Effects of Rosa roxburghii &edible fungus fermentation broth on immune response and gut microbiota in immunosuppressed mice
Dechang Xu,Jielun Hu,Yadong Zhong,Yanli Zhang,Wenting Liu,Shaoping Nie,Mingyong Xie*
State Key Laboratory of Food Science and Technology,China-Canada Joint Laboratory of Food Science and Technology (Nanchang),Key Laboratory of Bioactive Polysaccharides of Jiangxi Province,Nanchang University,Nanchang 330047,China
Keywords:Fermented foods Immunosuppressed mice Immune response Gut microbiota Short-chain fatty acids
ABSTRACT With the rise of probiotics fermentation in food industry,fermented foods have attracted worldwide attention.In this study,protective effects of Rosa roxburghii &edible fungus fermentation broth (REFB) on immune function and gut health in Cyclophosphamide induced immunosuppressed mice were investigated.Results showed that REFB could improve the immune organ index,and promote the proliferation and differentiation of splenic T lymphocytes.In addition,it attenuated intestinal mucosal damage and improved intestinal cellular immunity.REFB administration also up-regulated the expression of IL-4,INF-γ,TNF-α,T-bet and GATA-3 mRNA in small intestine.Furthermore,administration of REFB modulated gut microbiota composition and increased the relative abundance of beneficial genus,such as Bacteroides.It also increased the production of fecal short-chain fatty acids.These indicate that REFB has the potential to improve immunity,alleviate intestinal injury and regulate gut microbiota in immunosuppressed mice.
1. Introduction
The immune system is the most effective defense against invading pathogens in the body and relies on a variety of immune organs(spleen and thymus),immune cells (splenocytes and macrophages)and immune molecules to enhance immunity.The spleen is an organ in which T and B lymphocytes accumulate,and can regulate immune function through cellular and humoral immunity[1].
The intestine is the largest immune organ and the main organ for nutrient absorption in the body.The intestinal mucosal immune system consists of the mucus layer,the intestinal epithelium and the lamina propria.The large number of goblet cells and lymphocytes in the intestinal epithelial layer synthesis and secrete proteoglycans and antimicrobial peptides to form the mucus layer,which could prevent pathogens from invading[2].Immune cells in the lamina propria are mainly T cells and B cells,which respond rapidly to intestinal signals and participate in intestinal mucosal immunity[3].
Gut microbiota is an integral part of the body,which could reduce the risk of infections and autoimmune diseases,and act as an immune stabilizer to stimulate the immune response and protect the barrier function of the gut[4].Gut microbiota plays an important role in the health state of the host as well as the protection against disease,and it is easily influenced by diet and environment factors[5].There is substantial evidence that dietary treatment could enhance immunity,modulate gut microbiota and maintain gut homeostasis[6].
RosaroxburghiiTratt is a member of the Rosaceae family,and growing interest in studyingR.roxburghiihas been emerging due to its nutritional and energetic components,such as organic acids,vitamin C,polysaccharides,polyphenols and minerals[7].These components have a variety of biological activities,such as antioxidant,anti-diabetic,anti-atherosclerosis and other functions[8].As the promising function food,R.roxburghiifruit can be consumed in the fresh or processed form,such as juice,dried and canned products.However,the fresh fruit containing tannin has an astringent taste,and the shelf life is short,which greatly reduces the utilization of prickly pear[9].Fermented plant foods are attracting more and more attention as healthy foods due to their special flavour and health-promoting potential.Probiotic fermentation is an emerging food processing method that could not only extend food storage life,improve food quality and enhance the functionality of food,but also play an important role in relieving disease and keeping healthy.For example,Lactobacillusplantarum-fermentation could enhance the anti-diabetic effects ofMomordicacharantiapolysaccharides in rats[10],Asparagusofficinalispolysaccharides fermented byL.plantarumhas a better recovery effect on reducing liver damage caused by Cy[11],and probiotic-fermented carrot pulp exerts stronger effect in reducing blood glucose than unfermented one[12].
A growing number of studies have shown that fermented fruit and vegetable juices have immune-boosting,antioxidant and gastrointestinal protective effects,such as the Ouli fermented juice,co-fermented peptide-jackfruit juice,and fermented litchi juice[13-15].In this paper,the potential protection effects on immune function and gut health ofR.roxburghii&edible fungus fermentation broths(REFB) were systematically investigated by cyclophosphamide (Cy)-induced immunosuppressive mice model,which aims to explore new kind of immunomodulatory healthy food from probiotic fermentation and provide new ideas for the utilization ofR.roxburghiiand edible fungus resources.
2. Materials and methods
2.1 Materials and reagents
R.roxburghiiand edible fungus (Hericiumerinaceus,Pleurotus ostreatusandLentinulaedodes) were mixed in the ratio of 5:5,and then fermented with microorganisms (mainlyLactobacillusreuteriandLactobacillusacidophilus).After sterilization and centrifugation,REFB was obtained.The main nutritional composition table of REFB were listed in Supplementary Information Table 1.The mixed fermentation microorganism was provided by Guizhou Qianzhi Ai Biotechnology Co.Ltd.(Guizhou,China).

Table 1 The main nutritional composition table.
Cy,lipopolysaccharide (LPS) and concanavalin A (Con A) were purchased from Sigma-Aldrich (St.Louis,MO,USA).Levamisole hydrochloride (LH) was obtained from Renhe Tang Pharmaceutical Co.Ltd.(Shandong,China).Cell Counting Kit-8 (CCK-8) was bought from Dojindo Molecular Technologies Co.(Shanghai,China).PBS buffer dry powder and RPMI1640 medium were from Solarbio Life Science Co.(Beijing,China).The fetal bovine serum (FBS)was obtained from Biological Industries Co.(Israel).Revere aid first strand cDNA synthesis kit was purchased from Thermo Fisher Scientific (Vilnius,Lithuania).SYBR premix ex taq II kit was from Takara Biotechnology (Dalian,China).Primers were designed by Genecript China,Ltd.,(Nanjing,China).
2.2 Animals and experimental design
Female BALB/c mice (NO.SCXK (Xiang) 2016-0002),6 weeks old and (20 ± 2) g,were purchased from the Hunan Slac Jingda Laboratory Animal Co.Ltd (Changsha,China).Animals were acclimatized for one week with an ambient temperature (22 ± 1) °C,humidity (50 ± 5)%,12 h:12 h light/dark cycle,free access to food and water.All mice used in this study were cared for according to the Guidelines for the Care and Use of Laboratory Animals published by the United States National Institutes of Health (NIH Publication 85–23,1996),and approved by the Animal Feeding Review Board of Nanchang University,China (Animal Experiment License No.0064257).
The experimental cycle including 7 days of adaptation was 40 days in total.After adaptation,the experimental mice were radomly divided into 6 groups (n=10),included normal control group (NC),model control group (MC),positive control group (PC),and low-,medium-,and high-dose REFB groups (REFB-L,REFB-M,REFB-H).Except NC group,all other groups were intraperitoneal injected with Cy (80 mg/kg bw) from day 8 to day 10 and also on day 25 and 33 to establish the immunosuppressive mouse model.From day 11,mice in the REFB-L,REFB-M and REFB-H groups were orally given 3.9,7.8 and 15.6 mL/kg bw of REFB for 30 consecutive days,respectively.The NC and MC groups were gavaged with equivalent volume of saline instead.The PC group was administered with levamisole hydrochloride (LH) at a dose of 40 mg/kg bw.Mice were sacrificed by cervical dislocation 24 h later after the last administration.Thymus,spleen and other organs were collected for further use.The experimental design is shown in Fig.1.
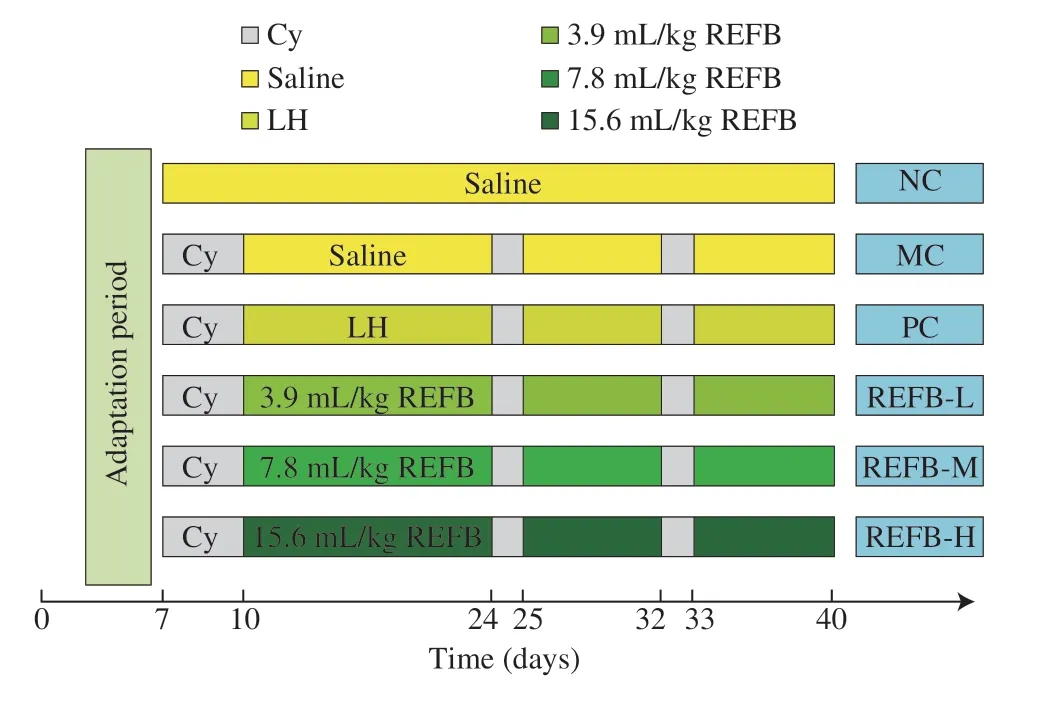
Fig.1 Schematic diagram of the animal experimental design.Cy:cyclophosphamide;LH: levamisole hydrochloride;REFB: Rosa roxburghii &edible fungus fermentation broth.
2.3 Immune organ indexes
The excised spleen and thymus were rinsed in saline,drained on filter paper and accurately weighted.The spleen and thymus indexes were calculated as equation 1:
2.4 Preparation of lymphocyte cell
Spleens were taken under aseptic conditions,moistened with PBS(0.01 mol/L,pH 7.4),ground,passed through a 200-mesh cell sieve,rinsed onto plates with PBS,and washed three times with PBS to obtain single cell suspension.The cells were re-suspended in Roswell Park Memorial Institute (RPMI) 1640 medium with 10% FBS and the cell concentration adjusted to 5 × 106cells/mL[16].
2.5 Lymphocyte proliferation assay
The detection of lymphocyte proliferation was assayed by the Cell Counting Kit-8 (CCK-8) method[17].The cell suspension(100 μL) was placed in every well of a 96-well flat-bottomed.An equal volume of Lipopolysaccharide (LPS) (10 μg/mL) or Concanavalin A (ConA) (5 μg/mL) was added and a blank control group was set up with an equal volume of medium and 6 replicate wells were set up for each group.The 96-well flat-bottomed were incubated in an incubator at 37 °C with 5% CO2for 48 h.A mixture of CCK-8 and medium was then prepared according to the instructions of CCK-8 kit.The mixture of CCK-8 (100 μL) was added to each well of the culture plate,incubated in the incubator for 30 minutes and the absorbance was measured at 540 nm.The stimulus index (SI) was calculated as equation 2:
2.6 Determination of CD3+,CD4+ and CD8+ T lymphocytes in spleen
The prepared single cell suspension (1.5 mL) with the cell concentration of 5 × 106cells/mL was taken.After washing and centrifuging twice with PBS,20–50 μL of PBS was left in the tube after the last centrifugation to make cell suspension.The cell suspension was mixed with 0.625 μL of anti-CD3,anti-CD4 or anti-CD8 antibodies respectively and incubated for 30 min at 4 °C protected from light.The reaction was terminated by adding 1.0 mL of PBS,centrifuged,washed twice with PBS to remove excess antibody and resuspended in 0.5 mL of PBS.The counts of CD3+,CD4+and CD8+T lymphocytes were detected by flow cytometry and measured as percentages of the total number of T-lymphocytes in each.
2.7 Histological observation
Tissue specimens of the small intestine were prepared based on the method described previously[18].The small intestine was fixed with 10% formalin solution for more than 24 h,and paraffin sections were prepared.The tissue structure was examined by using Hematoxylin and stained (H&E),the number of goblet cells was detected by Alcian blue/periodic acid-Schiff staining (AB-PAS) and the state of tight junctions was detected by Immunohistochemical Staining (IHC).The histological differences were observed under an optical microscope.ImagePro software was used for measurement analysis.
2.8 Real-time qPCR
Total RNA was extracted from small intestine tissue immersed in RNA wait by TRIzol Bacterial RNA Isolation Kit (Solabio,Beijing,China).The cDNA was obtained by reverse transcription using the Revert Aid First Strand cDNA Synthesis Kit,and the RT-qPCR was performed based on the SYBR Premix Ex Taq II kit by the Quant Studio 7 Real-Time PCR System.Data analysis was performed by the 2-ΔΔCtmethod.The reference gene Gapdh was used for normalization.The primer sequences used were listed in Table 2.
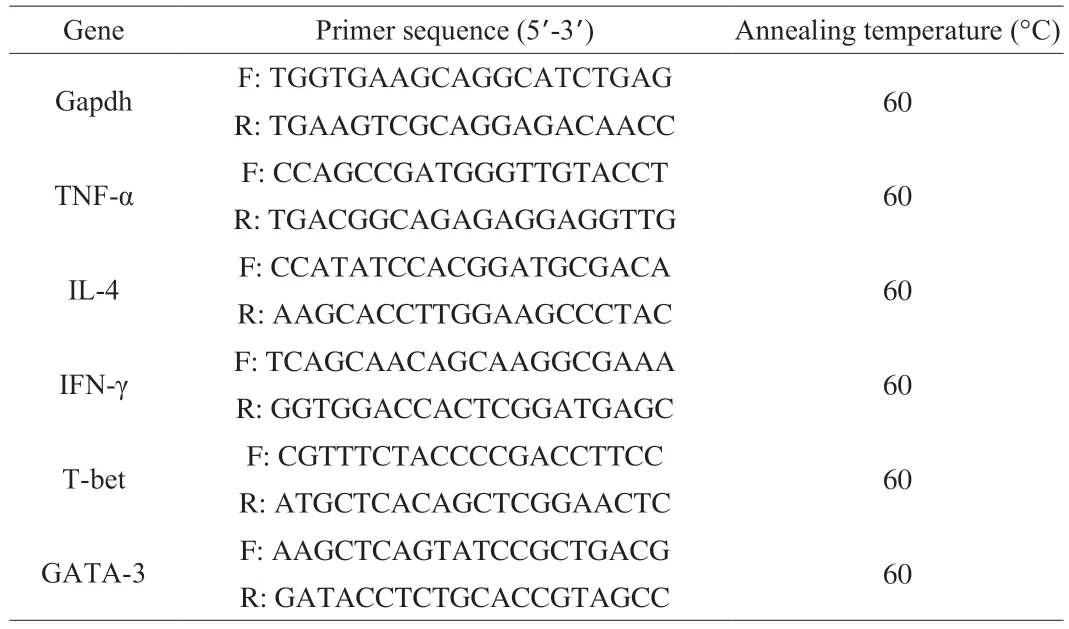
Table 2 Sequence and annealing temperature of primers
2.9 16S rRNA sequencing of gut microbiota
DNA was extracted from cecal contents by using the QIAamp DNA Stool Mini Kit (Qiagen,Hilden,Germany) according to the manufacturer’s instruction.Extracted DNA were subjected to 2% agarose gel electrophoresis and ultraviolet spectroscopy for checking the quality and concentration (Nano Drop ND 2000,Thermo Fisher Scientific,Waltham,USA).The library preparation kits (TruSeq Nano DNA LT kit,U.S.A.) and cluster generation and sequencing reagents (MiSeq Reagent Kits v2,500 cycles PE,U.S.A.) were purchased from Illumina.The bacterial 16S rRNA gene V4 hypervariable region was amplified by primers 515F 5’-barcode-GTGCCAGCMGCCGCGGTAA-3’ and 806R 5’-GGACTACHVGGGTWTCTAAT-barcode-3’.Amplicons were extracted from 2% agarose gels and purified using the Common Agarose Gel DNA Recovery Kit (Tiangen,China).Purified PCR products were quantified by Qubit 3.0 (Life Invitrogen).The Agilent 2100 Bioanalyzer System (High Sensitive DNA Chip) was used for analyze the quality control of the library.The pooled DNA product was paired-end sequenced on the Illumina MiSeq platform according to the standard protocols.
QIIME 2 (http://qiime2.org) software was used for quality control splicing,filtering,and other preprocessing of the raw data,and DADA2 was used to sequence splicing and removal of chimeric sequences.Alpha diversity and microbial taxon distribution analysis was performed.Beta diversity analysis was performed using principal coordinate analysis (PCA).Genus-level relative abundance matrices were submitted on the Galaxy online analysis platform for LEfSe analysis (http://huttenhower.sph.harvard.edu/galaxy/).
2.10 Determinations of short-chain fatty acids (SCFAs) in feces
Feces (100 mg) were diluted 10 times with saline,and then homogenated and centrifuged.0.4 mL of anhydrous ether was added to 0.6 mL of the supernatant,mixed well,stood for 5 minutes,centrifuged,and filtered (0.22 μm).The level of SCFAs were determined using gas chromatography (GC) according to our published method[19].
2.11 Statistical analysis
Results were expressed as means ± standard deviation (SD) and the data analysis were performed using SPSS 23.0 software.Twoway analysis of variance (ANOVA) with Tukey’s test was used,andP<0.05 was considered to be statistically significant.
3. Results
3.1 Effects of REFB on the immune organ indexes
Thymus and spleen play an important role in the immune system of the body.The thymus and spleen indices reflect to a certain extent the immune capacity of the body[1].As shown in Figs.2A-B,the spleen and thymus indices of the MC group were significantly lower than those of the NC group (P<0.05),suggested that Cy could significantly suppress the immune function of mice and the immunosuppression model was successfully established.The three REFB groups had a significant higher spleen and thymus indices compared to the MC group (P<0.05).The results indicated that REFB could reduce the atrophy of immune organs caused by Cy to different degrees and effectively restore immune organ damage in immunosuppressed mice.
3.2 Effects of REFB on the proliferation of splenic lymphocytes
The spleen could exert immunomodulatory effects through T cellmediated cellular immunity and B cell-mediated humoral immunity.As shown in Figs.2C-D,T-lymphocyte proliferation was significantly inhibited in the MC group ((67.42 ± 2.00)%) compared with the NC group ((100.00 ± 8.34)%) (P<0.05),indicating that Cy inhibited the specific immunity in mice.The proliferation of T-lymphocytes in the REFB-L ((78.92 ± 13.13)%),REFB-M ((79.25 ± 7.40)%) and REFB-H ((80.35 ± 3.78)%) groups was significantly higher than that of the MC group (P<0.05).The proliferation of B-lymphocytes was significantly lower in MC group ((49.72 ± 2.58)%) compared to NC group (100.00 ± 14.80)%) (P<0.05),indicating that Cy could cause damage to humoral immunity in mice.These results suggest that REFB had a promoting effect on the proliferation of T-lymphocytes.
3.3 Effect of REFB on T lymphocyte subset
T-lymphocytes are the most important group of cells in the body for cellular immunity,including CD3+,CD4+and CD8+,etc.,and they could respond to the cellular immune state[20].The effects of REFB on the expression of CD3+,CD4+and CD8+spleen T lymphocytes are presented in Fig.3.The two-dimensional plot shown in Fig.3A illustrated the relative intensity values of the fluorescence signal or scattered light signal.In addition,the percentage of CD4+,CD8+,and CD3+T lymphocytes was significantly lower in the MC group((35.10 ± 1.98)%,(15.07 ± 3.13)% and (50.96 ± 2.59)%) compared with the NC group (P<0.05) (Figs.3B-D),indicating that Cy suppressed the specific immune function of the mice.Compared with the NC group,the percentage of CD3+and CD4+T lymphocytes increased significantly in the REFB-L ((71.87 ± 3.04)%,(53.53 ± 2.94)%),REFB-M ((70.89 ± 1.69)%,(53.91 ± 0.89)%),and REFB-H ((81.14 ± 6.90)%,(58.29 ± 4.60)%) groups (P<0.05),and the REFB-L and REFB-H groups had a significant increase in the percentage of CD8+T lymphocytes ((18.17 ± 0.47)%,(22.49 ± 2.59)%) compared with MC group (P<0.05).CD4+/CD8+is an important indicator of T lymphocyte activity,and its ratio is usually stable under normal physiological conditions.An increase of the ratio indicates dominant helper immune response and enhanced immune function,while a decrease indicates low immune function[21].According to Fig.2E,the ratio of CD4+/CD8+was decreased in the MC group (2.40 ± 0.31) compared with the NC group (2.72 ± 0.10) (P<0.05).Compared with the MC group,REFB-L group (3.04 ± 0.16) and REFB-M (3.36 ± 0.17) group showed a significant higher CD4+/CD8+ratio (P<0.05).These results showed that REFB could suppress the decrease in CD3+,CD4+and CD8+T lymphocytes percentages caused by Cy and increase the CD4+/CD8+ratio.It was indicated that REFB could effectively improve impaired immune function by up-regulating T lymphocyte subsets.Interstingly,it has been reported thatGanoderma lucidumfruiting body extracts after probiotic fermentation showed similar results for T cell differentiation by Li et al[22].
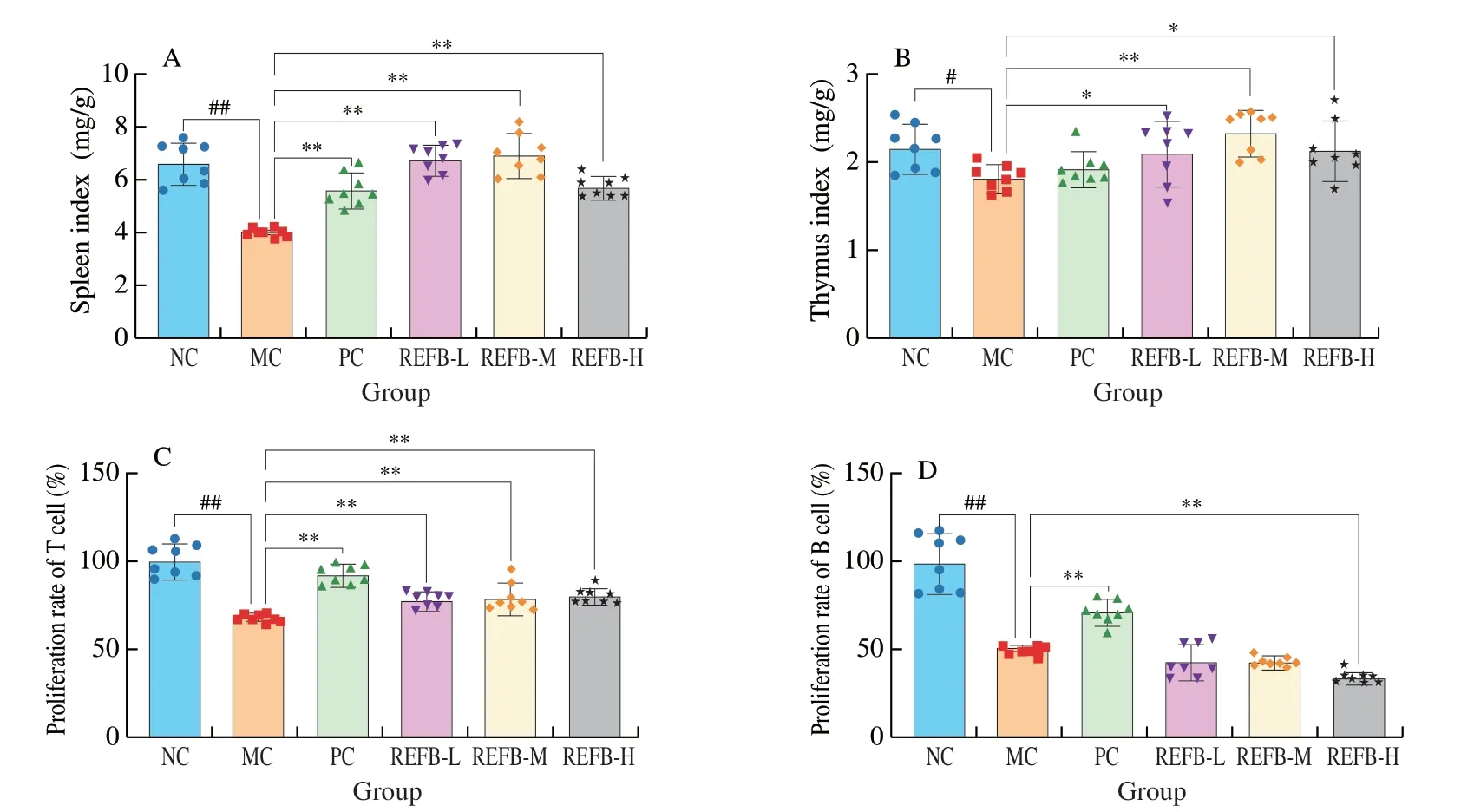
Fig.2 Effect of REFB on organ indiced and proliferation of splenic lymphocyte in the immunosuppressed mice.(A) Spleen index.(B) Thymus index.(C)The proliferation of T cell.(D) The proliferation of B cell.Data are expressed as the mean ± SD (n=8).Compared with the NC group: #P <0.05 and ##P <0.01;compared with the MC group: *P <0.05 and **P <0.01.
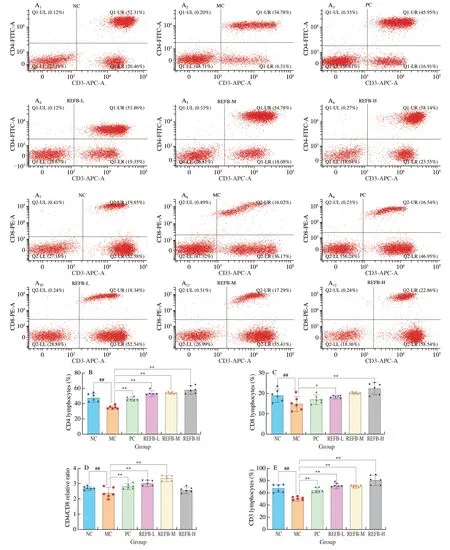
Fig.3 Effect of REFB on the T lymphocyte subset.(A) Relative intensity values of CD3+,CD4+ and CD8+ T cells.(B) The percentage of CD4+ T lymphocyte subset.(C) The percentage of CD8+ T lymphocyte subset.(D) The relative ratio of CD4+/CD8+.(E) The percentage of CD3+ T lymphocyte subset.Data are expressed as the mean ± SD (n=6).Compared with the NC group: #P <0.05 and ##P <0.01;compared with the MC group: *P <0.05 and **P <0.01.
3.4 H&E staining of small intestine
The intestinal mucosa is the body’s first line of defense against pathogens[23].The basic functional unit of the intestinal mucosa is the villi-crypt axis,which integrity and functions are fundamental to assure tissue and whole-body homeostasis,the ratio of villus length/crypt depth (V/C) is also considered to be one of the indicators of intestinal morphology[24].To detect the effect of REFB on intestinal morphology,jejunum tissues were collected for histopathological analysis (Fig.4A).H&E staining showed that the small intestinal villi of the NC group were complete and smooth without any inflammation or epithelial cell damage.The length of the small intestinal villi of the MC group was significantly reduced and shortened.The small intestinal villi length (V) and crypt depth (C) measurements results are shown in Figs.4F-H.Compared with NC group (V: (372.97 ± 42.66)μm;C: (98.30 ± 9.54) μm;V/C: (3.79 ± 0.43),the length of small intestinal villi (V: (281.61 ± 16.20) μm) was significantly shorter,the depth of crypts (C: (147.25 ± 9.65) μm) was significantly increased,and the V/C value (1.91 ± 0.11) was significantly reduced in the MC group (P<0.05).Cy can cause serious damage to the structure of the small intestine.Compared with the MC group,each dose group of REFB could significantly increase the length of small intestine villi and reduce the depth of crypts,and significantly increase the V/C value (P<0.05).The result showed that the structural damage of the small intestine was effectively repaired after administration of REFB.
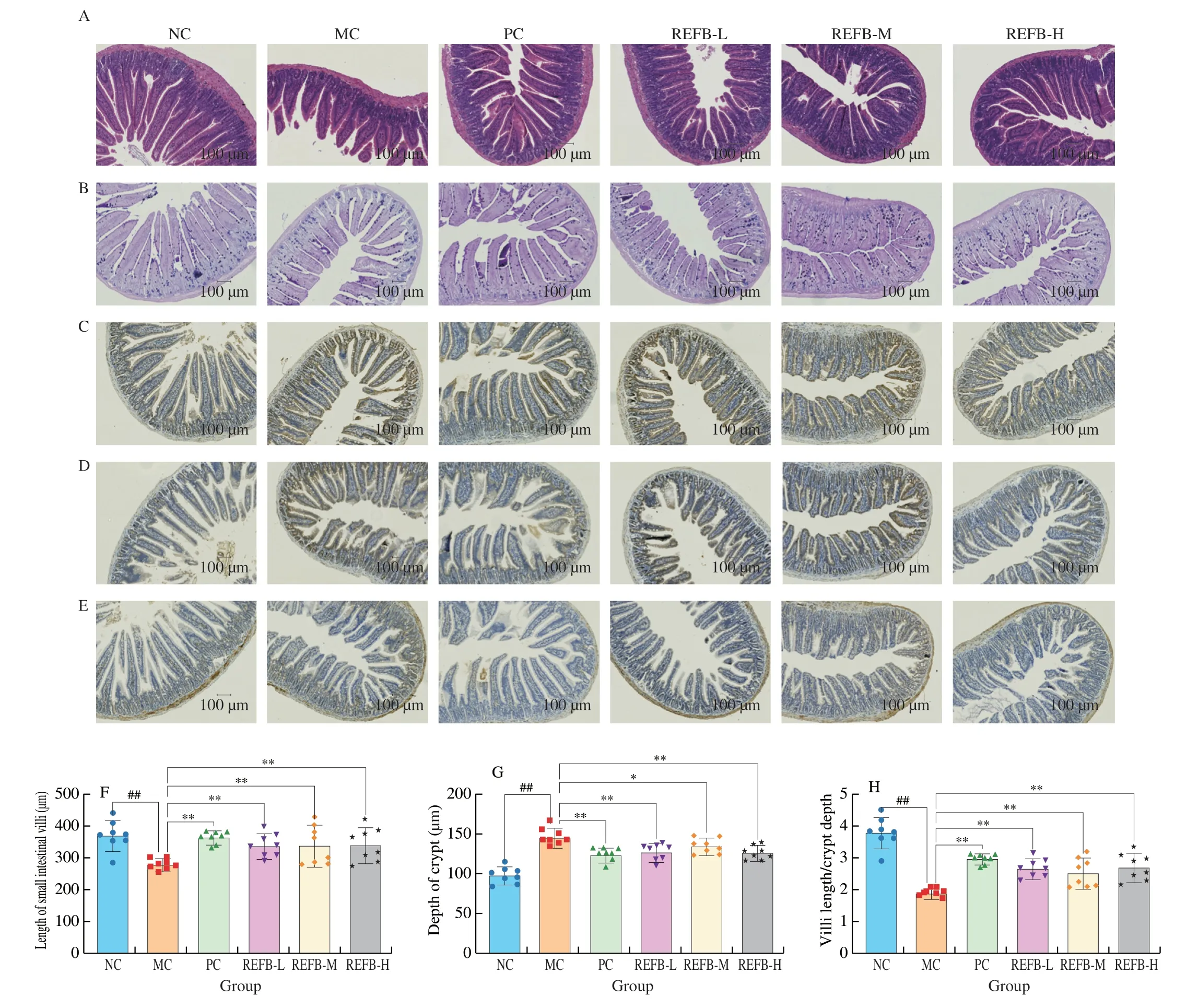
Fig.4 Effect of REFB on the histology of the small intestine of immunosuppressed mice (200× magnification).(A) H&E stained small intestine tissues.(B) AB-PAS stained small intestine tissues.(C-E) ICH stained for Claudin-1,Occludin and ZO-1 tight junction protein.(F) Villi length.(G) Crypt depth.(H) Value of V/C.(I) Number of goblet cells.(J-L) The expression of Claudin-1,Occludin and ZO-1.Data are expressed as the mean ± SD (n=6).Compared with the NC group:##P <0.01;compared with the MC group: *P <0.05 and **P <0.01.

Fig.4 (Continued)
3.5 AB-PAS staining of small intestine
Goblet cells are mucus-secreting cells in the intestinal epithelium that could secrete gel-forming mucins and prevent the pathogens from adhering to the intestinal epithelium[25].After AB-PAS staining,the goblet cells appeared dark blue (Fig.4B).As shown in Fig.4I,the number of goblet cells in the MC group (706.50 ± 34.08 cells) was significantly lower than that in the NC group (929.67 ± 30.06 cells)(P<0.05),indicating that Cy could destroy the intestinal mucosal barrier by interfering with the secretion of goblet cells to some extent.Cy-induced intestinal injury was alleviated to some extent after administration of REFB,with a significant increase in goblet cells in the REFB-M and REFB-H groups (767.67 ± 42.18,829.67 ± 33.65 cells) (P<0.05).The results indicating that REFB could improve intestinal injury induced by Cy.
3.6 ICH staining to detect the state of tight junctions in small intestine
Tight junctions,as the part of intestinal barrier,are essential in the permeability of the intestinal barrier and the maintenance of epithelial cell integrity[26,27].The expression of three tight junction proteins(Claudin-1,Occludin and ZO-1) were determined by ICH staining(Figs.4C-E).Compared with the NC group,the expression of the three tight junction proteins was reduced in the MC group (Figs.4J-L),among which the expression of ZO-1 was significantly lower(P<0.05),indicating that Cy was able to disrupt the intestinal mucosal integrity by inhibiting the expression of tight junction proteins.Compared with the MC group,the expression of the three tight junction proteins was increased to different degrees in the REFB dose groups,among which the expression of ZO-1 was significantly increased in all REFB dose groups (0.10 ± 0.01,0.10 ± 0.00 and 0.11 ± 0.02) (P<0.05),the expression of Claudin-1 was significantly increased in the REFB-M and REFB-H groups (0.15 ± 0.03,0.15 ± 0.06) (P<0.05),and the expression of Occludin was significantly increased in the REFB-M group (0.13 ± 0.01) (P<0.05).In addition,the expression of Claudin-1 and Occludin was higher than that of ZO-1,which was consistent with the trend of the effect of fermented Cordyceps powder on Cy induced intestinal damage of tight junction proteins studied by Ying et al[28].The above results suggest that REFB had some repairing effect on intestinal injury and intestinal barrier induced by Cy.
3.7 Number of T lymphocytes in lamina propria of the small intestine
There are two main subsets of T cells in the gut,CD4+and CD8+T cells,and disorders of T cell immunity can lead to intestinal inflammation and impaired intestinal barrier function[29,30].The number of CD4+and CD8+T lymphocytes in the small intestine were determined by the ICH method (Fig.5A).The number of CD4+T cell in the MC group (394.50 ± 32.54 cells) significantly decreased compared with that in the NC group (558.00 ± 37.80 cells) (P<0.01),and the number of CD8+T cell in the MC group (573.17 ± 42.61 cells)was also significantly reduced compared with the NC group (871.33 ±91.14 cells) (P<0.01) (Figs.5B-C).REFB-L,REFB-M and REFB-H group obviously increased the number of CD4+T cells in the intestinal injury mice (473.83 ± 16.40,493.17 ± 44.05,562.50 ± 19.99 cells)(P<0.01).There was a tendency to increase the number of CD8+T cells in all dose groups of REFB,with the REFB-M and REFB-H groups had a significant effect in increasing the number of CD8+T cells (714.33 ± 30.02,742.67 ± 22.96 cells) (P<0.01).The above data showed that REFB could promote intestinal immunity and repair Cy-induced intestinal injury by increasing the level of CD4+and CD8+T cells in the lamina propria of the small intestine.
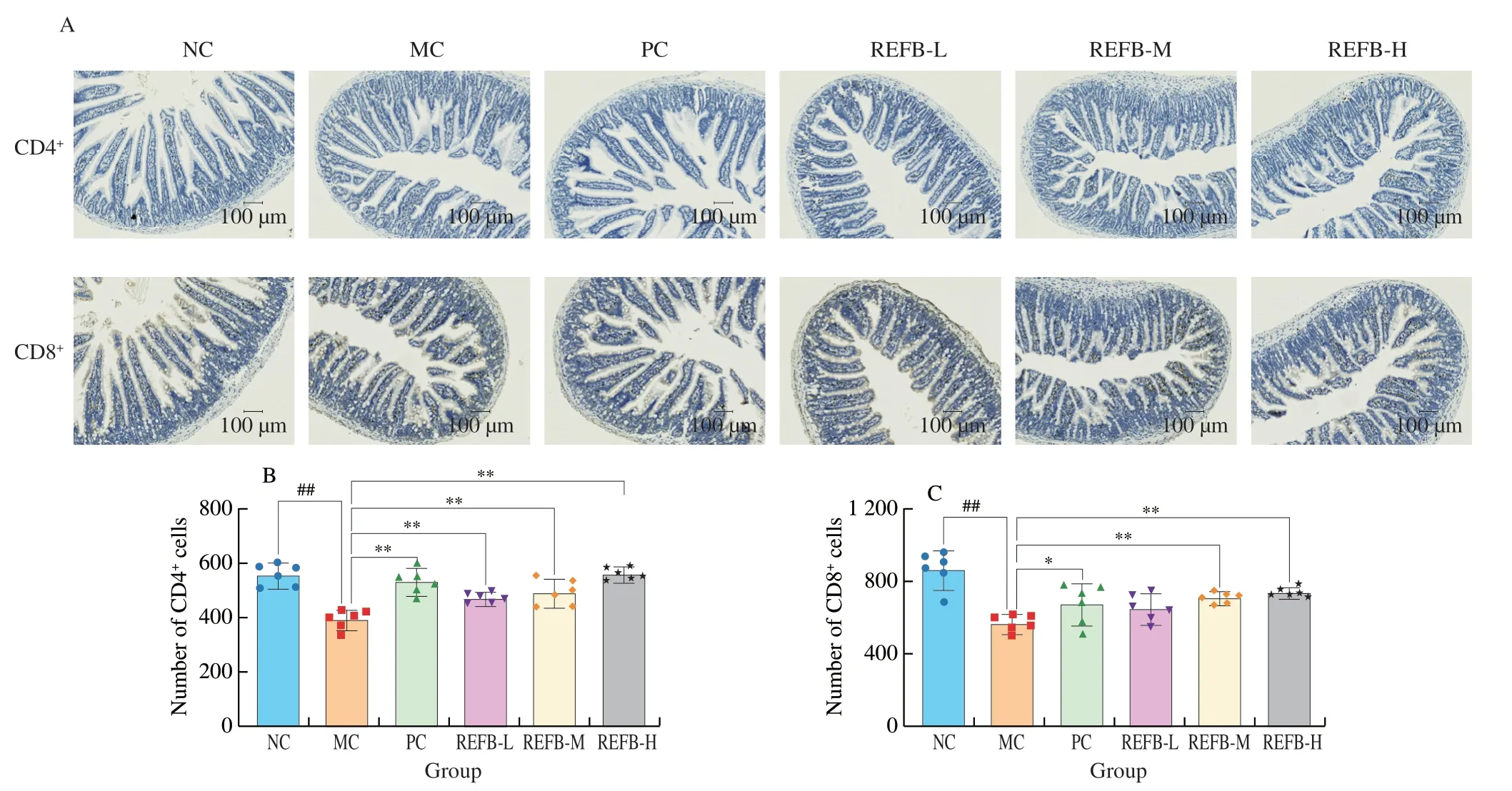
Fig.5 Effect of REFB on the number of T cells in small intestine.(A) ICH stained for CD4+ and CD8+ T cells in lamina propria of intestine.(B) The number of CD4+ cells.(C) The number of CD8+ cells.Data are expressed as the mean ± SD (n=6).Compared with the NC group: ##P <0.01;compared with the MC group:*P <0.05 and **P <0.01.

Fig.6 Effects of REFB on mRNA expression levels of cytokines and related transcription factors in the small intestine.(A) TNF-α.(B) IL-4.(C) INF-γ.(D)T-bet.(E) GATA-3.Data are expressed as the mean ± SD (n=6).Compared with the NC group: ##P <0.01;compared with the MC group: *P <0.05 and **P <0.01.
3.8 Effect of REFB on mRNA expression of cytokines in small intestine
CD4+T cells consist of functionally diverse subsets,including Th1,Th2 and other T cell subsets.Th1 cells secrete IL-2,TNF-α and IFN-γ,which mainly mediate cellular immunity,and their transcription factor is T-bet.Th2 cells secrete IL-4,IL-6 and IL-10,which mainly participate in humoral immunity,and their transcription factor is GATA-3[31].The effect of REFB on the mRNA expression of cytokines (IL-4,IFN-γ,TNF-α,T-bet,and GATA3) were shown in Figs.6A-E.The mRNA expression levels of IL-4,IFN-γ,TNF-α,T-bet and GATA-3 in mouse intestine of the MC group (0.41 ± 0.05,0.49 ± 0.11,0.61 ± 0.14,0.43 ± 0.13 and 0.39 ± 0.11) were significantly lower than those in the NC group,indicating that Cy could cause intestinal injury.Compared with the MC group,the mRNA expression of IL-4,IFN-γ,TNF-α,T-bet and GATA-3 in all dose groups increased after gavage of REFB.The REFB-H group had the best effect,with 1.90,1.62,2.57,1.92 and 1.56 fold increase in IFN-γ,TNF-α,IL-4,T-bet and GATA-3,respectively,compared to the MC group.Therefore,REFB could have a positive effect on intestinal cytokine production,which in turn enhances intestinal immunity.
3.9 Effect of REFB on gut microbiota
Gut microbiota plays an important role in the functional maintenance of the immune system as a participant in immune metabolism and they are closely associated with intestinal mucosal immunity.Gut microbiota can stimulate the proliferation of intestinal epithelial cells to influence the development of the immune system,and can also affect the metabolic pathways,such as short-chain fatty acids and bile acids pathways to regulate immune function.Disturbances in the gut microbiota could cause autoimmune diseases[32].16S rDNA amplicon sequencing was used to evaluate the changes in the gut microbiota.Different alpha diversity indexes (shannon and observed otus indices) were used for analyzing the gut microbial diversity.As shown in Fig.7A,shannon index was higher in the REFB group than in the MC group,and the observed otus index was also higher in the REFB-M group than in the MC group.The results ofαdiversity indicated that gavage of REFB affected the microbial diversity of mice with intestinal injury.Theβdiversity was analyzed by PCA (Fig.7B).Gut microbiota of the MC group was significantly different from that of the NC group,and the structure of the gut microbiota was similar to the NC group after the administration of REFB.Among them,the REFB-M group were closer to the NC group,when compared to the other groups.The results suggested that REFB improved the changes in the gut microbiota destroyed by Cy,and positive regulated the gut microbiota structure of Cy-induced intestinal injury in mice.

Fig.7 Effect of REFB on the gut microbiota of immunosuppressed mice.(A) α-diversity index (Shannon and observed_otus) of gut microbiota.(B) PCA of gut microbiota.(C) Relative abundance of microbiota at the phylum level.(D) Relative abundance of microbiota at the genus level.(E) Relative abundance of Firmicutes.(F) Relative abundance of Bacteroidetes. (G) Relative abundance of Proteobacteria.(H) LDA score of groups with LDA >2.(I) Cladogram diagram of the LEfSe analysis.Data are expressed as the mean ± SD (n=8).Compared with the MC group: *P <0.05.

Fig.7 (Continued)
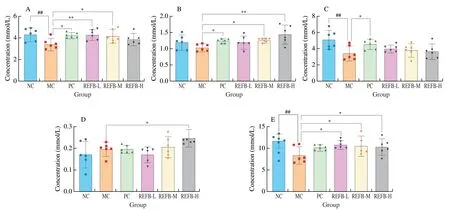
Fig.8 Effect of REFB on the SCFAs in the feces.(A) Acetic acid.(B) Propionic acid.(C) Butyric acid.(D) Valeric acid.(E) Total SCFAs.Data are expressed as the mean ± SD (n=6).Compared with the NC group: ##P <0.01;compared with the MC group: *P <0.05 and **P <0.01.
At the phylum level,Bacteroidetes has been reported to be the cornerstone of healthy intestinal homeostasis and could be involved in the host immune regulation by promoting the production of cytokines[33].The changes of the relative abundance at the phylum level for microbiota were shown in Fig.7C.The relative abundance of Firmicutes,Bacteroidetes and Proteobacteria in the NC group was 58.94%,37.77% and 1.91% respectively (Figs.7E-G),while in the MC group the relative abundance of Bacteroidetes and Proteobacteria was reduced to 32.10% and 1.31%,respectively,and the relative abundance of Firmicutes increased to 63.59%.After gavage of REFB,the relative abundance of Firmicutes (47.81%) (P<0.05) were reduced and the relative abundance of Bacteroidetes were increased(43.46%) (P<0.05),compared with the NC group.This finding was consistent with other previously reported roles of fermented juices in regulating immunosuppression[14].
In addition,analysis of microbial composition at the genus level showed thatLactobacillus,S24-7,Clostridiales,Rikenellaceae,PrevotellaandBacteroideswere the representative microbes found in the gut of the mice in the NC group (Fig.7D).Compared to the NC group,the MC group showed a significant change in the composition of the gut microbiota.The REFB treatment increased the relative abundance ofClostridiales,Lachnospiraceae andBacteroides.Studies have shown thatBacteroideswere capable of fermenting dietary fiber and produce short-chain fatty acids[34],increased abundance ofClostridialescould prevent pathogens colonization[35],Lachnospiraceae could induce the production and accumulation of Th17 cells and exert immunomodulatory effects[36],which is beneficial for body health.It is well known thatLactobacillushas a beneficially regulatory effect on the immune system of the intestine and the host.Interestingly this study found that Cy increased the abundance ofLactobacillus,while the REFB treatment decreased the relative abundance ofLactobacillus(P<0.05).The study by Mesa et al.similarly found that cyclophosphamide-induced immunosuppression increased the abundance of intestinal lactobacilli in chickens[37].The body’s defense mechanisms against diseases include innate immunity,mucosal barriers and intestinal colonization resistance.Intestinal colonization resistance refers to resistance to colonization by exogenous pathogens or inhibition of the overgrowth of resident bacteria normally present at low levels in the intestine[38].This inhibition was generally maintained by the beneficial effects of the major anaerobic bacteria in the gut,such asLactobacillus,When the intestinal environment changes,it will promote the growth of specific strains,thereby providing colonization resistance.Therefore,the increased abundance ofLactobacillusin the MC group found in this study suggests colonization resistance as a possible mechanism of REFB promoting health and counteracting the damage caused by Cy to the gut.
LEfSe was used to analyze the differential gut microbe among different groups (Figs.7H-I).Compared to the NC group,the differentials microb in the MC group mice were TM7,TM7_3,CW040,F16,Gammaproteobacteria,Enterobacteriales,Enterobacteriaceae,Bacilli,Lactobacillales and Lactobacillaceae(LDA >2,P<0.05).The key bacteria in REFB-M group were Porphyromonadaceae,Parabacteroides,Butyricmonas,RuminococcusandDesulfovibrio(LDA >2,P<0.05),and REFB-H enriched the abundances ofAlistipes,ParaprevotellaandArthromitus(LDA >2,P<0.05).
3.10 Effect of REFB on the levels of SCFAs in feces
SCFAs are the major final products of complex carbohydrates fermented by intestinal microbiota,they play an important role in the maintenance of epithelial barrier function and regulation of immune function in the body[39].As shown in Figs.8A-E,the concentration of total SCFAs in the feces of the MC group ((8.51 ± 1.73) mmol/L) was significantly lower than that of the NC group mice ((11.76 ± 1.37) mmol/L)(P<0.01).Meanwhile,the concentrations of fecal acetic acid and butyric acid were significantly lower in the MC group ((3.34 ± 0.48),(3.47 ± 0.81) mmol/L) than that in the NC group ((4.35 ± 0.54),(5.16 ± 1.03) mmol/L) (P<0.01).After administration of REFB,the concentrations of acetic acid,propionic acid and valeric acid in the feces were increased to various degrees in the REFB-L,REFB-M and REFB-H groups compared to the MC group.In addition,the total SCFAs levels of the REFB-L ((10.86 ± 0.84) mmol/L),REFB-M((10.65 ± 1.97) mmol/L) and REFB-H ((10.41 ± 1.67) mmol/L) groups were significantly higher than the MC group (P<0.05).Studies have shown that SCFAs could stimulate the proliferation of intestinal goblet cells and also directly or indirectly regulate the differentiation of T cells[40].Another study showed that the relative abundance of Bacteroidetes was positively correlated with the level of propionic acid[14].This was consistent with the results of SCFAs,goblet cells,T cell differentiation and gut microbial in this study.It was suggested that REFB could increase the level of SCFAs in the faces of mice with intestinal damage,which may be related to its regulation of intestinal gut microbiota.
4. Conclusions
In this study,oral administration of REFB was found to increase the immune organ index,promote the proliferation and differentiation of splenic lymphocytes,and exert immunomodulatory effects in the immunosuppressed mice.It also improved the length of villi and crypt depth,increased the number of goblet cells,improved the expression of tight junction protein,and repaired intestinal damage in mice.REFB administration increased the number of CD4+and CD8+cells in the intestine,up-regulated the expression of Th1 and Th2 cellrelated cytokines and transcription factors,and regulated intestinal immunity.In addition,REFB administration could modulate gut microbiota and increase fecal SCFAs levels.This study could provide useful information for the effects of fermented foods on the immune response and gut health.
Declaration of competing interest
Shaoping Nie and Mingyong Xie are the editorial board members forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgments
The financial supports from the Key Program of the National Natural Science Foundation of China (32130082),Jiangxi High Level Talent Cultivation Project (20204BCJ24006),Project of State Key Laboratory of Food Science and Technology (SKLF-ZZA-201911),and Central Government Guide Local Special Fund Project for Scientific and Technological Development of Jiangxi Province(20212ZDD02008) were gratefully acknowledged.
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing

