lab Chip 13,2879–2882(2013).(b)Microfluidic of ZnO nanowires for separation of low-concentration EVs from urine.74 Reproduced from Yasui et al.,Sci.Adv.3,e1701133(2017).(c)Nanoscale micropillar array chips with gap sizes ranging from 25 to 235 nm.76 Reproduced from Wunsch et al.,Nat.Nanotechnol.11,936–940(2016).(d)Chip composed of 1024 parallel nano-DLD arrays.77 Reproduced from Smith et al.,Lab Chip 18,3913–3925(2018).
Besides utilizing nanowires,researchers have also employed carbon nanotubes for the separation of EVs.Yehet al.75reported a microfluidi platform consisting of 3D carbon nanotube arrays that separated differently sized EVs in a label-free and high-throughput manner.These arrays were made up of 3D nitrogen-doped carbon nanotubes(CNxCNTs)that formed two regions with different pitches,capturing larger(∼300 nm diameter)and smaller(∼80 nm diameter) EVs.However,both types of EVs had low capture rates.In summary,while separating EVs using nanowires or carbon nanotubes offers label-free and highly efficien separation benefits the manufacturing process can be complex,and issues with clogging also pose a challenge for this technique.
4. Nanoscale deterministic lateral displacement method(nano-DLD)
Deterministic lateral displacement(DLD)is a flui dynamicsbased method through which particles of varying sizes are separated according to differences in their trajectories between periodically spaced micropillars.78This method uses rectangular,circular,or triangular arrays of micropillars distributed throughout the channel of a microfluidi chip.78,79Nano-DLD involves setting the distance between the microcolumns at the nanoscale to achieve higher separation accuracy and resolution.The critical cutoff diameter(critical dimension)of DLD is determined by the distance between micropillars and the displacement angle,which refers to the ratio of the lateral offset of the micropillar array to the center-to-center distance between two pillars.While particles smaller than the critical size exhibit a zigzag pattern as they pass through the array of micropillars,larger particles undergo lateral displacement due to the collision mode.79Nano-DLD facilitates the separation of particles that are larger and smaller than the critical size along the direction of flui motion.The DLD technique has been employed to separate circulating tumor cells (CTCs),80blood cells,81bacteria,82and EVs.83Santanaet al.83designed a DLD platform to study the separation of EVs from the culture supernatant of cancer cells.Their experiments revealed that particles larger than the critical size (250 nm)displayed lateral displacement,while those smaller than 250 nm exhibited minimal lateral displacement.Although the efficienc of particle recovery from this microfluidi platform was only about 39%,its purity was 98.5%.However,owing to diffusion,this nonnanostructured device faced difficultie in accurately separating EVs with a diameter less than 200 nm.To effectively separate EVs,nanostructures featuring smaller gaps and fine structures or external field-assiste separation measures are necessary.Zeminget al.84exploited an electrostatic force in conjunction with adjustments to the ionic strength of the solution.This allowed them to achieve the separation of particles measuring 51 and 190 nm by modifying the electrostatic force between the particles and microcolumns.Wunschet al.76developed a nano-DLD technique that exploited a passive microfluidi action instead of relying on an external field They created nanoscale micropillar arrays on silicon substrates with gap sizes ranging from 25 to 235 nm[Fig.3(c)].Their experiments revealed that at low Péclet numbersPe,where deterministic displacement and diffusion compete,the resolution of the nano-DLD arrays was adequate enough to separate particles sized between 20 and 110 nm.Although the platform had the potential to separate biocolloids on a chip,the flo rate remained limited to 0.1–0.2 nl/min.To address the challenge of sample throughput,Smithet al.77transformed a single-array nano-DLD device into a parallel array comprising 1024 separate nano-DLD arrays integrated on a single chip [Fig.3(d)].Following calibration with fluorescen particles,intact EVs were successfully separated from human serum and urine.The platform demonstrated a recovery rate of 50%,surpassing conventional techniques such as UC and SEC.Wunschet al.85developed i-nanoDLD,an integrated nano-DLD device.They fabricated 31 160 parallel arrays at high density,resulting in a throughput of 17 ml/h,a firs for this technique.They then utilized the device to enrich and purify EVs smaller than 200 nm from urine,significantl reducing protein content and other impurities.While the recovery rate of the nano-DLD technique is high,its clinical applicability is limited by the complexity of the manufacturing process,and crucial concerns remain regarding equipment clogging and co-precipitation of EVs and proteins.
B.Nanostructure with external fields
Integrating acoustic and electric field into nanostructures provides higher-throughput separation techniques that do not harm EVs (Table II).The acoustic fiel is comparable to some medical ultrasound techniques and can efficientl separate biological particles of interest.86Electric field typically exploit the electrical properties of EVs.The zeta potential of EVs differs slightly depending on their origin,with EVs from plasma and those from breast cancer cells (MCF-7) displaying zeta potentials of approximately-11 and -13.4 mV,respectively.87,88These property differences facilitate the separation of EVs.
1. Acoustic field
The combination of an acoustic fiel with nanotechnology provides a label-free,reagent-free,and contact-free EV separation method.Acoustic fiel methods perform separation based on differences in mechanical properties such as size,density,or compressibility.89,90This approach enhances cell and bioparticle manipulation accuracy while maintaining biocompatibility.86The sample solution is subjected to acoustic streaming effects when acoustic waves are applied,allowing the acoustic radiation force and acoustic drag force to act on particles.91–93Nanostructures such as microtips,microcolumns,and filte membranes have been paired with acoustic waves to achieve EV separation functionality.Chenet al.94developed an ultrafast EV separation device called EXODUS by combining piezoelectric transducers (PZTs) with porous alumina(AAO)membranes.This device utilized a negative-pressure oscillation system and a double-coupled harmonic oscillationsystem to generate high-and low-frequency vibrations.EXODUS not only effectively circumvented the clogging problem caused by the accumulation of proteins or large vesicles on the membrane surface,but also provided enhanced purificatio and separation efficiency Moreover,compared with traditional EV separation methods,this platform displayed significan improvements in processing throughput (1 ml/min),purity (99%),and recovery (90%).EVs were extracted from the urine of 113 patients displaying urinary system diseases via RNA sequencing analysis using EXODUS,demonstrating its high efficiency effectiveness,and repeatability[Fig.4(a)].

TABLE II.Nanostructure-based EV separation method with external fields
2. Electric field
In a homogeneous electric field only charged particles migrate toward oppositely charged electrodes,leaving neutral and uncharged particles unaffected by the electric fiel forces.98When a particle is in a nonuniform electric field it becomes polarized,inducing a directional movement under the dielectrophoretic (DEP) force,irrespective of the particle’s charge state.99,100Generally,the magnitude of the DEP force is affected by the volume,dielectric constant,electrophoretic mobility,and electric fiel intensity of a particle.87,101
In EV separation methods based on the DEP effect,EVs are also subjected to thermal effects caused by the electrodes.To avoid direct contact occurring between electrodes and EVs,Ibsenet al.102used 400 alternating current electrokinetic (ACE) electrodes on a microfluidi platform.They overlayed the electrodes with a porous hydrogel layer,enabling concentration of larger cells and EVs under a low fiel strength and the effective recovery of separated EVs.Nanostructures such as membranes can also effectively minimize the contact between electrodes and EVs.Davieset al.95developed a filtratio system based on a pressure-driven tangential flo filtratio method along with a DC electrophoresis drive for effi cient separation of EVs from whole blood samples collected from melanoma mice [Fig.4(b)].They synthesized a porous polymer monomer (PPM) into membrane structures and integrated these within PMMA microfluidi channels viain situphotolithography.On application of a DC voltage,the separation of EVs based on their distinct differences in electrophoretic mobility compared with proteins increased both the purity and efficienc of the separated EVs and enabled greater extraction of RNA.Choet al.96proposed an electromigration-based EV separation technique [Fig.4(c)].They employed an electrical fiel together with a dialysis membrane,featuring a pore size of 30 nm,to extract EVs from diluted mouse blood samples.pH and solution osmolarity buffers were employed to prevent the potential problems caused by Joule heating and electrochemical reaction products.The platform extracted ∼65%of the EVs and excluded about 84%of the related proteins.Chenet al.103developed a microfluidi platform(ExoSMP)based on a dual filte device driven by an electrical fiel to separate EV subpopulations via size-selectivity.Mogiet al.104proposed a separation mechanism based on electrophoresis and employing a microfluidi chip design,utilizing a synthetic Nafio membrane sandwiched between two channels.Cations were drawn into this membrane,ultimately generating an ion-depletion region that imposed an electric fiel force on EVs,resulting in their concentration within the main channel of the sample batch.Marczaket al.97developed a microfluidi platform incorporating ion-exchange membranes and gel electrophoresis to separate EVs[Fig.4(d)].In a combination of pressure-and electric field-base approaches,agarose gel was used to filte out large-scale material such as cell debris.A lateral local electric fiel of 100 V/cm was applied to the sample flo within the microchannel,separating EVs from other components.Ultimately,the enriched EVs permeated the ion-exchange membranes and were separated within the agarose gels.
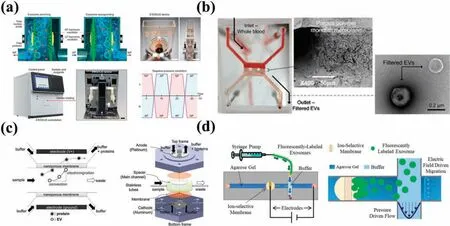
FIG.4.Nanostructures combined with external field for EV separation.(a)Schematic of the EXODUS device.94 Reproduced from Chen et al.,Nat.Methods 18,212–218(2021).(b)Platform for the separation of EVs by synthesizing porous membranes combined with electric fiel filtratio in microfluidi channels.95 Reproduced from Davies et al.,Lab Chip 12,5202–5210(2012).(c)Electromigration-based EV separation chip.96 Reproduced from Cho et al.,Sensors Actuators B Chem.233,289–297(2016).(d)Microfluidi platform combining ion-exchange membrane and gel electrophoresis.97 Reproduced from Marczak et al.,Electrophoresis 39,2029–2038(2018).
3. Surface-modified nanostructures
Immunologically based separation techniques are extensively utilized for EV separation because of their high specificity selective character,and biocompatible compatibility.105,106In contrast to traditional immunological methods,this strategy can be applied on a microfluidi platform.Specifi antibodies targeting antigen features on the EV surface can be immobilized on the surfaces of either magnetic beads or microfluidi channels.Generally,nanostructures amalgamated with magnetic nanomaterials demonstrate advantages with regard to diagnostic applications,since they can function as effective carriers or probes that capture intended EVs directly.107
Utilizing a chemical co-precipitation approach,Changet al.108synthesized PEG-coated Fe3O4magnetic nanoparticles with a size of 20 nm that reduced the protein content(e.g.,serum albumin and immunoglobulins) of fetal bovine serum by 39.89%,without damage to EV integrity[Fig.5(a)].Koet al.109designed the ExoTENPO chip containing six series-connected membranes.A PC membrane including a layer of magnetic material was adopted for extracting nucleic acids,proteins,and cellular debris.By utilizing biotinylated antibodies conjugated with streptavidin-coated magnetic nanoparticles(MNPs),this innovative method successfully reduced antibody amounts at a low cost.The formation of a magnetic trap by creating a wedge-shaped nanopore edge rather than cylindrical nanopores increased capture efficienc by generating a high nanoscale magnetic fiel gradient.Zhanget al.110deposited gold and NiFe successively on track-etched membranes,forming a structure capable of detecting monopoles and dipoles and consequently elevating trapping forces by ∼10 times,resulting in a 99% capture efficienc on magnetic beads functionalized with anti-rabbit IgG antibody.The device was able to separate EVs from human plasma mixed with high-density lipoproteins(HDL)[Fig.5(b)].
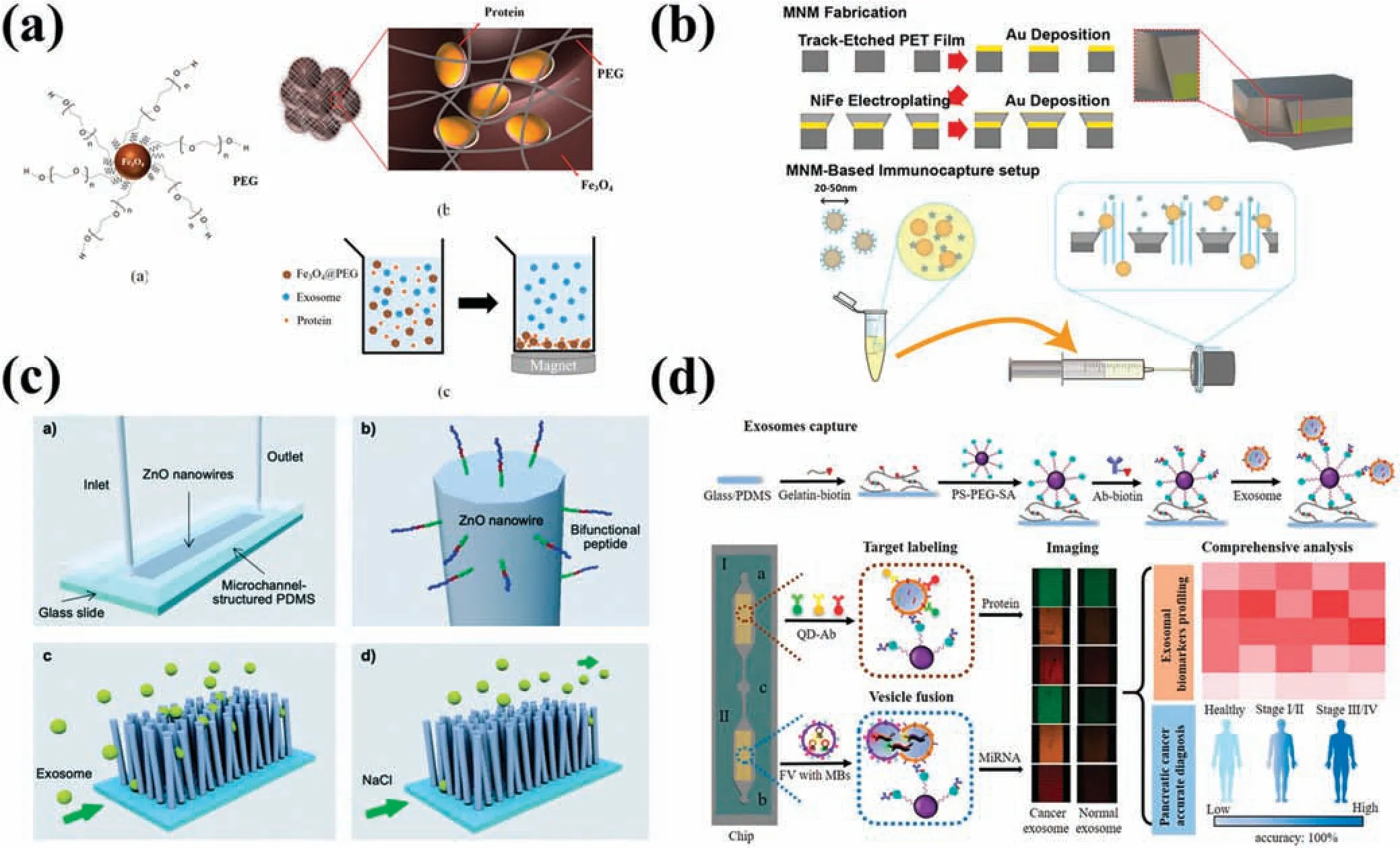
FIG.5.Platforms for surface-modifie nanostructures to separate EVs.(a)EV purificatio method using PEG-coated Fe3O4 MNPs.108 Reproduced from Chang et al.,PLoS One 13,e0199438(2018).(b)Separation of EVs and HDL by wedge-shaped magnetic nanoporous membranes.110 Reproduced from Zhang et al.,Commun.Biol.5,1358(2022).(c) Microfluidi chip with bifunctional peptide-modifie ZnO nanowires.111 Reproduced from Suwatthanarak et al.,Lab Chip 21,597–607 (2021).(d) Device with Y-shaped micropillars and EV trapping through a gelatin layer and PS spheres.114 Reproduced from Zhou et al.,Small 16,2004492(2020).
The abovementioned nanowire configuration can generally be combined with immunoaffinit techniques to separate EVs.Suwatthanaraket al.111developed a microfluidi chip with bifunctional peptide-modifie ZnO nanowires,featuring EWI-2 amino acid sequence-functionalized arrays constructed via the bonding sequence P238.By using CD9 and CD81 markers,the chip was able to separate breast cancer cell-secreted EVs [Fig.5(c)].Inspired by the microvilli on the surfaces of intestinal epithelial cells,Donget al.112fabricated an array of nanowires on a silicon substrate called NanoVilli.Anti-EpCAM (epithelial cell adhesion molecule) modificatio intensifie the binding force,leading to reproducible separation of tumor cell-derived EVs from the serum of non-small-cell lungs cancer patients.Cho and co-workers113demonstrated a magnetic polypyrrole nanowire combining different types of EV antibodies with high-density MNPs.Elongated and biotinylated polypyrrole nanowires facilitated CD9-,CD63-,and CD81-specifi antibody binding and consequently EV separation.Supplementing nanowires with magnetic materials or surface modificatio can specificall separate EVs effectively with reproducibility and convenience.However,distinguishing EV subtypes remains challenging.
Immobilizing EV-specifi antibodies on the surface of microfluidi devices is another promising approach.Zhouet al.114designed a microfluidi platform comprising Y-shaped micropillars.Both a biotinylated gelatin layer and streptavidinmodifie polystyrene (PS) microspheres with a diameter of 50 nm were integrated into the microchannel.Utilizing the Y-shaped structure boosted the contact rate between EVs and CD63 antibodies,while the modifie 50 nm PS spheres enhanced the binding sites for CD63 antibodies.Taking advantage of the thermoresponsive properties of the gelatin membrane,Zhouet al.114elevated the chip temperature to 37○C to release the captured EVs [Fig.5(d)].Although introducing antibodies improves the capture rate of EVs,the high cost of antibodies limits their practical application,and the complicated modificatio process is another drawback.
III.EV DETECTION METHODS BASED ON NANOSTRUCTURES
Nanotechnology has enabled the development of novel approaches to EV detection.Over the past few decades,numerous methods have emerged that combine nanotechnology and characterization of EVs based on their physical or biological properties.Given the complexity of components carrying EVs,detection methods tend to involve multiple techniques working in tandem tocharacterize EVs.Recently,EV-based point-of-care testing(POCT)techniques have been introduced,offering advanced cancer diagnosis,treatment monitoring,and assessment of prognosis.Lab-onchip systems have been devised that incorporate nanostructures for EV separation,enrichment,purification and detection.These systems include those based on detection using immunofluorescence surface plasmon resonance(SPR),surface-enhanced Raman scattering(SERS),and electrochemical methods(Table III).Additionally,single-EV detection techniques have great potential for addressing the question of EV heterogeneity.

TABLE III.Exosome detection methods based on nanostructures.
A.Nanostructure-enabled immunofluorescence detection
Fluorescence detection is a widely used EV detection technique in biochemical analysis and clinical diagnosis,boasting high accuracy,sensitivity,and rapid response.115,116It typically works in tandem with highly specifi immunoaffinit in detecting EV surface proteins or constructing surface protein maps.117EVs are frequently labeled with nonspecifi lipophilic membrane fluorescen dyes such as PKH26.However,these dyes are not ideal markers for detecting and analyzing surface proteins.118Both antibodies and nucleic acid aptamers can label EV surface proteins.Given the low cost and excellent chemical stability of nucleic acid aptamers,they serve as potential substitutes for traditional antibodies in high-throughput protein analyses.119Combining fluorescen immunoassays with nanostructures facilitates the detection of EV subpopulations.
Graphene oxide (GO) is a 2D material with the capacity for fluorescenc quenching.The π–π stacking interaction facilitates solid affinit binding of single-stranded DNA (ss-DNA) to the GO surface,and fluorescenc quenching takes place by fluores cence resonance energy between conjugated dyes and GO.120Jinet al.121employed GO as a nanointerface to develop ExoAPP,an EV analysis system predicated on aptamer nanoprobes.Following GO membrane quenching of specificall labeled FAM aptamers,target EVs bound these aptamers preferentially.The efficac of both EpCAM and prostate-specifi membrane antigen (PSMA) in facilitating epithelial–mesenchymal transition detection was demonstrated through identifying EVs coming from prostate cancer cells.EpCAM and CD63 were also proven to be effective in differentiating tumor-derived EVs from benign tissues[Fig.6(a)].Ohet al.122quenched FAM-labeled peptide nucleic acid probes to detect miR-193 expression in EVs,enabling precise visualization of intercellular EVs.Carbon nanotubes serve as another nanostructure.Tayebiet al.123employed MoS2multiwalled carbon nanotubes (MWCNTs) as a fluorescenc quenching material to quench anti-human CD63PE and produced a fast,sensitive,and quantifiabl EV detection platform.Following the combination of CD63PE antibody with EVs,the complex subsequently reacted with MWCNTs.
Zeng’s group124devised an EV analysis platform that featured modifie nanointerfaces on microchannel surfaces.For the firs time,they integrated microfluidic by embedding polydopamine(PDA) film onto GO,thereby significantl increasing the deposition rate of PDA.A Y-shaped micropillar array along with the GO/PDA nanointerface structure served to elevate the contact area in the flo channel,thereby elevating EV capture efficienc along with suppressing nonspecifi adsorption.Utilizing this novel nanostructure,captured EVs were labeled using biotinylated antibodies,which subsequently underwent reaction with streptavidinconjugated β-galactosidase (SβG).The catalytic action of SβG on 2-β-D-galactopyranoside produces fluorescen signal amplification This assay is ultrasensitive and elegantly detected EVs at volumes as low as 2 μl of plasma from women suffering from ovarian cancer.Building upon their previous work,the same research group created an integrated self-assembled 3D nanopatterned microfluidi chip with highly sensitive capabilities for detecting EV surface proteins.125In this new work,the authors replaced GO/PDA with a silica colloid.Nanocolloids exhibit the capability of autonomous assembly into 3D porous structures in microfluidi channels,while facilitating low hydrodynamic resistance to enable extensive EV capture and detection.The authors proceeded to employ SβG again for enzymatic signal amplificatio to detect EVs.Finally,EV detection in patient plasma determined that folate receptor α on EVs is a potential biomarker for early diagnosis and monitoring of ovarian cancer patients[Fig.6(b)].
Hydrogels serve as a biocompatible materials that can be employed in the creation of nanostructures for detecting EVs.126Limet al.126developed a microfluidi device,the exoNA sensor chip,to detect EV mRNA.They engineered hydrogels equipped with 3D nanostructures that enabled mRNA detection in EVs from HER2+breast cancer mice blood within 2 h.The vacuum-driven exoNA sensor chip enabled precise control over flo rate.Their 3D nanostructured hydrogel contained two probes,ERBB2 and GAPDH,which triggered enzyme-free amplificatio of the fluorescen signal viain situhybridization chain reaction at room temperature.Encapsulation of the probe in liposomes resulted in no fluorescen signal being generated under static conditions and ensured stability and response time during detection.Upon contact between liposomes and EVs,membrane degradation ultimately amplifie the generated fluorescen signal.Comparison of mRNA expression levels between diseased mice and control groups was carried out using their platform,with outcomes that corresponded with those of qRT-PCR analysis[Fig.6(c)].
In another study,127Liu’s group proposed an integrated EVin situdetection device.They utilized ion sputtering to deposit gold nanoparticles on an AAO membrane to produce 50 nm nanoporous gold nanoclusters (AuNCs).Captured modifie CD63 antibody was then used to hybridize EVs on the AuNCs with a secondary antibody-conjugated gold nanorod (AuR) probe.In a fluorescen field AuNCs scatter green light,whereas AuRs scatter red.However,when the gap between the two scattered wavelengths becomes less than 200 nm,resonance coupling occurs between them,with the creation of plasmons.This shift of the spectrum of scattered light to yellow amplifie the scattering intensity.The device detected EVs directly in urine samples (500 μl) obtained from lungs cancer patients and controls [Fig.6(d)].Donget al.64developed a nanostructure based on a photonic crystal for EV detection.EVs were captured by an AAO nanofiltratio membrane,which was then exposed to CD63-targeting aptamers(AptCD63).Some of the aptamers bound to the EVs,while the remainder passed through the membrane and were captured by a nitrocellulose membrane endowed with a photonic crystal nanostructure that boosted the fluorescen signal.
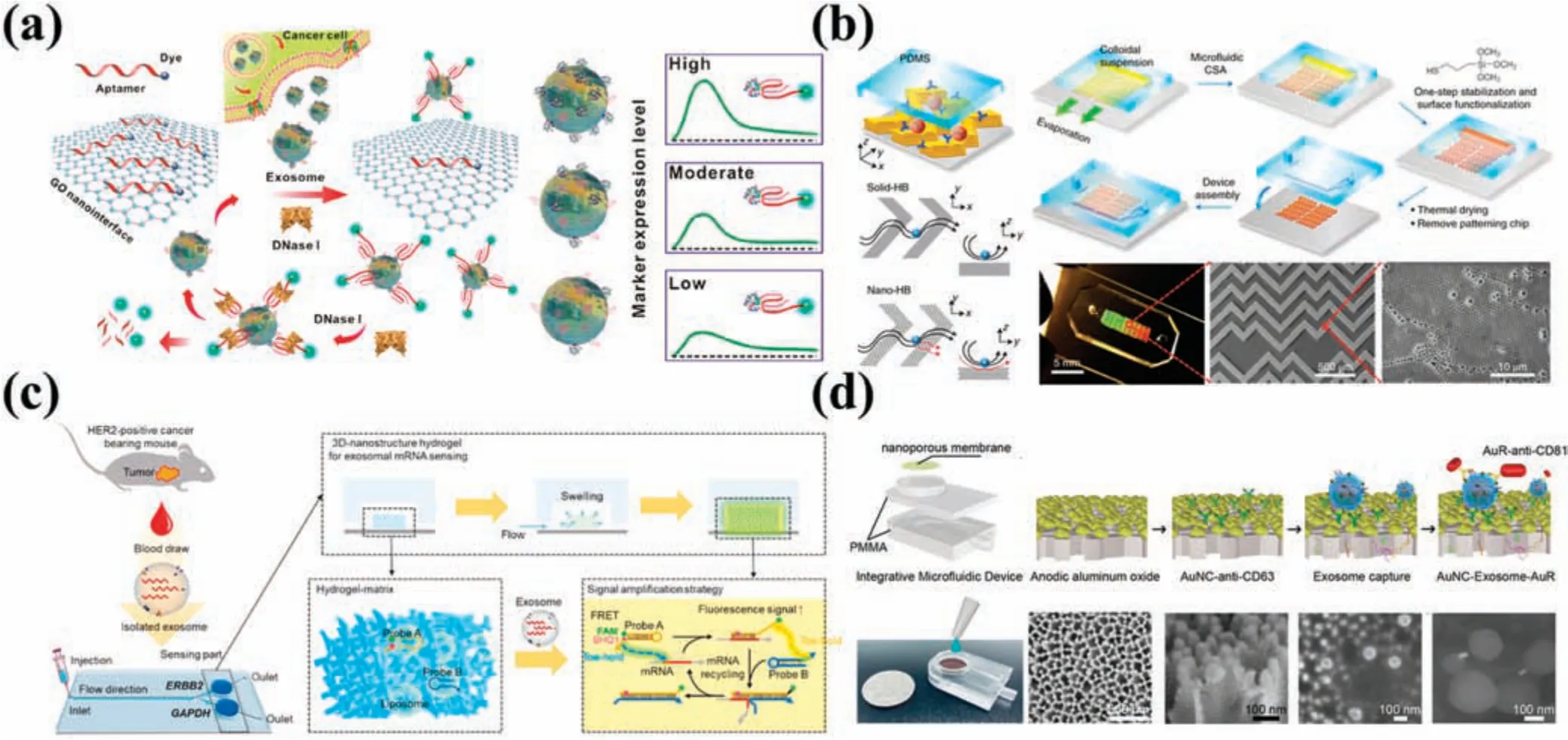
FIG.6.Platforms for immunofluorescenc detection of EVs.(a)Aptamer nanoprobe-based EV analysis system(ExoAPP).121 Reproduced from Jin et al.,Anal Chem 90,14402–14411(2018).(b)GO/PDA as a nanointerface EV analysis platform.125 Reproduced from Zhang et al.,Nat.Biomed.Eng.3,438–451(2019).(c)Schematic of exoNA sensor chip.126 Reproduced from Lim et al.,Biosens.Bioelectron.197,113753(2022).(d)Integrated urine EV in situ detection device.127 Reproduced from Yang et al.,Biosens.Bioelectron.163,112290(2020).
B.Nanostructure-enabled surface plasmon resonance(SPR) detection
Surface plasmon resonance (SPR) has emerged as an optical technique to detect molecular interactions at sensing interfaces by tracking surface refractive index fluctuations52This technique facilitates label-free and real-time monitoring of optical contrast transformations caused by the adsorption of EVs on the plasma layer.SPR represents a novel potential approach for detecting EV subsets with increased sensitivity and specificity128
SPR devices are ideal for EV detection owing to their sensitivity to contact responses that occur within 200 nm of the surface.Zhuet al.129developed nanowall array-based microfluidi chips bound to specifi antibodies to extract and purify EVs from cell culture supernatants.Antibodies to CD9,glycoprotein CD41b,and the tyrosine kinase receptor MET were adhered to gold-coated glass chips and used to detect EVs through SPR imaging [Fig.6(a)].Weissleder’s team130incorporated periodic gold nanopore arrays(nPLEX)into microfluidi chips using a light interference lithography technique to provide high-throughput and label-free EV detection through SPR.When functionalized with CD63 antibody,EVs could be detected from the ascites of ovarian cancer patients on specifi binding to the nPLEX sensor.Measurement of the wavelength shift in the spectrum or of the intensity change at fixe wavelengths enabled observation of changes in the local refractive index around pore complexes,giving a limit of detection of ∼3000 EVs.This technique was faster and more sensitive and required a smaller sample size when compared with the conventional gold standard ELISA.Drawing on the results of their previous work,Weissleder’s team131also developed an automated detection and analysis system for EVs derived from circulating tumor cells that could diagnose pancreatic cancer via measurement of fiv protein markers.This approach proved efficient with 84% accuracy,81% specificity a price of only$60,and a mere ten-minute measurement and analysis time.Chen’s team132utilized a hydrothermal synthesis approach to create a 2D metal–organic framework(MOF),copper tetrakis(4-carboxyphenylporphyrin)(Cu-TCPP),which they deposited on the surface of a gold chip to provide improved optoelectronic properties.This was then used to manufacture an SPR biosensor with substantially enhanced detection accuracy,sensitivity,and quality factor.A multifunctional peptide,comprising four structural domains,was employed as a probe in an experiment to detect programmed death ligand 1(PD-L1)EVs[Fig.6(b)].
Shao’s team133created an amplifie plasma exosome (APEX)platform based on enzymatic deposition of insoluble optical precipitates and subsequent signal amplificatio through SPR for detection of EVs.This platform included a silicon nitride surface with a patterned array of gold nanoholes.EVs were captured on an array surface modifie by CD63 antibody,displaying∼400% spectral improvement in signal by binding amyloid (Aβ).The detection of EV-bound Aβ provided an improved reflectio of PET imaging of plaques within the brain,enabling diagnosis of Alzheimer’s disease in under an hour.Following this success,the same group increased the structural complexity of the platform by modifying the nanohole array into a nanoring array.134Using molecular reactions within a plasmonic nanoring resonator and bio-orthogonal probe amplification in a technique they referred to as ExoSCOPE,they were able to measure EVs in the blood of a patient undergoing targeted cancer therapy,accurately classify the patient’s condition,and effectively determine treatment outcomes within 24 h,representing an improvement compared with conventional pharmacokinetic/pharmacodynamic analysis methods[Fig.7(c)].
C.Nanostructure-enabled surface-enhanced Raman scattering(SERS) detection
Raman spectroscopy is a molecular spectroscopy technique that relies on the inelastic scattering of photons to identify biomolecules such as nucleic acids,lipids,and proteins.135Nevertheless,the Raman signal is commonly faint owing to the minute inherent Raman scattering sizes of the molecules involved and hindrance by fluorescen signals in the target sample.By employing surfaceenhanced Raman scattering (SERS) techniques to analyze coarse metal surfaces or structures,the Raman signal of the molecule being measured can be intensifie (103–1015-fold),thereby improving detection sensitivity.136Consequently,an integrated microfluidi chip based on SERS techniques has the advantages of high specificity multiplexing,and sensitivity.
For the detection of nanoscale EVs,Jalaliet al.137devised a microfluidi platform with butterfly-shape structure to differentiate and characterize non-immune and label-free EVs through SERS.In this approach,PS spheres were employed in bottomup self-assembly for the manufacture of plasmonic nano-bow-tie configurations This structure boosted optical and electrical properties while bolstering Raman signals.EV monolayer dispersion was accomplished using the microfluidi system.A miniature portable Raman device was combined with a microfluidi chip to distinguish EVs from two different glioma cells and noncancerous glial cell EVs[Fig.8(a)].Wanget al.138reported an EPAC-II-based microfluidi chip incorporating multiplex SERS detection.This platform identifie 28 serum samples (5 μl per sample) in ∼65 min,with an asymmetrical ring-shaped electrode formation enhancing the effi cacy and sensitivity of antibody capture of EVs.Enrichment of EVs on the surface of a modifie electrode through antibodies was followed by multiplex SERS detection and analysis.EV variations in sera from early-stage melanoma patients and healthy individuals were analyzed,leading to detection of high expression levels of melanoma-associated chondroitin sulfate proteoglycan (MCSP),melanoma cell adhesion molecule (MCAM),CD61,and CD63 in EVs in the former in comparison with the latter.Thus,on the basis of these four selected biomarkers,early-stage melanoma patients were successfully distinguished from healthy individuals[Fig.8(b)].

FIG.7. Detection of EVs by surface plasmon resonance techniques.(a) Detection platform for EVs in cell culture supernatant combined with antibody microarray and SPR.129 Reproduced from Zhu et al.,Anal.Chem. 86,8857–8864 (2014).(b) Schematic of SPR sensor based on 2D MOF structure.132 Reproduced from Wang et al.,Biosens.Bioelectron.201,113954(2022).(c)ExoSCOPE detection platform with nanoring structure.134 Reproduced from Pan et al.,Nat.Nanotechnol.16,734–742(2021).
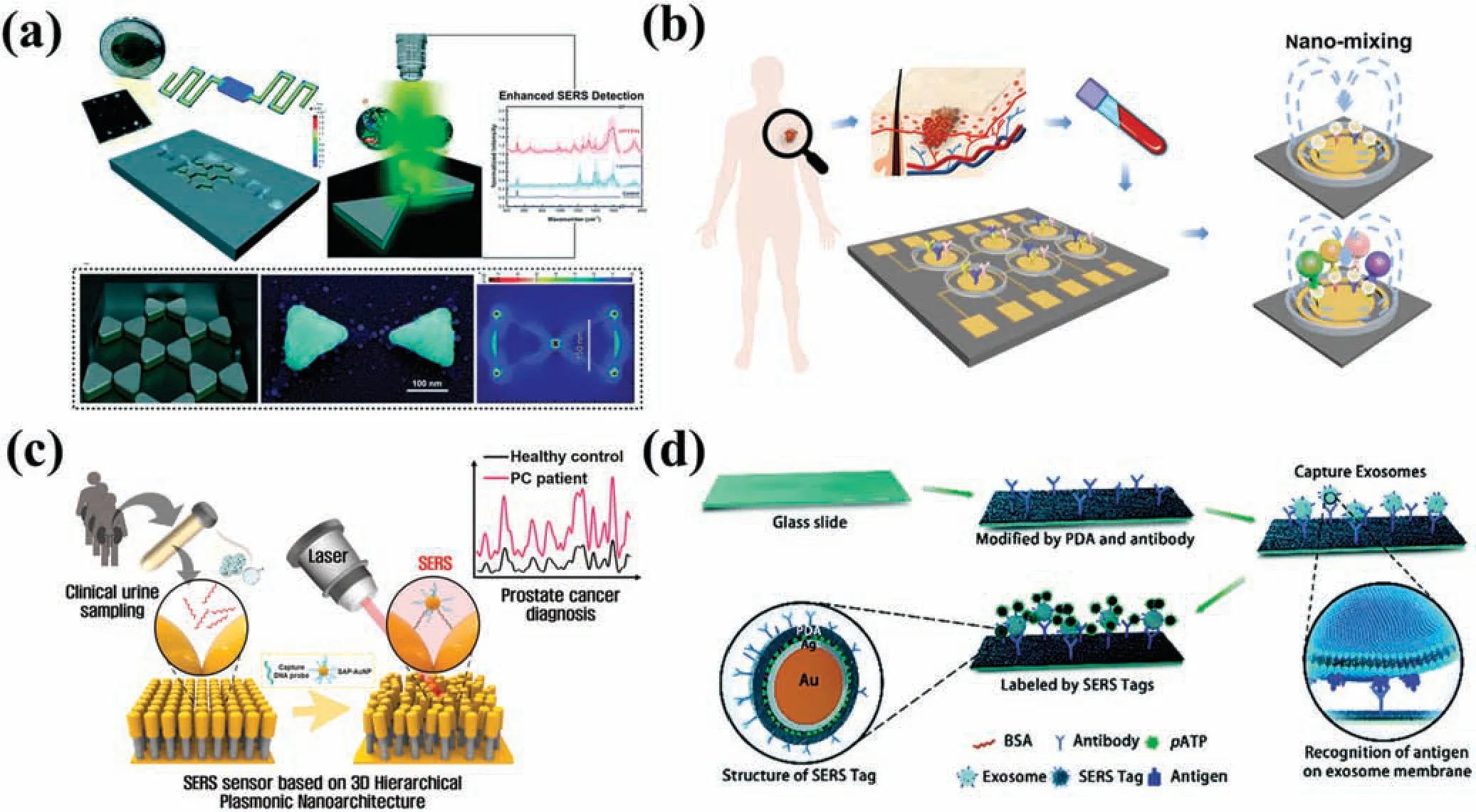
FIG.8. Detection of EVs by surface-enhanced Raman scattering (SERS) techniques.(a) Microfluidi platform with butterfly-shape structure.137 Reproduced from Jalali et al.,Lab Chip 21,855–866(2021).(b)EPAC-II platform combining microfluidi chip and multiplex SERS detection.138 Reproduced from Wang et al.,Adv.Funct.Mater.32,2010296(2022).(c)SERS system for detecting miRNA in urine.139 Reproduced from Kim et al.,Biosens.Bioelectron.205,2010296(2022).(d)SERS detection of EVs captured in a microfluidi channel.140 Reproduced from Li et al.,Chem.Sci.9,5372–5382(2018).
miRNAs are commonly low in abundance in body fluid and exhibit a high degree of sequence homology.Kimet al.139developed a SERS system utilizing 3D plasmonic nanostructures for detecting miRNAs in urine.Gold nanopillars and self-assembled DNA probe-conjugated gold nanoparticles(SAP-AuNPs)were employed to construct a 3D nano hierarchical structure that significantl boosted the SERS signal of miRNAs present in urine.This hierarchical plasmonic nanostructure served to extend the plasmonic hotspot between SAP-AuNPs and nanopillars,thereby increasing sensor sensitivity and enhancing clinical applicability.When the target miRNA was present,it was confine within a 3D plasmonic hotspot formed between a nanopillar head and a AuNP bearing a semi-complementary DNA probe.The sensor exhibited an exemplary detection performance,with a detection limit as low as 10 aM and a linear detection range from 10 aM to 100 nM for miRNAs(miR-10a and miR-21)in EVs of prostate cancer,surpassing that of qRT-PCR[Fig.8(c)].
A traditional technique for EV detection involves modifying specifi antibodies in microchannels.Liet al.140developed a pioneering SERS nanotag for sensitive EV detection utilizing a polydopamine-coated antibody–reporter–Ag(layer)–Au(core)multilayer structure.By injecting EVs derived from pancreatic cancer into the flo channel along with antibody to the macrophage migration inhibitory factor(MIF),a high-intensity SERS signal was achieved.Remarkably,only 2 μl of clinical serum samples were required to differentiate pancreatic cancer patients from healthy individuals [Fig.8(d)].Inspired by honeycombs,Donget al.141developed a 3D gold-coated titania microporous inverse opal(MIO)structure exhibiting remarkable EV-enhanced SERS signal performance.The MIO could directly separate and detect EVs from cancer patients’plasma.This method has the potential for swift and labelfree diagnosis of cancer by tracking the protein phosphorylation process in EVs.
D.Nanostructure-enabled electrochemical detection
Electrochemical detection analyzes changes in the electrical signals of target EVs and has the advantages that it is highly sensitive and low in cost,requiring only simple equipment and small samples.Typically,electrochemical detection amplifie the electrical signal of the targeted molecule through aptamer or antibody binding.142,143Zhouet al.144developed an aptamer-based CD63-specifi EV electrochemical biosensor to quantitatively measure EVs in cell supernatants.They integrated CD63 aptamers and modifie gold electrodes into a microfluidic chip and applied a methylene blue label to the aptamers via DNA probing strands.After the CD63 aptamer was hybridized into a DNA duplex,displacement of the antisense strand led to a weakened electrochemical signal without the need for antibody labeling or fluorescen image acquisition[Fig.8(a)].Li and co-workers145proposed a label-free EV sensor that utilized the characteristics of antibody and aptamer binding to amplify the electrochemical signal.The sensor used CD63 antibodymodifie gold electrodes and specifi aptamers for binding gastric cancer EVs.The addition of a heme/G-quadruplex system with rolling circle amplificatio (RCA) allowed for sensitive and selective detection of EVs in gastric cancer.Exposure of gastric cancer EVs to RCA stimulated abundant production of G-quadruplexes.After incubation of the reaction product with heme,the resulting heme/G-quadruplex structure catalyzed the reduction of H2O2,with the consequent generation of electrochemical signals.Donget al.146utilized a similar strategy in which aptamer-modifie magnetic beads were used to bind EVs from tumor cells.Messenger DNA (mDNA) released by these EVs then hybridized with probe DNA immobilized on gold electrodes,generating an electrochemical signal.
The use of nanostructures for electrochemical detection of EVs is a novel technique.Mathewet al.147developed a microfluidi electrochemical detection system that integrated nano-interdigitated electrodes,utilizing both a redox cycle and enzymatic amplifica tion to augment electrochemical signals.The sensor comprised two sets of interdigitated nanoelectrode arrays with nanometer-scale spacing,which improved the signal amplificatio capability of the redox cycle.The method used an anti-EpCAM antibody on the electrode surface to capture EVs,together with a reporter anti-EpCAM antibody conjugated to alkaline phosphatase(ALP)via biotin–SAV interactions.Subsequently,captured EVs were selected sequentially twice.Reaction of the ALP with ap-aminophenyl phosphate substrate provided a firs electrochemical signal amplification which was followed by a second amplificatio due to redox cycling of the product of this reaction.This approach was successful in analyzing different concentrations of prostate cancer cells [Fig.9(b)].Jung’s group148introduced a detachable aptamer-based electrochemical sensor,known as the DeMEA chip.They prepared a composite of chitosan,graphene nanosheets,and MoS2nanosheets.This composite material enhanced electrode conductivity and improved the EV capture rate owing to the increased surface area.The sensor was modifie with an EpCAM aptamer and utilized a minute sample size of only 10 μl for detection purposes.
E.Nanostructure-enabled piezoelectric biosensor detection
Advances in acoustic technology have provided opportunities for the development of novel detection strategies based on piezoelectric biosensors and the fact that the frequencies of acoustic waves change in response to fluctuation in the propagation medium.Acoustic techniques that generate acoustic pressure and streaming effects in fluid have the advantage of good biocompatibility.Piezoelectric biosensors enable capture of extracellular vesicles (EVs),as well as functioning as tools for processing EVs(e.g.,lysing EVs).149Wanget al.150developed a surface acoustic wave(SAW)sensor based on the use of gold nanoparticles as signal enhancers for the highly sensitive detection of EVs in blood samples obtained from cancer patients.After pre-treating the sensor,they modifie its surface with an anti-CD63 antibody to immobilize EVs.Streptavidin-modifie AuNPs and biotin-conjugated anti-EpCAM antibodies were then utilized to bind and recognize EVs.The SAW phase shift caused by the introduction of the AuNPs amplifie the signal,giving this sensor an improved detection sensitivity(through the mass effect).Testing demonstrated that this sensor was able to detect EVs in blood samples within 30 min[Fig.9(c)].
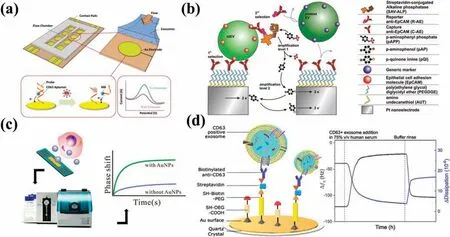
FIG.9.Platforms for detecting EVs based on nanostructures.(a)Electrochemical detection platform with modifie aptamers on the electrode surface.144 Reproduced from Zhou et al.,Methods 97,88–93(2016).(b)Electrochemical method for the detection of tumor-derived EVs using nano-interdigitated electrodes.147 Reproduced from Mathew et al.,Nano.Lett.20,820–828(2020).(c)SAW sensor with gold nanoparticles as signal amplificatio materials.150 Reproduced from Wang et al.,ACS Sensors 5,362–369(2020).(d)Acoustic immunodetection of EVs with QCM-D.152 Reproduced from Suthar et al.,Anal.Chem.92,4082–4093(2020).
Conventional methods for EV nucleic acid examination suffer from laborious operation,excessive time consumption,and limited sample retrieval rates.151Sutharet al.152developed a bulk acoustic wave(BAW)resonator-based sensor for EVs that employed a quartz crystal microbalance with dissipation detection(QCM-D)for labelfree capture and analysis of CD63-positive EVs.An affinity-base method was used to functionalize the sensor surface with anti-CD63 antibodies.Selective separation of EVs was achieved by tracking changes in sensor resonance frequency (which depended on surface antigen species,mass,and viscoelasticity) when analytes were adsorbed onto the sensor surface.Following binding of EVs expressing CD63 to the sensor surface,the resonance frequency underwent specifi changes that correlated with the physical properties and concentration of the EVs.By utilizing dissipation monitoring and frequency,this technique enabled direct evaluation of EVs at their native concentrations in complex biological samples[Fig.9(d)].
F.Nanostructure-enabled EV analysis at the level of individual particles
The majority of the available techniques mentioned above focus on analyzing bulk EVs rather than individual particles.However,EVs show pronounced heterogeneity in their sources,compositions,and functions.Therefore,analyzing individual EVs is essential to unveil their diversity and complexity.In-depth analysis of single EVs can provide crucial insights into their biological roles,intercellular communication,and potential as diagnostic and therapeutic tools.153Moreover,single-EV analysis is a more effective strategy than bulk analysis in determining specifi molecular and phenotypic features of cancer.154Consequently,innovative methods that enable precise and comprehensive single-EV analysis are in great demand.
Research on single EVs also focuses on their proteins,nucleic acids,and physical and chemical properties,similar to studies on EV populations.However,examining single EVs presents technical challenges that differ from those encountered when dealing with EV populations.The minute size of EVs (30–150 nm) and their low abundance in body fluid necessitate specialized techniques for single-EV detection and trapping.Additionally,the limited cargo of a few molecules per marker poses a challenge for signal amplificatio and detection.155Microfluidi techniques based on nanostructures have demonstrated significan advantages in separating and detecting single EVs.On the one hand,nanostructures can provide tiny channels and pores with similar sizes to EVs,thus enabling the separation and capture of a single EV.On the other hand,nanostructures provide superior optical detection capabilities for digital-based techniques.156
Capturing and separating single EVs is a prerequisite for their analysis.157Techniques based on nanostructures and microfluidic can be divided into two categories: those based on physical properties and those based on immune affinity Physical characteristicbased techniques generally employ nanoscale structures directly.Nelson’s group158developed a novel approach to allow visualization and ion-sensitive dye measurement of single EVs [Fig.10(a)].A filtration-base platform was used to capture and stabilize EVs based on their size.After selective capture of secreted EVs within the pores of ultrathin nanoporous silicon nitride(NPN)membranes based on their size,real-time fluorescenc imaging was used to measure the kinetics of pH changes in single vesicles brought about by the activity of the Na/H antiporter.This study has provided a new platform for studying possible mechanisms for cargo loading and maintenance at the single-EV level.In addition to single-size nanostructures,a combination of multiple-size nanostructures enabled the preparation of subpopulations of EVs in three different size fractions and an analysis of the presence of HER2 and PSMA in breast and prostate cancer cell-derived EVs,respectively,at the single-EV level.159Along with passive flui interaction,active techniques have also been utilized to capture single EVs.160Nanostructures triggered by acoustic waves generate robust nanoscale force gradients for the trapping of single EVs [Fig.10(b)].A structured elastic layer placed between a microfluidi channel and a traveling SAW device provided submicrometer acoustic traps capable of capturing individual submicrometer particles.The acoustically driven deformation of the nanocavities produced time-averaged acoustic field that directed suspended particles toward the nanocavities and trapped them there.SAWs permit massively multiplexed particle manipulation with deterministic patterning at the single-particle level.
Immune-affinit traps capture EVs by binding a selected membrane protein to its corresponding antibody immobilized on a nanostructure.161The small size of the nanostructure and an appropriate optical enhancement effect then enable a lower detection limit to be reached.130Trau and co-workers162introduced the DECODE chip,which captured EVs on a nanostructured pillar chip,confine individual EVs,and used SERS to detect three lungs cancer-associated biomarkers as well as a generic single-EV biomarker [Fig.10(c)].The DECODE chip was used to generate digitally acquired single-EV molecular profile in a cohort of 33 subjects including both those with malignant and benign lungs nodules,as well as healthy individuals.DECODE was able to provide specifi molecular profile for single EVs that enabled differentiation between malignant and benign nodules with an area under the curve(AUC)of 0.85.
Single-EV analysis utilizing nanostructures presents several advantages over traditional bulk analysis methods.By analyzing EVs individually,researchers can obtain more detailed information about their characteristics and better understand the relationship between different subtypes of vesicular molecules and diseases.The high-precision counting and improved detection limits provided by nanostructure-based methods can also help detect disease biomarkers with greater accuracy,potentially leading to earlier and more accurate diagnoses.Additionally,these methods may be useful for monitoring disease progression and treatment response.While current nanostructure-based methods may be cumbersome and have low throughput,ongoing research is focused on developing more efficien and scalable techniques.As these methods continue to improve,they hold promise as valuable diagnostic tools for cancer and other diseases.
IV.CONCLUSION AND OUTLOOK
As essential biomarkers in liquid biopsies,EVs have shown significan potential in clinical diagnosis.Much work has been done on the separation and detection of these complex nanoscale particles and the detailed biological information that they encapsulate.This review commenced by outlining the constituents and biogenesis of EVs,and the traditional methods used for their separation and detection.This was followed by a summary of the latest advances in nanostructure-based procedures for EV separation and detection,as well as their application in bioanalysis and disease diagnosis.Thanks to advances in nanofabrication technology,novel techniques based on nanostructures are able to handle complex bodily flui samples and provide extensive insights into the function and structure of EVs.Nevertheless,these nanostructure-based techniques are still in their early stages of development.
1.Owing to the inherently small sample volume of microfluidic in nanostructures,the resulting separation throughput is very low(of the order of just microliters per minute),which falls far below the liter per minute requirement of clinical applications.Nonetheless,even such low sample sizes and throughputs have minimal impact on detection results.Furthermore,EVs in samples (especially bodily fluids are biologically heterogeneous,with a mixture of multiple subtypes of EVs.Thus,the combined implementation of several separation techniques will be needed to achieve high-resolution separation.However,separating EVs of pathological cells from those of normal cells poses a challenge.Moreover,employing passive or active methods that combine acoustic and electric field during EV separation inevitably results in EV damage.Amplifie electric and acoustic field can produce thermal effects,which irreversibly affect the biological activity of EVs.Overcoming these limitations will enable a deeper appreciation to be achieved of the roles of different EV subsets in early-stage cancer and in disease prognosis,allowing for personalized therapy development.
2.Regarding detection,conventional methods often necessitate an all-inclusive EV characterization through NTA,western blotting,SEM,and qRT-PCR.Contemporary studies require greater EV purity.The integration of nanostructures and optical detection significantl reduces the detection limits,enabling more precise and sensitive EV detection.Different research groups utilize different antibodies or aptamers for cancer diagnoses,and hence have varying views on analysis and characterization techniques.Thus,there is a pressing demand to establish standardized EV detection processes.
3.Integrating nanostructures with microfluidi technology in the biomedical domain assists in system miniaturization.The use of lab-on-chip equipment,including sample pretreatment,separation,and detection,efficientl reduces excessive processing periods,exorbitant costs,and laborious clinical sample management.Physicians have acknowledged the advances that can be achieved through the use of EV-based point-of-care(POC)devices.Also,notably,machine-learning algorithms and other artificia intelligence techniques can provide novel approaches for high-dimensional EV data processing and analysis.Such algorithms should enable enhanced cancer detection,diagnosis,and classification boosting the potential for clinical application of multifunctional POC devices.
ACKNOWLEDGMENTS
The authors gratefully acknowledge financia support from the National Key R&D Program of China (Grant No.2018YFE0118700),the National Natural Science Foundation of China (NSFC Grant No.62174119),the 111 Project (No.B07014),and the Foundation for Talent Scientists of Nanchang Institute for Micro-technology of Tianjin University.
AUTHOR DECLARATIONS
Conflict of Interest
The authors have no conflict to disclose.
DATA AVAILABILITY
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
- 纳米技术与精密工程的其它文章
- Stress distribution variations during nanoindentation failure of hard coatings on silicon substrates
- Electrode design for multimode suppression of aluminum nitride tuning fork resonators
- Effects of simulated zero gravity on adhesion,cell structure,proliferation,and growth behavior,in glioblastoma multiforme
- Design and analysis of longitudinal–flexuralhybrid transducer for ultrasonic peen forming
- An advanced cost-efficient IoT method for stroke rehabilitation using smart gloves
- Electrical characterization of an individual nanowire using flexible nanoprobes fabricated by atomic force microscopy-based manipulation

