D-Psicose intake exacerbates dextran sulfate sodium-induced colitis in mice through alteration in the gut microbiota and dysfunction of mucosal barrier
Xuejio Zhng,Ang Li,Yunyifei Wng,Jin Wng,Bowei Zhng,Yn Zhng,Jingmin Liu,Shuo Wng,*
a Tianjin Key Laboratory of Food Science and Health,School of Medicine,Nankai University,Tianjin 300072,China
b College of Food Science and Technology,Hebei Agricultural University,Baoding 071001,China
Keywords:D-Psicose Colitis Gut microbiota Short chain fatty acids Mucin 2
ABSTRACT D-Psicose,as a low-calorie rare sugar,has attracted a lot of attention in recent years for alternating to sucrose.The anti-obesity effect of D-psicose has been extensively confirmed in previous studies,however,the impact of D-psicose on colitis remains vague.Here,we firstly evaluated the effect of the D-psicose prophylactic intervention on dextran sulfate sodium-induced colitis in C57BL/6 mice.The pathological symptoms,inflammatory cytokines levels,gut microbiota composition,short chain fatty acids (SCFAs) production and colonic barrier integrity were comprehensively evaluated.The results confirmed that D-psicose intervention aggravated colitis,characterized by the exacerbation of colon shortening,increase of colonic inflammatory infiltration,and marked exaltation of disease activity indices and IL-6,IL-1β and TNF-α levels.Further,the dysfunction of gut microbiota was identified in the psicose group.The abundance of pro-inflammatory bacteria Lachnospiraceae_NK4A136_group was significantly up-regulated while the abundance of probiotics Akkermansia and Lactobacillus were significantly down-regulated in the psicose group compared to the model group.Moreover,the production of SCFAs was suppressed in the psicose group,accompanied by a decrease in the level of mucin 2 (Muc-2).Collectively,the underlying mechanism of the exacerbation of colitis by D-psicose intervention might be attributed to microbiota dysfunction accompanied by the reduction of SCFAs,which leads to the damage of the mucosal barrier and the intensification of inflammatory invasion.
1. Introduction
D-Psicose (also namedD-allulose),referred as a special carbohydrate,is a rare sugar that exists in nature[1].SinceD-psicose is a C-3 epimer ofD-fructose,that a great deal of research has been devoted to producingD-psicose fromD-fructose through chemical,enzymatic and genetically engineered methods[2,3].At present,D-psicose has been licensed in several countries and widely used in the food industry[4].With the sweetness nearly 70% of sucrose,D-psicose only have 0.4 kcal/g in calories[5].It can be used as an ideal sugar substitute in candies or beverages.Researches have shown that approximately 70% ofD-psicose will be absorbed by the small intestine and then excreted in the urine,and the remaining 30% will reach the colon[6].
Over the past decades,the physiological function ofD-psicose has been studied.Many randomized controlled and acute exposure trials have been conducted,indicating thatD-psicose intake can alleviate obesity,prevent type 2 diabetes,and accelerate lipid consumption[7-9].The composition of gut microbiota would be profoundly altered underD-psicose intervention for 16 weeks in C57BL/6J mice[10].There are also concerns about the safety ofD-psicose consumption,becauseD-psicose can promote growth or infectivity of certain pathogenic bacteria[11].Consequently,the association betweenD-psicose and gut microbiota remains ambiguous.The influence ofD-psicose on diseases closely related to gut microbiota,such as inflammatory bowel disease (IBD),urgently need to be investigated.
IBD is a non-communicable digestive tract disease with global consequence.IBD as we know it is usually composed of Crohn’s disease and ulcerative colitis[12].Although the etiology of IBD is still unknown,increasing studies have confirmed that there is a close relationship between diet and IBD[13-15].The association between dietary ingredient and the prevalence of IBD has been repeatedly emphasized[16].The gut microbiota and its metabolites play a key role in the prevention and treatment of IBD.IBD patients tend to have many consistent changes in the gut microbiota including an increase abundance in Firmicutes,a change in the ratio of Firmicutesto Bacteroidetes,and a decrease in short chain fatty acids (SCFAs)[17,18].
As a new substitute for sucrose,more physiological functions ofD-psicose should be explored and confirmed.Furthermore,considering the possible future application ofD-psicose in food,and the close association between diet and colitis,studies have been conducted to explore the interactive effect ofD-psicose on the gut microbiota and colitis.We thus evaluated the effect of 21 days intervention ofD-psicose in drinking on dextran sulfate sodium(DSS)-induced colitis in mice.The pathological condition of colitis and the level of inflammation were detected.At the same time,gut microbiota composition,SCFAs and the intestinal mucosal barrier were determined.To our knowledge,D-psicose worsened colitis,exacerbated colon inflammation.Mechanistically,D-psicose altered the composition of gut microbiota,reduced the production of SCFAs,leading to the disruption of the colonic mucosal barrier.Overall,the present research firstly identified the effect ofD-psicose administration in DSS-induced colitis,and provided new perspectives and evidence for evaluating the microbiological safety ofD-psicose and its safe use.
2. Material and methods
2.1 Material and reagent
D-Psicose (Cas# 551-68-6,molercular weight: 180.16 Da,purity ≥ 98%) was purchased from Beijing Chemsynlab Co.,Ltd.(Beijing,China).DSS (Cat# 0216011090,molercular weight:36 000−50 000 Da) was obtained from MP Biomedicals Co.,Ltd.(Santa Ana,USA).Antibodies used for immunofluorescence were purchased from Abcam company (Cambridge,UK).Anti-mucin 2(Muc-2) antibody (Cat# ab272692,dilution ratio: 1:500),anti-zonula occludens 1 (ZO-1) antibody (Cat# ab216880,dilution ratio: 1:100),anti-occludin antibody (Cat# ab216327,dilution ratio: 1:100).TRIzolTMreagent was purchased from Thermo Fisher Scientific(Invitrogen,Cat# 15596018).
2.2 Animals and experimental procedure
Thirty males C57BL/6 mice were obtained from the Vital River Laboratory Animal Technology Co.,Ltd.(Beijing,China).6–8 weeks old mice with similar body weight were randomly assigned to 3 groups (control group,model group and psicose group).After a 7-day acclimation period,three groups of animals were housed under standard specific pathogen-free conditions following the protocols in the Nankai animal resources center (SYXK-2019-0001).All animals had free access to water and food,mice in the control group and model group were fed with purified water as drinking,while the psicose group were given water contained 0.75 mg/mLD-psicose.
After 21 days of intervention with different types of drinking water,the animals of model group and psicose group were undergoing a 7-day continuous challenge of 2.5% DSS to induce colitis.During induction of the colitis model,body weight,rectal bleeding and stool status were detected and recorded daily for assessment of disease activity index (DAI) as previously described (Table S1)[19].The final macroscopic score for each animal is the average of these three separate scores.At the end of the trial,animals were euthanized to obtain colonic tissue for subsequent physiological and pathological index determinations.The details of the experimental procedure can be seen in Fig.1A.
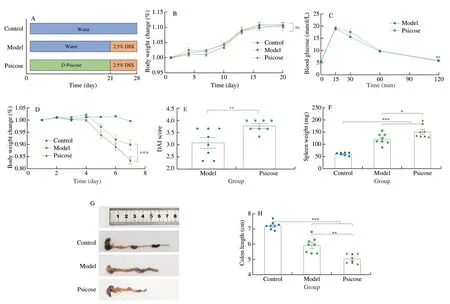
Fig.1 D-Psicose intervention exacerbated DSS-induced colitis in C57BL/6 mice.(A) Schematic diagram of animal experiment.(B) Body weight change throughout the 21-day intervention.(C) OGTT result;(D) Body weight change during 2.5% DSS treatment;(E) DAI score;(F) Spleen weight;(G) Colon length photographs;(H) Colon length result.Data expressed as the mean ± SEM (n =8).The ns means no significance,* P <0.05,** P <0.01,*** P <0.001 significantly different between groups.
2.3 Oral glucose tolerance test (OGTT)
Each mouse was gavaged with 2 g/kg glucose at 0 min,and then venous blood was obtained by tail clipping.The OGTT curve was obtained by measuring blood glucose at the 0,15,30,60,and 120 min respectively.
2.4 Histopathology
Histopathological samples were the distal colon sections of mice cleaned with PBS.Samples were fixed in 10% formalin,sectioned at 5 μm,and paraffin-embedded for hematoxylin and eosin (H&E)staining.Histopathological results were obtained by light microscopy.
2.5 Real-time quantitative polymerase chain reaction (RT-qPCR)
Colon total RNA was extracted by TRIzol method following the manufacturer’s instructions.The cDNA template was obtained by LunaScriptTMRT SuperMix Kit (Cat# E3010L,New England BioLabs).The ChanQ Universal SYBR qPCR Master Mix(Cat# Q71102,Vazyme) was subsequently used for qPCR in accordance with the manufacturer’s instruction.The expression of colonic inflammatory cytokines and genes related to the mucosal barrier was determined by the 2−ΔΔCtmethod.β-Actinwas used as the internal reference gene.The specific oligo nucleotide primers sequences are shown in Table S2.
2.6 Enzyme linked immunosorbent assay (ELISA) for inflammatory cytokines
The inflammatory cytokines levels of interleukin (IL)-6,tumor necrosis factor (TNF)-α and IL-1β in serum were detected by ELISA kits (Biocalvin,#CK-E20012M,#CK-E20220M,#CK-E20533M)according to the manufacturer’s instructions.
2.7 Gut microbiota analysis
Colon contents were used to extract total DNA by the Power Soil DNA Isolation Kit,followed by bacterial 16S rRNA amplification of the V3–V4 fragment by primers 338F(5’-A C T C C T A C G G G A G G C A G C A-3’) a n d 8 0 6 R(5’-GGACTACHVGGGTWTCTAAT-3’).Then the Illumina Hiseq 2500 system (PE250) was used for high quality sequencing.The acquisition and analysis of data was performed with the analysis platform of BMKCloud (http://www.biocloud.net/).
2.8 SCFAs production analysis
The fecal samples were vacuum freeze-dried for 24 h and weighed.Then the fecal samples were homogenized and crushed,and centrifuged at 13 000 r/min for 10 min to obtain the supernatant.Subsequent extraction was carried out by 10% sulfuric acid and diethyl ether.Finally,after centrifugation,the samples were filtered through a 0.22 μm organic filter,and the content of SCFAs was determined by gas chromatography Agilent 7890A with the DB-FFAP capillary column.
2.9 Immunofluorescence
Paraffin sections of colon tissue were used for immunofluorescence staining experiments.Samples were incubated with primary antibodies overnight at 4 °C after samples were blocked with 5% BSA for 30 min.The subsequent secondary antibody incubation conditions were 30 min at 37 °C.Anti-fluorescence decay sealant containing 4’,6-diamidino-2-phenylindole (DPAI) was used for nuclear staining.
2.10 Statistical analysis
Results in this study were performed and visualized by GraphPad Prism 8.Datas were expressed as means ± standard errors of the means (SEM).Significances of different groups were conducted by one-way analysis of variance (ANOVA) or two-way ANOVA followed by Tukey’s post hoc test.Pvalues less than 0.05 (P<0.05)was considered statically significant.
3. Results
3.1 D-Psicose intervention exacerbated DSS-induced colitis in C57BL/6 mice
A 28-day animal experiment was carried out according to the procedure shown in Fig.1A.The amount of water consumption was measured (approximately 4–7 mL/day per mice) duringD-psicose intervention and 2.5% DSS treatment,and no significant difference between the different groups was observed.During theD-psicose intervention,the body weight of the mice was measured every 3 days.The results showed thatD-psicose did not alter the weight gain of the mice with no significant difference in data between the three groups(Fig.1B).Previous research suggested thatD-psicose might affect oral glucose tolerance in mice,the OGTT was thus performed on 21thday.The results showed that theD-psicose intervention did not alter the glucose tolerance of the mice (Fig.1C).Subsequently,mice were treated with 2.5% DSS for 7 days and body weights were recorded daily.The results presented in Fig.1D showed that consumption ofD-psicose aggravated the weight loss in mice compared with the model group.The result of DAI score was displayed in Fig.1E.The DAI score in the psicose group was significantly higher compared to the model group (P<0.01).In addition,similar results were found in the spleen weight of mice,the spleen weight of the psicose group was significantly higher than that of the model group (P<0.05) (Fig.1F).Colon length in mice was measured and the results demonstrated thatD-psicose administration significantly accelerated colon shortening compared to the model group (P<0.01) (Fig.1G–H).Taken together,the results indicated thatD-psicose intervention aggravated the symptoms of DSS-induced colitis in C57BL/6 mice.
3.2 D-Psicose intake promoted colonic inflammation and increased levels of pro-inflammatory cytokines
H&E staining of the colon was performed to exhibit the histopathology of the mouse colon.As shown in Fig.2A,after 2.5%DSS treatment in the model group and psicose group,the colonic epithelial layer of mice was destroyed,and there was more serious crypt disappearance and extensive inflammatory cell infiltration in the colonic epithelium and lamina propria compared with the control group.Notably,higher levels of inflammatory infiltration of the mucosal was observed in psicose group than in model group.Inflammatory cytokine levels played a critical role in the occurrence and progression of IBD.The expression levels of pro-inflammatory cytokinesIL-6,IL-1βandTNF-αin colon tissue were determined by RT-qPCR (Fig.2B–D).When compared to the model group,the psicose group showed a statistically significant higher expression level in all these pro-inflammatory cytokines (P<0.01,P<0.001).To further explore the effect ofD-psicose intake on the systemic inflammatory of mice,the level of pro-inflammatory cytokines in serum was detected by ELISA.As showed in Figs.2E–G,the levels of IL-6,IL-1β and TNF-α in the model group andD-psicose group were significantly higher than that in the control group due to the treatment of 2.5% DSS (P<0.001).Consistent with the gene expression,D-psicose intervention promoted the expression of pro-inflammatory factors in serum compared with the model group.In summary,D-psicose intake promoted colonic inflammation in DSS-induced colitis.

Fig.2 D-Psicose intervention promoted colonic inflammation.(A) Representative images of H&E;The scale bar of each row is 200,100 and 50 μm,respectively.(B–D) Relative expression of IL-6, IL-1β and TNF-α mRNA in colon.(E–G) The protein level of IL-6,IL-1β and TNF-α in serum.Data expressed as the mean ± SEM (n =8).* P <0.05,** P <0.01,*** P <0.001 significantly different between groups.
3.3 D-Psicose altered gut microbiota composition
To further determine the effect ofD-psicose intervention on the gut microbiota,microbiological analysis was performed.α-Diversity of the microbiota characterized by Chao1,ACE,and Simpson index was determined (Figs.3A–C).The results showed that consumption ofD-psicose reduced the Chao1 and ACE indices compared to the model group,indicating lower levels of species richness in psicose group.Moreover,Simpson index represented the microbial homogeneity was elevated in the psicose group,with no significant difference compared to the model group (P=0.1) (Fig.3C).The β-diversity of gut microbiota was illustrated by principal coordinate analysis (PCoA)and Binary-Jaccard distance-based similarity analysis (ANOSIM)between operational taxonomic units (OTUs) (Fig.3D).The results indicated that the three groups were clustered independently,proving that the administration of 2.5% DSS andD-psicose resulted in a significant separation of the gut microbiota.The relative abundance of the top 10 microorganisms was further analyzed at the phylum level(Fig.3E).Firmicutes and Bacteroidetes are the dominant flora in these three groups.Firmicutes accounted for 45.6% and 54.2% of the model and psicose groups,respectively (Fig.3F).Bacteroides accounted for 26.4% and 29.1% (Fig.3G).Firmicutes/Bacteroidetes ratio was significantly higher in psicose group than that in model group(P<0.01) (Fig.3H).In summary,D-psicose treatment significantly altered the composition of the gut microbiota.

Fig.3 Effects of D-psicose intervention on gut microbiota diversity and species abundance at the phylum level.(A) Chao 1 index;(B) ACE index;(C) Simpson index;(D) PCoA analysis;(E) Relative abundance of gut microbiota at phylum level;(F) Relative abundance of Firmicutes;(G) Relative abundance of Bacteroidetes;(H) Firmicutes/Bacteroidetes ratio.Data expressed as the mean ± SEM (n =8).* P <0.05,** P <0.01,*** P <0.001 significantly different between groups.
3.4 D-Psicose induced gut microbiota dysfunction and reduced SCFAs production
To gain a clearer comprehensive understanding of the microbial profile,further analysis of the gut microbiota at the genus level was performed (Fig.4).Histograms of the top 20 genera in these three group were plotted to observe species distribution at the genus level (Fig.4A).uncultured bacterium_f_Muribaculaceae was the most dominant communities in the control group,followed byAkkermansia.The abundance of uncultured bacterium_f_Lachnospiraceae was highest in the psicose group.In the model group,the abundance ofBacteroideswas most prominent,except for the higher abundance of uncultured bacterium_f_Lachnospiraceae.Subsequently,8 genera with significant differences were screened out (Fig.4B).The abundances of uncultured bacterium_f_Muribaculaceae,Ruminococcaceae_UCG-014and Lachnospiraceae_NK4A136_group were significantly increased owing to theD-psicose intervention,relative to the model group.Meanwhile,the abundance ofAkkermansia,LactobacillusandEscherichia-Shigelladecreased in the psicose group compared to the Model group with significant differences in data (P<0.05,P<0.01).To further identify characteristic representative species of each group,line discriminant analysis effect size (LEfSe) was performed at LDA equal to 4.0 (Fig.4C).Consistent with the above results,the aggregation ofAkkermansiaandLactobacillusin the model group was confirmed.However,Lachnospiraceae_NK4A136_group,Ruminococcaceae_UCG-014 andRuminiclostridium_9were representative species of the psicose group.TheAkkermansiaandLactobacillushave been reported to be closely related to the production of SCFAs[20].And previous study has shown that high sugar diet could increase the abundance of Lachnospiraceae_NK4A136_group,thereby reducing the production of SCFAs and exacerbating colitis in mice[21].Therefore,the levels of SCFAs in the colonic contents were then measured (Figs.5A–D).The lowered content of acetate,propionate,butyrate and total SCFAs was found in psicose group compared with model group.Notably,there were significant differences in the reduction of propionate and butyrate levels (P<0.05),which are closely associated to mucosal barrier function and inflammation levels.
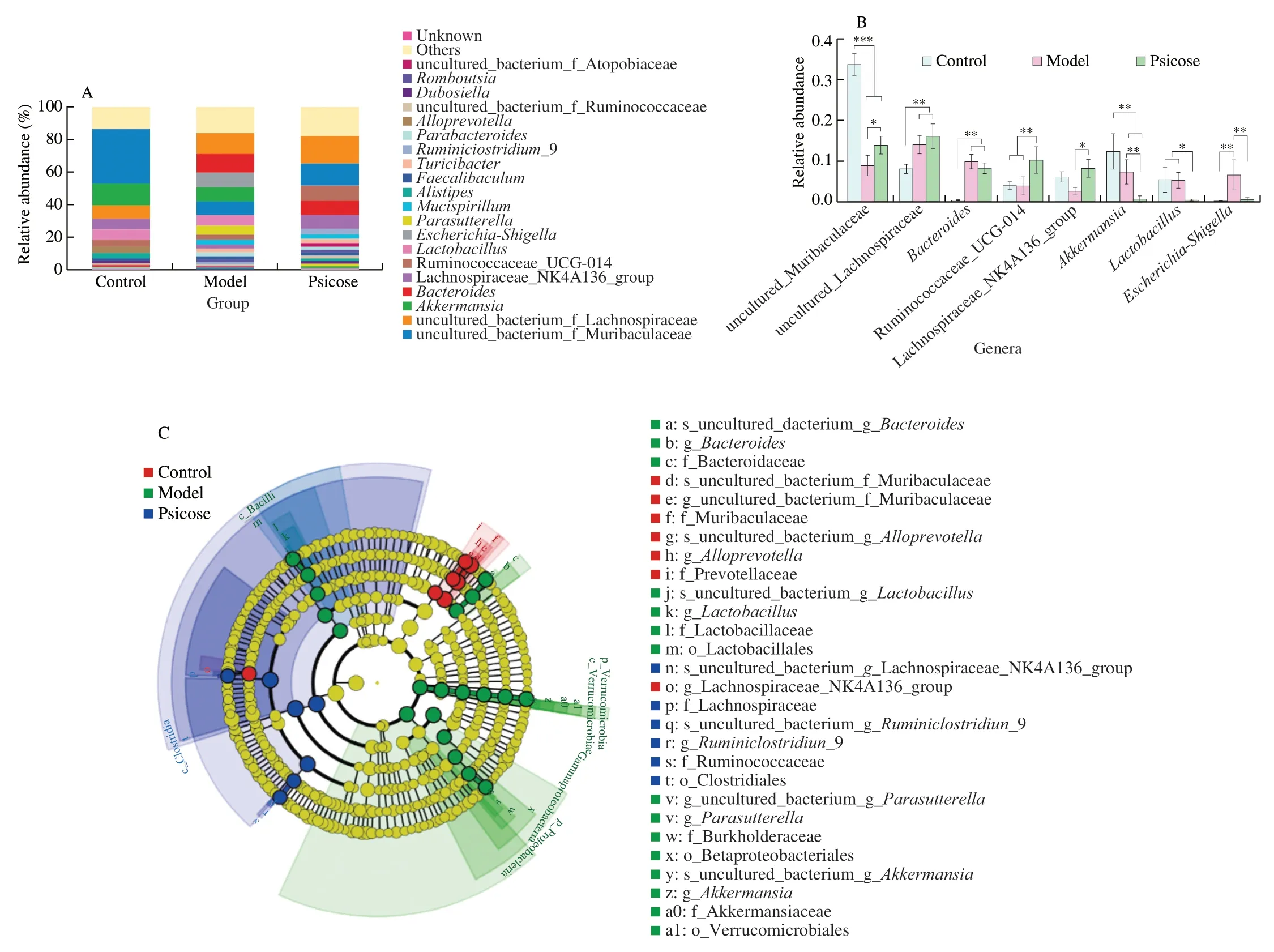
Fig.4 Effects of D-psicose intervention on gut microbiota composition at the genus level.(A) Relative abundance of gut microbiota at genus level;(B) Genera with significant differences;(C) LEfSe analysis.Data expressed as the mean ± SEM (n =8).* P <0.05,** P <0.01,*** P <0.001 significantly different between groups.
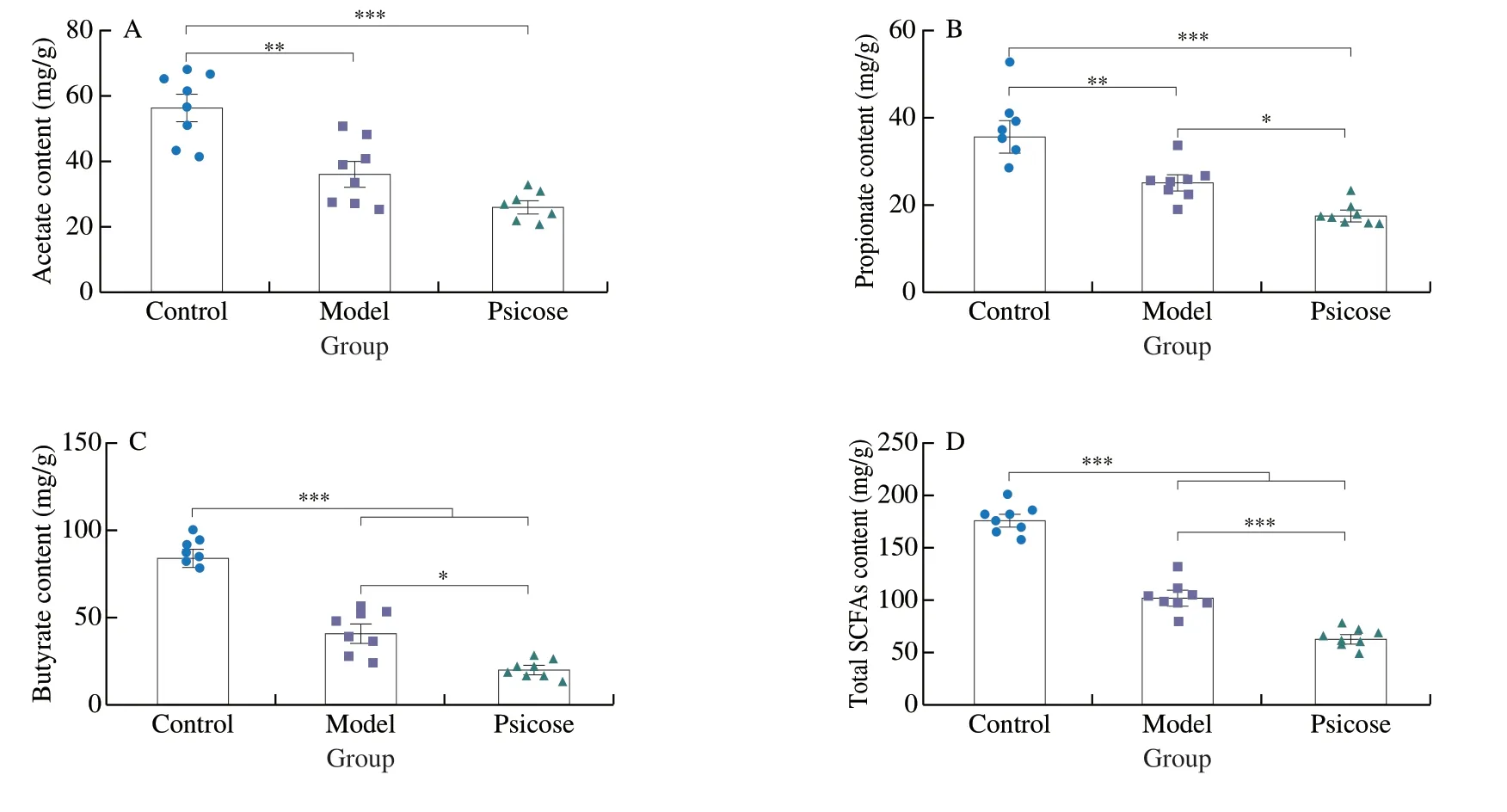
Fig.5 D-Psicose induced gut microbiota dysfunction and reduced short-chain fatty acid production.(A–D) The content of acetate,propionate,butyrate and total SCFAs.Data expressed as the mean ± SEM (n =8).* P <0.05,** P <0.01,*** P <0.001 significantly different between groups.
3.5 D-Psicose impaired the mucosal barrier
Colonic barrier dysfunction is a common symptom in IBD patients.To further explore the effect ofD-psicose consumption on barrier function,the relative gene expression levels of tight junctions and mucins in the colon were determined.Compared with the model group,the expression levels ofZO-1andOccludinwere significantly increased in psicose group (P<0.05,P<0.01) (Fig.6A–B).The expression level ofClaudin-1was decreased in psicose group but not statistically significant compared to model group (Fig.6C).In addition,the expression level ofE-cadherinwas not found to vary significantly among the three groups (Fig.6D).Most notably,Muc-2,a major member of the mucosal barrier,was significantly down-regulated in psicose group (Fig.6E).The protein expression of Muc-2,ZO-1 and Occludin in colon tissue was determined by immunofluorescence method to further evaluate the colonic barrier function more intuitively (Fig.6F).Similar to the results of H&E,the intake ofD-psicose seemed to maintain the expression of ZO-1 and Occludin,exerting a protective effect on the tight junctions of the colon.In agreement with the gene expression level,less fluorescence was detected in the expression of Muc-2 in psicose group compared to Model group.In conclusion,althoughD-psicose maintained tight junctions in the colon,it significantly impaired the mucosal barrier in DSS-induced colitis in mice.
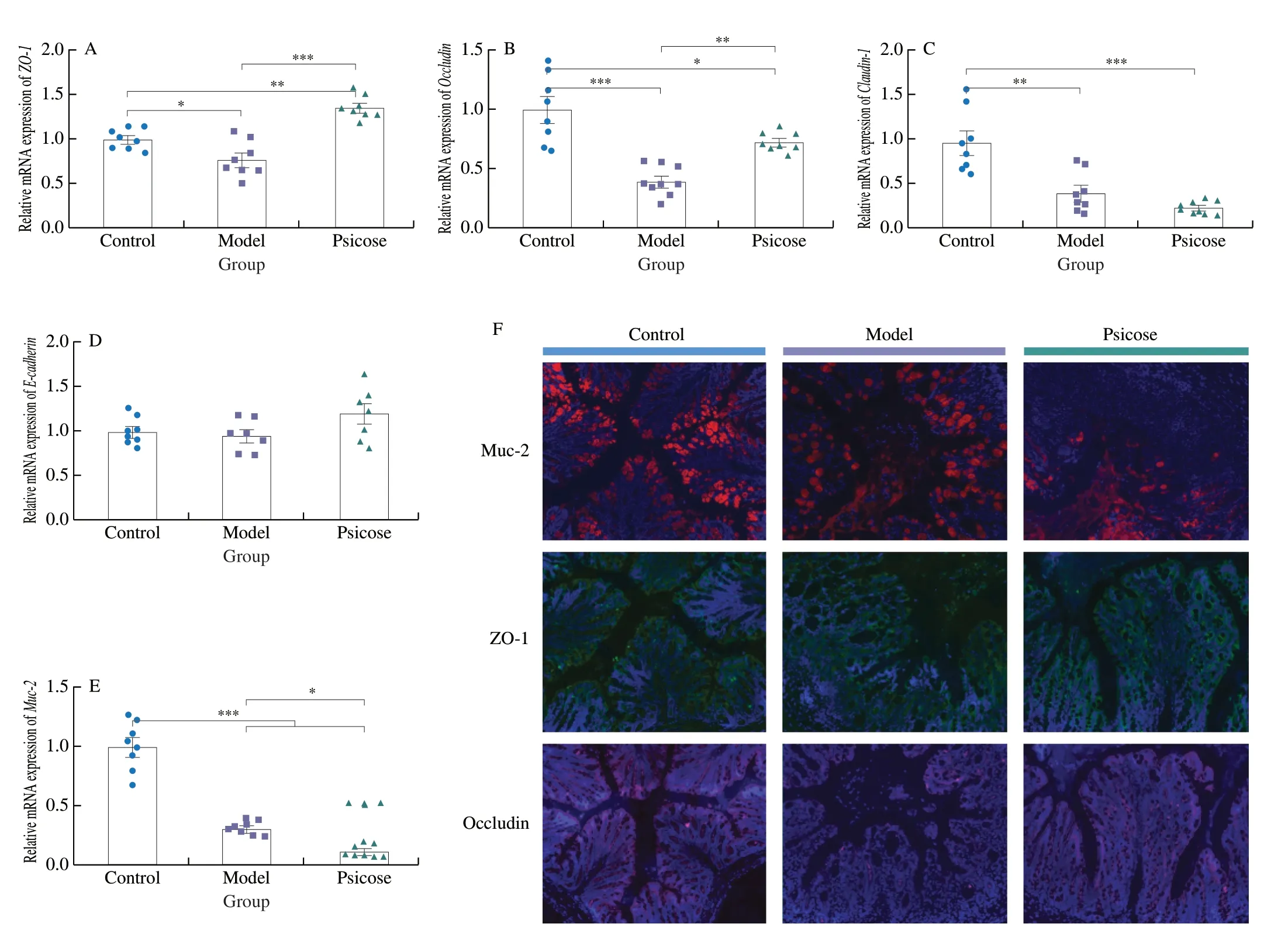
Fig.6 D-Psicose impairs the mucosal barrier.(A–E) Relative mRNA expression of ZO-1,Occludin,Claudin-1, E-cadherin and Muc-2.(F) Representative images of colonic barrier immunofluorescence (scale bar is 50 μm).Data expressed as the mean ± SEM (n =8).* P <0.05,** P <0.01,*** P <0.001 significantly different between groups.
3.6 Spearman correlation analysis
To identify the specific bacterial involving in the psicose-mediated deterioration on DSS-induced colitis,the Spearman correlation analysis was carried out (Fig.7).Nine indicators closely related to colitis,including inflammatory cytokines,SCFAs and colonic barrier,were screened for correlation between the relative abundance of gut microbiota (the top 20).Lachnospiraceae_NK4A136_group,uncultured bacterium_f_Lachnospiraceae,Ruminiclostridium_9 and uncultured bacterium_f_Ruminococcaceae,which were mainly enriched in the psicose group,showed a negative correlation with SCFAs as well as the level of Muc-2 and a positive correlation with inflammatory cytokines.Furthermore,AkkermansiaandLactobacillus,with low abundance in psicose group,showed completely opposite trends with a positive correlation with Muc-2 and SCFAs and a significant negative correlation with inflammatory cytokines.Moreover,Romboutsiawas significantly positively correlated with acetate.Meanwhile,the Rikenellaceae_RC9_gut_group and Muc-2 was positively correlated.Taken together,the above results implied that the high abundance of Lachnospiraceae_NK4A136_group and the low abundance ofAkkermansiaandLactobacillusmight play a critical role in psicose-mediated colitis exacerbation.Overall,these results demonstrated thatD-psicose intake could alter the microbiome composition,leading to a dysfunctional microbial profile,which in turn acted on SCFAs and the mucosal barrier.Of particular note,the high abundances of Lachnospiraceae_NK4A136_group coupled with low abundances ofAkkermansiaandLactobacillusmediated byD-psicose administration contributed to the exacerbation of colitis.

Fig.7 Spearman correlation analysis between representative gut microbiota and colitis-related parameters.The correlation is at the level of * P <0.05,**P <0.01,*** P <0.001.
4. Discussion
D-Psicose,a newly applied sweetener,has been defined by multiple previous studies as an ingredient with beneficial physiological effects on obesity and type 2 diabetes[22,23].However,some clinical cohort studies have confirmed that theD-psicose intake may cause some gastrointestinal disorders[24].Up until now,the mechanism by whichD-psicose caused gastrointestinal symptoms has yet been elucidated.Considering the possible wider application ofD-psicose in the future,its physiological characteristics should be rigorously further defined,whether for healthy individuals or patients.
Given this,we hypothesized thatD-psicose might play a role in colitis,and then an intervention trial in mice with DSS-induced colitis was conducted to investigate the influence ofD-psicose intake.We defined for the first time the effects ofD-psicose consumption on the inflammation level,colonic barrier function,gut microbiota composition and SCFAs production in mice.The results of present study demonstrated that the intervention ofD-psicose aggravated colitis.Mechanistically,D-psicose promoted the expression of inflammatory cytokines like IL-6,IL-1β and TNF-α,caused intestinal flora dysfunction,reduced the production of SCFAs and decreased the expression of Muc-2 resulting in mucosal barrier impaired.
Previous studies have demonstrated that elevated levels of inflammatory cytokines are the dominant characters in colitis[25-27].The increase of IL-6 also implied the over-activation of the cascade signaling pathway NF-κB,which played a critical role in the development of IBD[28].In this study,pro-inflammatory factors such as IL-6,IL-1β and TNF-α were detected after theD-psicose intervention.Inflammatory factor levels were almost 2-fold in the psicose group compared to the model group.Consistent with these findings,greater weight loss,colon shortening,and higher DAI score were observed in the psicose group.Meanwhile,H&E staining also exhibited more inflammatory infiltration afterD-psicose administration.Taking these results together,D-psicose intake exacerbated inflammation in DSSinduced colitis.
Although the etiology of IBD remains unclear,multiple lines of evidence have indicated that the disease was triggered by a concomitant disturbance in gut microbiota homeostasis[29,30].The composition of intestinal microbiota has been considered to be a key determinant of host susceptibility to IBD[31-33].Gut microbiota composition has been reported to be modulated afterD-psicose intervention in previous studies[34].However,studies on comprehensive analysis of the association betweenD-psicose and gut microbiota are still limited.To further define alterations in gut microbiome,16S rRNA high-throughput sequencing was performed in this study.As presented in Fig.3D,significant segregation of gut microbiota composition was found among the three groups.Consistent with previous results,the increased ratio of Firmicutes to Bacteroides has been found after 2.5% DSS treatment in both model and psicose groups[35].Of particular note was that Ruminococcaceae_UCG-014and Lachnospiraceae_NK4A136_group abundance significantly increased in the psicose group compared to the model group.Earlier findings have confirmed that enrichment of the Lachnospiraceae_NK4A136_groupinvolving in the promotion of inflammation,was found in rodent models of obesity and colitis[36,37].Coincidentally,Ruminococcaceae_UCG-014 was confirmed to be positively correlated with the inflammatory factor IL-1β[38].In the correlation analysis,Lachnospiraceae_NK4A136_group and Ruminococcaceae_UCG-014 respectively showed a significant positive correlation with inflammatory factors like IL-6,IL-1β and TNF-α.These results generally indicated that Lachnospiraceae_NK4A136_group and Ruminococcaceae_UCG-014played a key role in promoting inflammation in the psicose group.
Substantial causal evidence from both animal models and human validation experiments have indicated that the absence or decreased abundance of commensal bacteriumAkkermansiawas associated with a variety of diseases[39].Akkermansiahas been proved to repair the colonic mucosal barrier and suppressed inflammation[40].It has been commonly accepted that the mucus barrier is key to defense pathogens and inflammation for the colonic lamina propria[41].Furthermore,as a SCFAs-producing commensal bacteria,Akkermansiacould generate SCFAs by directly metabolizing carbohydrates.The production of SCFAs could effectively stimulate mucin expression,and thus maintained colon health and prevented the onset of inflammation[42].Another probiotic bacterium involved here,Lactobacillus,has been applied in several researches to prevent obesity due to effective prevention on inflammation and maintenance of the intestinal homeostasis[43].
Since our findings indicated that the abundance of theAkkermansiaandLactobacillussignificantly decreased in the psicose group,we speculated that corresponding changes should be reflected in the content of SCFAs.Sure enough,a significant decrease in the content of SCFAs was observed in the psicose group,especially the propionate and butyrate,which were strongly responsible for Muc-2 secretion.Consistent with previous findings[44,45],AkkermansiaandLactobacilluswere positively associated with the expression of Muc-2 coupled with the levels of propionate and butyrate,and significantly negatively associated with inflammatory cytokines.
Previous studies have shown that the intake ofD-psicose can significantly increase the expression level of genes related to tight junction function in the small intestine[46].Consistent with this result,in our experiment,the expression levels of Occludin and ZO-1 also increased afterD-psicose intervention compared with the model group.However,the damage of mucosal barrier would directly aggravate inflammatory infiltration and microbial dysfunction.The colonic mucosal barrier is a dynamic chemical complex barrier mainly composed of secreted Muc-2,which is secreted by goblet cells in response to the stimulation of SCFAs[47].Conversely,Muc-2 as a nutrient substrate for commensal bacteria,could be re-metabolized into SCFAs,which can further maintain the homeostasis of the mucosal barrier in this dynamic circulatory system.Considering previous studies on Muc-2–/–mice,it is reasonable to conclude that loss of the mucus layer may increase the susceptibility and accelerate the development of colitis[48].Consistent results shown in our study suggested the expression of Muc-2 was significantly reduced at both mRNA and protein levels in the psicose group compared with the model group.In conclusion,a correlation between gut microbiota dysfunction and disruption of the mucosal barrier in aggravated colitis followingD-psicose ingestion has been identified firstly in this work.
5. Conclusions
In conclusion,the intervention ofD-psicose disturbed the homeostasis of the gut microbiota,manifested as a decrease in the abundance of SCFAs-producing bacteria and an increase in the abundance of pro-inflammatory bacteria,resulting in damage to the colonic mucosal barrier and aggravation of colitis.Understanding the complex interactive effects ofD-psicose consumption and gut microbes on gastrointestinal diseases may provide compelling insights intoD-psicose’s physiological function and safe application.The present work emphasized that despite the beneficial effects ofD-psicose on obesity showed in previous studies,necessary biosafety assessments,such as the microbiome,should be taken into account when the scope of its application changed.
Conflicts of interest
Shuo Wang is an editorial board member forFood Science and Human Wellnessand was not involved in the editorial review or the decision to publish this article.All authors declare that there are no competing interests.
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No.32030083).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250046.
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing

