Apigenin ameliorates imiquimod-induced psoriasis in C57BL/6J mice by inactivating STAT3 and NF-κB
Xinshe Meng,Shihong Zheng,Zequn Yin,Xuerui Wng,Digng Yng,Tingfeng Zou,Huxin Li,Yunli Chen,Chenzhong Lio,*,Zhouling Xie,Xiodong Fn,Jihong Hn,c,Yjun Dun,*,Xioxio Yng,*
a Key Laboratory of Metabolism and Regulation for Major Diseases of Anhui Higher Education Institutes,College of Food and Biological Engineering,Hefei University of Technology,Hefei 230601,China
b Tianjin Key Laboratory of human Development and Reproductive Regulation,Department of General Gynecology,Tianjin Central Hospital of Gynecology and Obstetrics,Tianjin 300100,China
c Key Laboratory of Medicinal Chemical Biology,Key Laboratory of Bioactive Materials of Ministry of Education,College of Life Sciences,Nankai University,Tianjin 300071,China
Keywords:Psoriasis Apigenin Imiquimod Inflammation Signal transducer activator of transcription 3(STAT3)Nuclear factor-κB (NF-κB)
ABSTRACT Psoriasis is a chronic autoimmune disease featured by patches on the skin.It is caused by malfunction of immune cells and keratinocytes with inflammation as one of its key features.Apigenin (API) is a natural flavonoid with anti-inflammatory and immunoregulatory properties.Therefore,we speculated that API can ameliorate psoriasis,and determined its effect on the development of psoriasis by using imiquimod(IMQ)-induced psoriasis mouse model. Our results showed that API attenuated IMQ-induced phenotypic changes,such as erythema,scaling and epidermal thickening,and improved splenic hyperplasia.Abnormal differentiation of immune cells was restored in API-treated mice.Mechanistically,w e revealed that API is a key regulator of signal transducer activator of transcription 3 (STAT3).API regulated immune responses by reducing interleukin-23 (IL-23)/STAT3/IL-17A axis.Moreover,it suppressed IMQ-caused cell hyperproliferation by inactivating STAT3 through regulation of extracellular signal-regulated kinase 1/2 and nuclear factor-κB (NF-κB) pathway.Furthermore,API reduced expression of inflammatory cytokines through inactivation of NF-κB.Taken together,our study demonstrates that API can ameliorate psoriasis and may be considered as a strategy for psoriasis treatment.
1. Introduction
Psoriasis is a chronic autoimmune-mediated inflammatory skin disease that affects approximately 1%−4% of the global population[1].It is characterized by excessive proliferation and abnormal differentiation of keratinocytes,resulting in thickening of the stratum corneum and vascularization of the skin[2].The skin eventually forms white scales and/or red patches,which seriously impacts physical and mental health,as well as life quality of the patients[2,3].
The exact pathogenesis of psoriasis is complicated and has not been fully understood.Current researches suggest that the most important pathogenesis of psoriasis is the interaction of immune cells and keratinocytes through various cytokines[4].It is mainly manifested by abnormal immune activation,a sustained inflammatory response and excessive cell proliferation.During the development of psoriasis,abnormally activated dendritic c ells (DCs) secrete a large number of cytokines,such as interleukin-23 (IL-23),IL-6,IL-1β and tumor necrosis factor-α (TNF-α)[5].These cytokines promote polarization of CD4+T cells into T helper 1 (Th1) and Th17 cells.The activated Th1 cells excessively secrete interferon-γ (IFN-γ) and TNF-α,further leading to immune damage[6].Th1-type cytokines levels in skin lesions of psoriasis patients are positively correlated with psoriasis area and severity index (PASI)[7].On the other hand,IL-23-activated Th17 cells to secrete IL-17A,ultimately forming the IL-23/IL-17A axis,which is another main pathogenesis of psoriasis[8].Various cytokines secreted by immune cells further stimulate keratinocytes to secrete a large amount of growth factors,such as vascular endothelial growth factor A (VEGFA),which causes excessive cell proliferation and blood vessel formation,eventually leading to formation of erythema and scaling[9].Meanwhile,keratinocytes can also release inflammatory factors to regulate immune cells,maintaining and amplifying the inflammatory response through a positive feedback loop[10,11].
Currently,there are a few strategies for psoriasis treatment,including topical drugs (glucocorticoids and vitamin D3),systemic drugs (methotrexate and cyclosporine A),and biological agents (IL-23 inhibitor,utekinumab and IL-17A inhibitor,secukinumab)[12-14].However,these drugs may have severe side effects,such as high hepatic and renal toxicity,skin atrophy and drug dependence[15].Novel biologics have been shown particular efficacy in relieving psoriasis symptoms,but with limitations as well,such as expensive and inconvenient to use[16].Therefore,it is still an urge to identify safer and more effective anti-psoriasis drugs.
Apigenin (API) is a natural flavonoid widely found in various vegetables and fruits,with anti-inflammation,anti-oxidative stress and anti-cancer functions[17].To date,it is reported that API has strong activity against a variety of cancers by inhibiting cell proliferation and migration,modulating immunity,and attenuating inflammatory responses[18,19].In addition,several studies have shown that API alleviates chronic inflammation induced colitis by inhibiting activation of nuclear factor-κB (NF-κB)[20].Meanwhile,API protects against neurodegenerative diseases by increasing brain-derived neurotrophic factor levels[21].Our group has shown that API ameliorates 3,5-diethoxycarbonyl-1,4-dihydrocollidineinduced cholestasis by reducing inflammation and oxidative stress[22].Therefore,we speculated that API may be a novel active ingredient to treat psoriasis.In this study,we used imiquimod (IMQ)-induced psoriasis mouse model to determine the protective effects of API against psoriasis,and unveiled the involved mechanism using human keratinocytes (HaCaT cells)in vitro.
2. Materials and methods
2.1 Reagents
API,PD98059 and Bay11-7082 were purchased from Medchemexpress (New Jersey,USA).IMQ cream was purchased from Sichuan Med-shine Pharmaceutical Co.,Ltd.(Chengdu,China).Total RNApure reagent (TRIzol) was purchased from Beijing Zomen Biotechnology Co.,Ltd.(Beijing,China).Cocktail of protease inhibitors,phenylmethylsulfonyl fluoride (PMSF) and enhanced chemiluminescence (ECL) kit were purchased from Tanon (Shanghai,China).Bovine serum albumin (BSA) was purchased from Sigma Aldrich (St.Louis,MO,USA).Bromphenol blue,heparin,triton X-100 and sodium dodecyl sulfate (SDS) were purchased from Solarbio (Beijing,China).4’,6-Diamidino-2-phenylindole (DAPI)was purchased from Santa Cruz Biotechnology (Paso Robles,CA,USA).Hematoxylin and eosin (H&E) staining solution was purchased from Biosharp (Hefei,China).
Rabbit anti-extracellular regulated protein kinases 1/2 (ERK1/2,CAT# AF6240),phosphorylated ERK1/2 (p-ERK1/2,CAT#AF1014),phosphorylated-NF-κB (p-NF-κB,CAT# AF2006),IL-17A (CAT# DF6127),IFN-γ (CAT# DF6045),IL-6 (CAT#DF6087),signal transducer activator of transcription 3 (STAT3,CAT# AF6294),phosphorylated STAT3 (p-STAT3,CAT# AF3293),and cyclinD2 (CAT# AF5410) antibodies were purchased from Affinity Biosciences (Changzhou,China).Mouse anti-TNF-α (CAT#60291-1-Ig),IL-23 (CAT# 10253-2-AP),rabbit anti-heat shock proteins 90 (HSP90,CAT# 13171-1-AP),glyceraldehyde-3-phosphate dehydrogenase (GAPDH,CAT# 60004-1-Ig),Ki-67 (CAT# 27309-1-AP) and FITC-goat anti-rabbit IgG (H+L) (CAT# SA00003-2)antibodies were purchased from Proteintech Group Inc.(Illinois,USA).Rabbit anti-NF-κB (Cat# A16271) and IL-1β (Cat# A16271)antibodies were purchased from Abclonal (Wuhan,China).
2.2 Cell culture
HaCaT cells (an immortalized human keratinocyte cell line)were purchased from ATCC (Manassas,VA,USA),and cultured in complete RPMI 1640 medium (containing 10% FBS,50 mg/mL penicillin and streptomycin) at 37 °C in an atmosphere of 5% CO2.Cells at~90% confluence were cultured in serum-free medium before treatment. The concentration of API usedin vitroexperiment is correlate with previous studies[23,24].
2.3 In vivo study
C57BL/6J mice (male,~8-week-old) were purchased from the GemPharmatech Co.,Ltd.(Nanjing,China).The protocol for animal(#HFUT20210405001) was approved by the Institution Animal Ethics Committee of Hefei University of Technology.All animal experiments were conducted with the guidelines published in the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals.
API is extremely insoluble in water,while can be dissolved in DMSO.However,more than 2% of DMSO usedin vivostudy can cause toxicity.Compared to other solvents,olive oil is less likely to induce inflammation,and wildly used as a solvent forin vivostudies[25,26].Therefore,we used olive oil to dissolve API.According to previous studies,we choose the dose of 25 mg/(kg·d) API forin vivostudy[27,28].C57BL/6J mice were randomly divided into 6 groups (5 mice/group).In PBS groups,mice were received intraperitoneally(i.p.) injection of PBS daily for 14 days;in olive oil groups,mice were received i.p.injection of olive oil daily for 14 days;in API groups,mice were received i.p.injection of API (25 mg/(kg·d),dissolved in olive oil) daily for 14 days.From day 8,mice in vehicle or IMQ groups were treated with vaseline (62.5 mg/(mouse·d)) or IMQ cream (62.5 mg/(mouse·d)),respectively,on the shaved back skin for a week.All mice were sacrificed at day 15 with collection of tissue samples individually.
2.4 Evaluation of skin lesion by PASI score
At the end of experiment,the severity of mouse skin lesions was measured according to the PASI score as described[29].The PASI score was determined by three indicators: erythema,induration and desquamation.Score of these indicators were divided into 4 grades: 0,none;1,mild;2,moderate;3,marked;and 4,very marked.We used the sum of erythema,induration and desquamation score as the total score to indicate the severity of skin lesion.
2.5 Flow cytometry
At the end of experiment,spleen and axillary lymph nodes were collected and cut into fine pieces for grinding into single cells by glass slides,then filtrated with a 70 μm cell strainer.Cells from spleen or lymph nodes were incubated in 3 mL of red cell lysis buffer for 5 min,then centrifuged at 1 200 r/min for 3 min at 4 °C.The obtained single cell suspension was counted,and 1 × 106cells were used for the following experiment.The cells were re-suspended with 100 µL PBS and incubated with APC anti-mouse CD4,APC anti-mouse CD11c or FITC anti-mouse Gr1 antibody (Biolegend,California,USA) at 4 °C for 30 min in the dark.Finally,the cells were centrifuged at 1 200 r/min for 3 min at 4 °C,followed by washing with PBS for twice,then analyzed by flow cytometry[30].Gate strategy was used to analyze different immune markers in spleen and lymph nodes[31].We first identified lymphocytic or granulocyte gate by forward scatter (FSC) and side scatter (SSC),then analyzed CD4,CD11c or Gr1 positive cells by APC-or FITC-labeled antibody in indicated gate (Fig.S1).
2.6 H&E staining
The skin sections were conducted H&E staining to determine the pathological changes in skin tissues.The sections were observed and photographed using a ZEISS Scope A1 fluorescence microscope.The epidermal thickness (red height) was analyzed using Image J software by converting the pixels to the scale[22].
2.7 Determination of protein expression by Western blot and immunofluorescent staining
After treatment,total proteins were extracted from HaCaT cells or 30 mg skin tissue.Expression of IFN-γ,ERK1/2,p-ERK1/2,p-NF-κB,NF-κB,IL-23,IL-17A,STAT3,p-STAT3,IL-6,IL-1β,cyclinD2,HSP90,GAPDH and TNF-α protein was determined by Western blot as described[22].The signals were detected with Chemiscope 3000 mini (Qinxiang,Shanghai,China) and the band density was normalized by HSP90 or GAPDH in the corresponding samples and quantified using the Adobe Photoshop software.
Tissue sections or HaCaT cells were conducted immunofluorescent staining to determine expression of IL-17A,IFN-γ,p-STAT3,NF-κB,Ki-67 and cyclinD2 protein.Images were obtained using a ZEISS Scope A1 fluorescence microscope.The mean fluorescence intensity (MFI) was analyzed by Image J software.
2.8 Determination of mRNA expression by quantitative real-time PCR (qRT-PCR)
After treatment,total RNA was extracted from HaCaT cells using TRIzol as described[32].cDNA was synthesized by the Hiscript Q RT SuperMix for qPCR (+gDNA wiper) reverse transcriptase kit(Vazyme,Nanjing,China).Real-time PCR was performed using a SYBR Green PCR master mix and primers with sequences were listed in Table 1.mRNA expression was normalized byGAPDHmRNA in the corresponding samples.
2.9 Statistical analysis
All data were generated from at least three independent experiments.GraphPad Prism 7 was used for data statistical analysis.Data were analyzed by one-way ANOVA followed by Tukey’s multiple comparisons test.The significant difference was considered atP<0.05.
3. Results
3.1 API reduces IMQ-induced psoriasis-like skin lesions in mice
IMQ is wildly used to induce psoriasis-like lesions including erythema,skin thickening and scaling,the phenotypes are similar to human plaque psoriasis[2,11,33].To investigate the protection of API against psoriasis,mice were pre-treated with API for 7 days,followed by induction of psoriasis-like pathology with IMQ treatment on shaved back skin for one week (Fig.1A).As shown in Fig.1B,we showed that there are no significant changes in the dorsal skin of mice in vehicle groups.In contrast,mice in IMQ groups exhibitedextensive erythema and scaling,which was significantly attenuated by API,but not olive oil,indicating that API reduced IMQ-induced typical psoriasis-like lesions,while olive oil had little effects on IMQcaused symptoms (Fig.1B).The PASI score is commonly used to assess the severity of psoriatic lesions,which consists of the sum score of lesion area,erythema and scaling[11].The results in Fig.1C showed that PASI score was significantly increased in PBS and olive oil treated IMQ groups,while decreased in API group,suggesting that API reduces IMQ-caused psoriasis-like lesions.
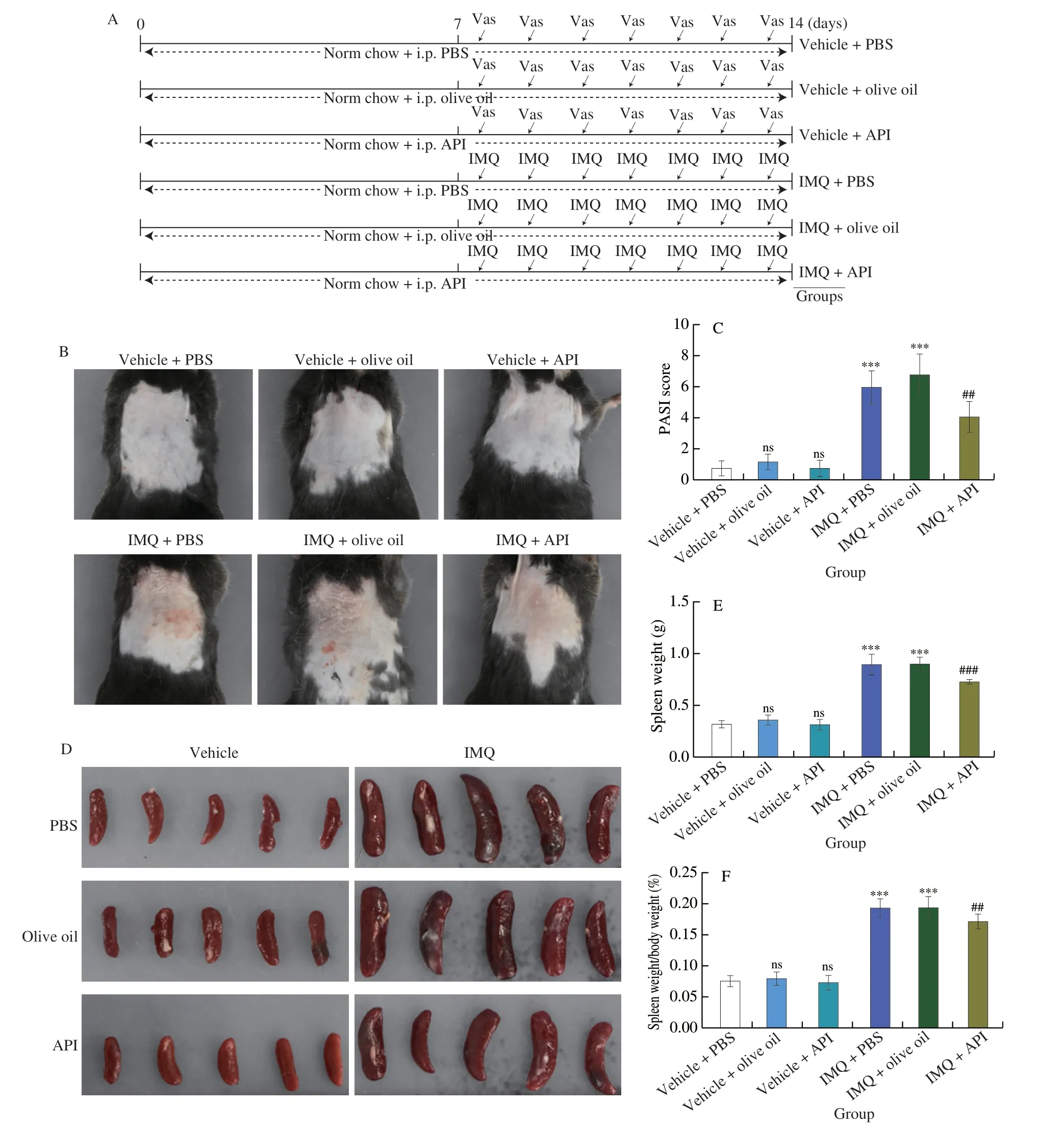
Fig.1 API reduces IMQ-induced psoriasis-like skin lesions in mice.A,The design of in vivo experiment.The 8-week-old male C57BL/6J mice were randomly divided into sit groups (5 mice/group) and received the following treatment.Mice were received i.p.injection of PBS,olive oil or API (25 mg/(kg·d),dissolved in olive oil) daily for 2 weeks.From day 8,mice were treated with Vaseline (62.5 mg) or IMQ cream (62.5 mg) on the shaved back skin daily for one week.At the end of experiment,all mice were used for following assay.B,Mice were photographed and the representative photos were presented.C,PASI score was calculated based on the images.D,Mouse spleen was collected and photographed.E and F,Spleen was weighed (E) and the ratio of spleen weight to body weight was calculated (F).G and H,skin sections were conducted H&E staining.Rete ridge (blue arrowheads),epidermal thickness of skin (red height) (G) and epidermal thickness of skin was measured (H).***P <0.001,ns,not significant vs.Vehicle+PBS group;## P <0.01,### P <0.001 vs.IMQ+PBS treated group (n=5).
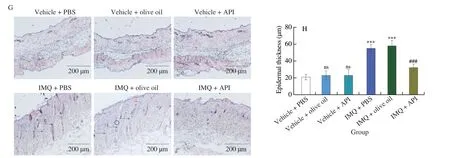
Fig.1 (Continued)
The enlargement of spleen indicates excessive activation of immune system.It is also an index of the severity of inflammatory response[33].Visually,the spleen volumes of in vehicle groups were approximately the same.However,the spleen volume of the mice in the IMQ groups was significantly larger than that of the vehicle groups,while the enlarged spleen was diminished by API treatment.However,olive oil treatment did not reduce IMQ-induced spleen enlargement (Fig.1D).Consistently,both the absolute spleen weight and the ratio of spleen weight to body weight were increased by IMQ treatment,whereas both were reduced after API but not olive oil treatment (Figs.1E and F),suggesting that API reduces IMQ-induced immune responses.
To further evaluate the effects of API on the histological changes in IMQ-induced psoriasis-like skin lesions,we performed H&E staining using 5-μm paraffin sections of skin.As shown in Fig.1G,the skin from both IMQ-PBS and IMQ-olive oil groups showed significant epidermal layer thickening and rete ridge (indicated by blue arrowheads).Statistical analysis further showed that the epidermal layer was thinner in API group than that in IMQ-PBS and IMQ-olive oil groups (Fig.1H).Taken together,the results above suggest that IMQ-enhanced psoriasis-like skin lesion can be reduced by API,but not affected by olive oil.Compared with PBS groups,oil would not cause other side effects.In this study,we used olive oil to dissolve API.Therefore,we used vehicle-olive oil (named as Ctrl),IMQ-olive oil (named as IMQ) and IMQ-API groups for further investigation in the following experiment.
3.2 API relieves IMQ-induced immune response
Lymphocytes,DCs and neutrophils are hyperactivated during the development of psoriasis[2].CD4 is a marker of Th lymphocytes that regulate immune response in psoriasis.It is especially the case in Th1 and Th17 cells.Gr1 is a marker of neutrophils.Excess neutrophils in psoriasis promotes secretion of IL-6 and IL-1β from activated DCs,thus amplifying inflammatory response.Our results showed that the percentage of Th lymphocytes and neutrophils was increased in mouse spleen of IMQ group.However,these changes were reversed by API treatment (Fig.2A).
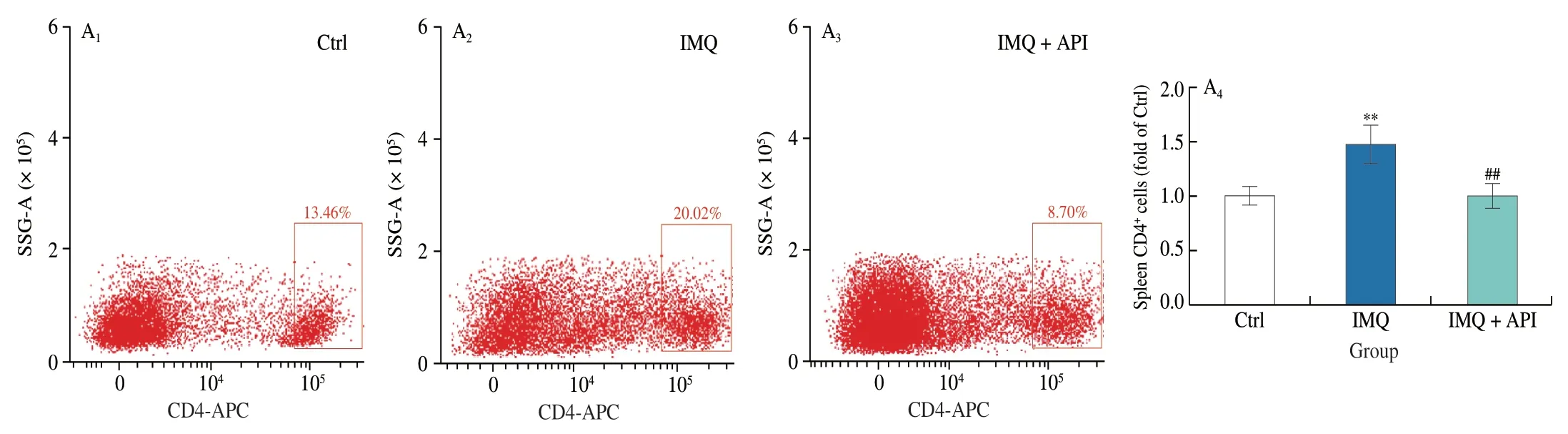
Fig.2 API modulates the immune response induced by IMQ.At the end of experiment,mouse spleen and axillary lymph nodes were collected.Cells isolated from these tissues were incubated with indicated antibodies and analyzed by flow cytometry.A,CD4+,CD11c+ and Gr1+ cells were detected in mouse spleen with quantitative analysis.B,CD11c+ cells were detected in lymph nodes with quantitative analysis.**P <0.01,*** P <0.001 vs.Ctrl group;# P <0.05,## P <0.01,### P <0.001 vs.IMQ treated group (n=3).
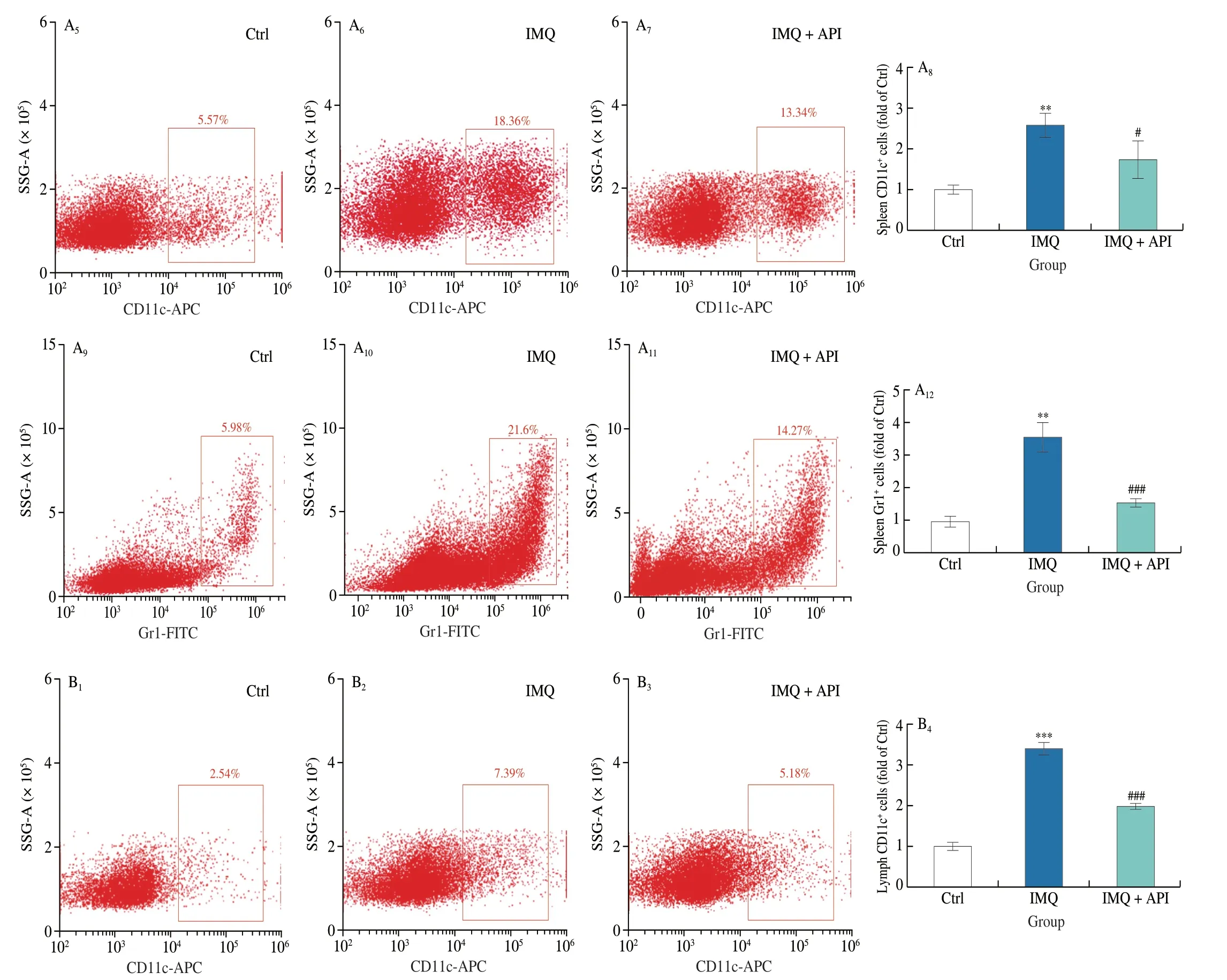
Fig.2 (Continued)
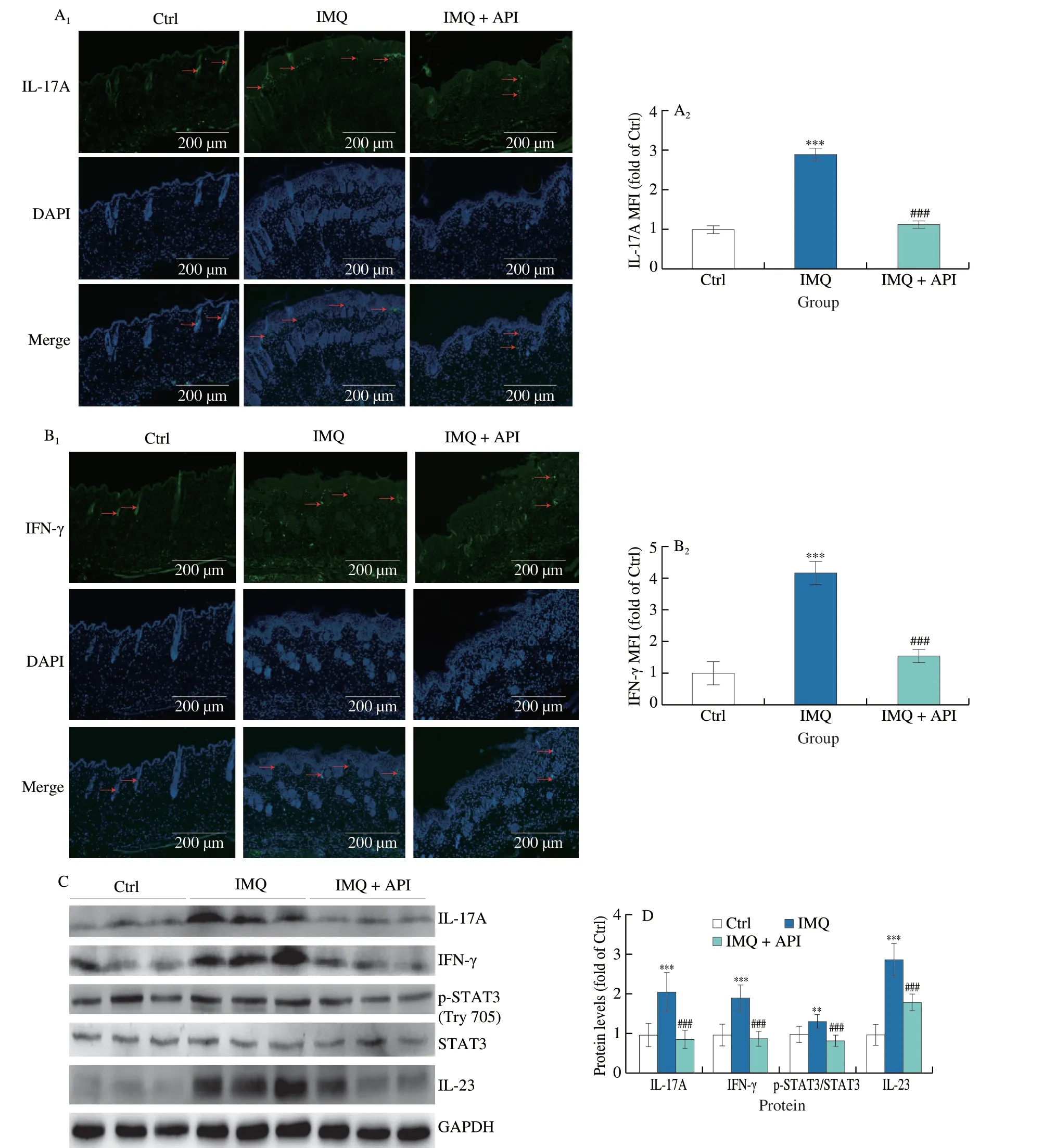
Fig.3 API regulates immune response by inhibiting IL-23/STAT3 signaling pathway.A and B,skin sections were conducted immunofluorescent staining to determine IL-17A (A) and IFN-γ (B) protein expression with quantitation of MFI (n=5).C and D,protein expression of IL-17A,IFN-γ,p-STAT3,STAT3 and IL-23 in skin tissues was determined by Western blot (C) with quantitative analysis of band density (D) (n=5).The red arrows represent the positive regions of IL-17A or IFN-γ.Scale bar: 200 μm;**P <0.01;*** P <0.001 vs. Ctrl group;### P <0.001 vs. IMQ treated group.
DC activation plays an important role in promoting pro-inflammatory factors production during psoriasis[5,30,34].We detected DCs using CD11c antibody,and observed that the percentage of DCs was increased in lymph nodes and spleen tissues of IMQ group mice (Figs.2A and B).However,API treatment substantially attenuated the increase of DCs.These results suggest that API can modulate IMQ-induced immune hyperreaction.
3.3 API regulates immune response by inhibiting IL-23/STAT3 pathway
We further explored the involved mechanisms by which API modulates immune response.Th17 and Th1 lymphocytes belong to a subpopulation of CD4+T lymphocytes,and are source for IL-17A and IFN-γ production,respectively.It has been reported that Th17 and Th1 lymphocytes or IL-17A and IFN-γ levels are increased in psoriatic lesions[11].In vivo,we observed that API treatment decreased IMQ-induced expression of IL-17A and IFN-γ (Figs.3A-D).
STAT3 acts as a central molecule mediating the differentiation of Th17 lymphocytes.IL-23 secreted by DCs mediates the phosphorylation of STAT3 at tyrosine 705 (Tyr705),which in turn promotes the production of IL-17A by Th17 lymphocytes.Consistent with changes in IL-17A,our results showed that IMQ-activated STAT3 was significantly decreased in API group.Moreover,API decreased IMQ-enhanced IL-23 levels (Figs.3C and D).Therefore,the results above indicate that API regulates immune response by inhibiting IL-23/STAT3/IL-17A pathway.
3.4 API attenuates IMQ-induced inflammation by inhibiting NF-κB activation
In the pathogenesis of psoriasis,various cytokines secreted by immune cells mediate a severe inflammatory response[35].Previous studies have shown that NF-κB is one of the key transcription factors that regulates inflammatory process in psoriasis[35].In vivo,we found IMQ significantly increased NF-κB protein expression in epidermal layer of skin,which was inhibited by API (Fig.4A).Moreover,API abolished IMQ-activated NF-κB phosphorylation (p-NFκB) in skin tissues (Fig.4B).Furthermore,we found that protein expression of inflammatory cytokines,TNF-α,IL-6 and IL-1β,was decreased in API group compared to IMQ group (Fig.4B).
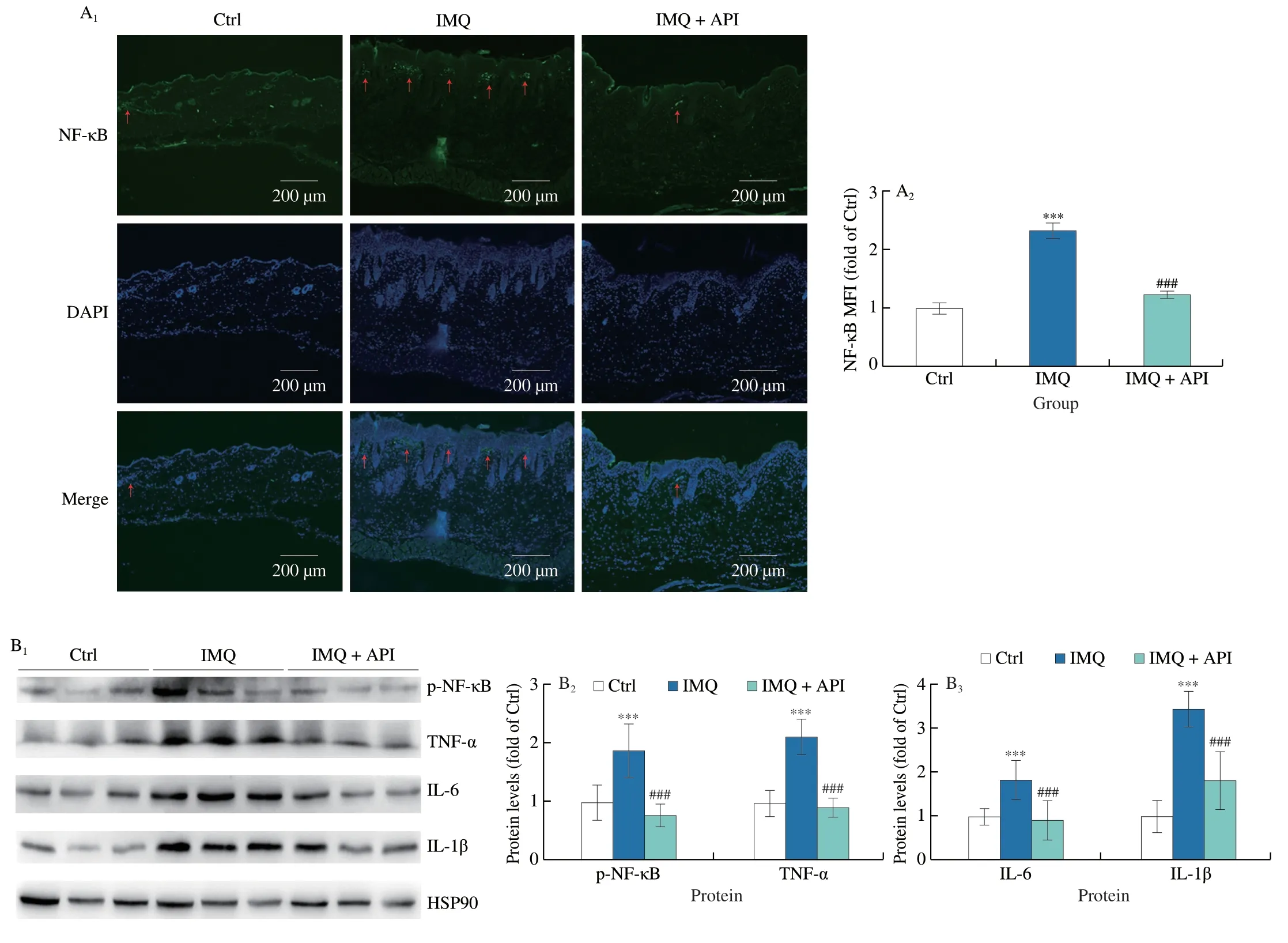
Fig.4 API attenuates IMQ-induced inflammation by inactivating NF-κB in vivo. A,skin sections were conducted immunofluorescent staining to determine NF-κB protein expression with quantitation of MFI (n=5).B,protein expression of p-NF-κB,TNF-α,IL-6 and IL-1β in skin tissues was determined by Western blot with quantitative analysis of band density (n=5).The red arrows represent the positive regions of NF-κB.Scale bar: 200 μm;***P <0.001 vs.Ctrl group;###P <0.001 vs. IMQ treated group.
To verify whether API also attenuates inflammatory responsein vitroby inactivating NF-κB,we used IMQ to induce inflammation in HaCaT cells.We first conducted MTT assay to determine the cytotoxicity of IMQ on HaCaT cells.As shown in Fig.S2,we found that cell viability was slightly increased by 5 and 10 μmol/L of IMQ,while reduced by high concentration.Moreover,our results showed that 15 or 20 μmol/L of IMQ has little effect on cell viability.Therefore,we chose IMQ at a concentration of 20 μmol/L forin vitroexperiments.Consistent with thein vivoresults,we found that protein expression of p-NF-κB and nuclear translocation of NF-κB was enhanced by IMQ treatment (Figs.5A and B).At the same time,protein expression of NF-κB downstream cytokines,TNF-α,IL-6 and IL-1β,was also increased by IMQ (Fig.5A),which suggests that NF-κB signaling pathway was activated by IMQ.In contrast,API treatment reduced IMQ-activated NF-κB signaling pathway (Figs.5A and B).In addition,qRT-PCR results showed that IMQ-induced mRNA levelsIL-6,IL-1β,TNF-αandIL-8were decreased by API treatment (Fig.5C).Thus,our results suggest that API attenuates IMQ-induced psoriasis-like skin inflammation by inhibiting NF-κB pathway bothin vivoandin vitro.
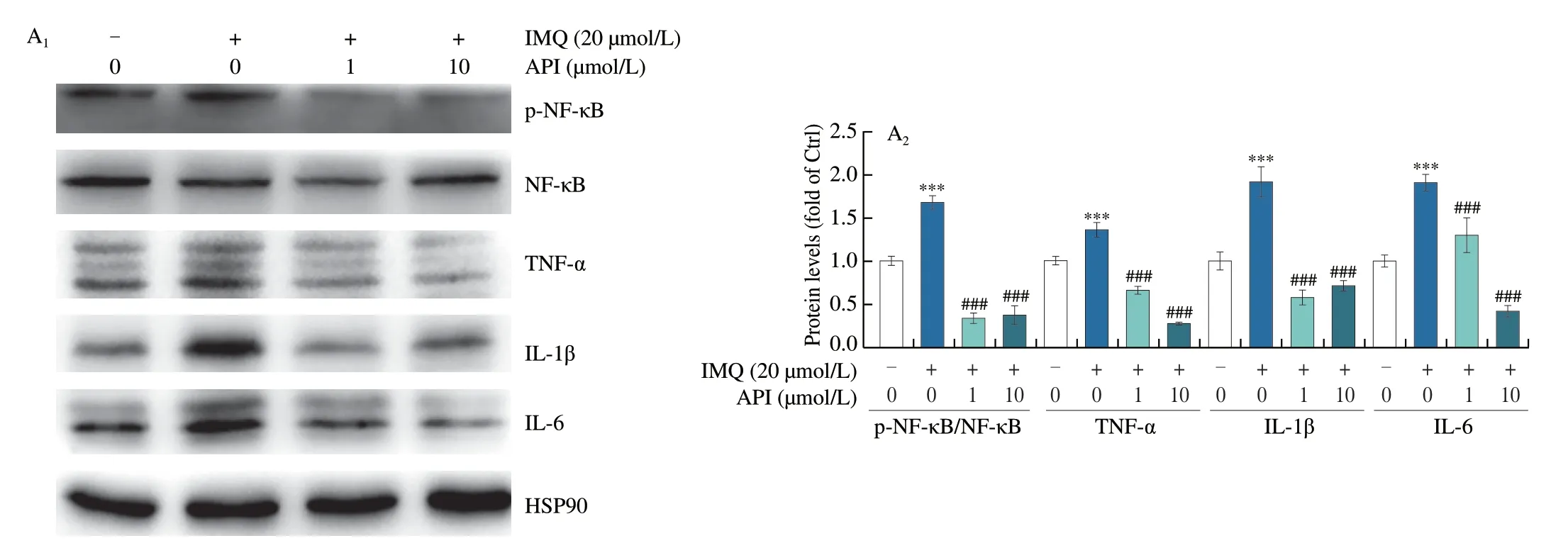
Fig.5 API inhibits expression of inflammatory cytokines by inactivating NF-κB in HaCaT cells.A-C,HaCaT cells in 6-or 48-well plates were pre-treated with API for 12 h,then treated with IMQ (20 μmol/L) for 6 h.Protein expression of NF-κB,p-NF-κB,TNF-α,IL-6 and IL-1β was determined by Western blot with quantitative analysis of band density (A),protein expression of NF-κB was determined by immunofluorescent staining with quantitation of nuclear translocation (B).mRNA expression of IL-6,IL-1β,TNF-α and IL-8 was determined by qRT-PCR (C).Scale bar: 100 μm;*P <0.05,*** P <0.001 vs. Ctrl group;# P <0.05,### P <0.001 vs. IMQ treated group (n=3).
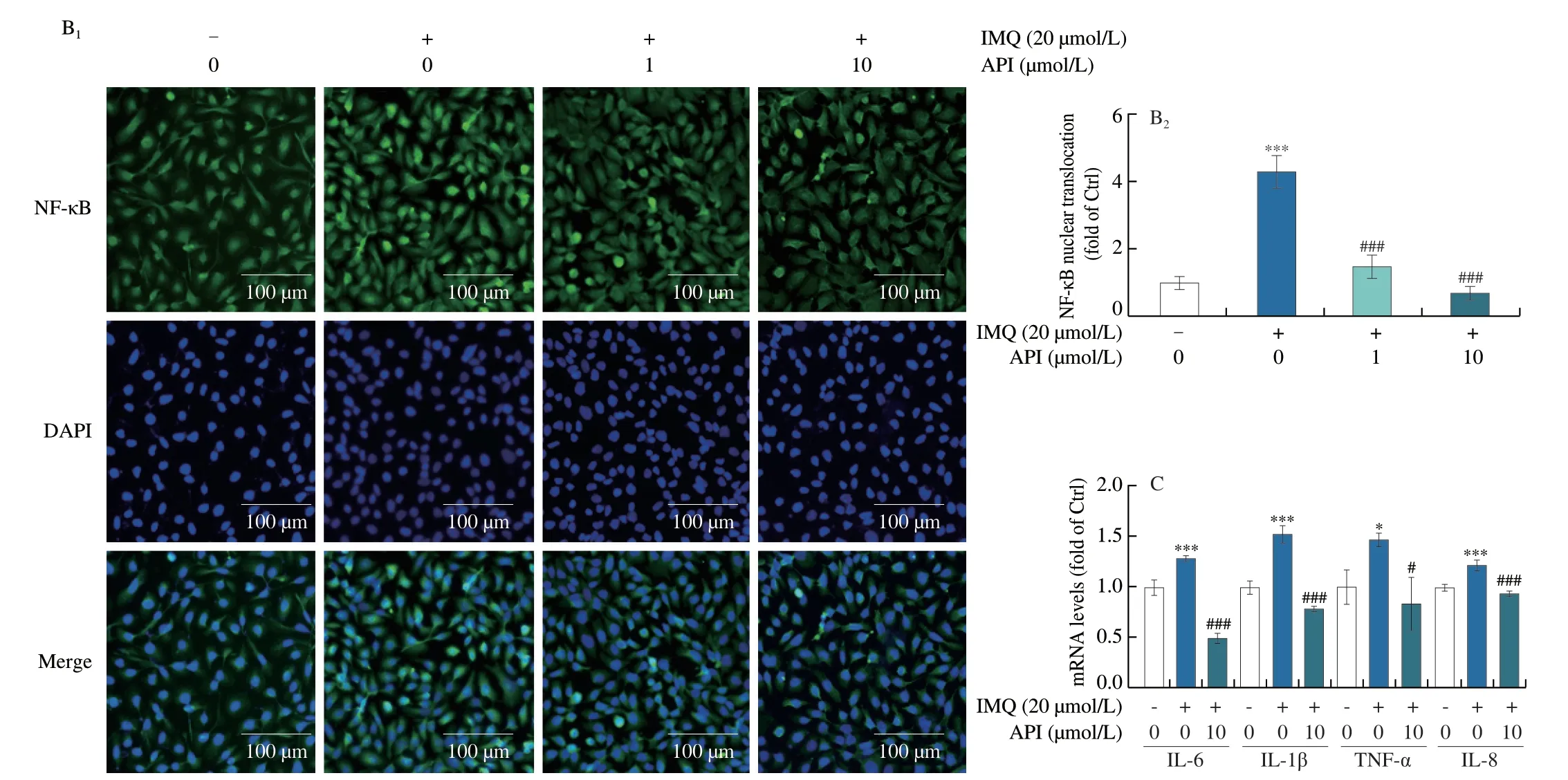
Fig.5 (Continued)
3.5 API inhibits IMQ-induced cell proliferation
Abnormal proliferation and differentiation of keratinocytes are the most important features of psoriasis[36].In vivo,we found that IMQinduced expression of proliferation markers,Ki-67 and cyclinD2,and the induction was inhibited by API treatment (Figs.6A-C).It has been shown that IL-17A activates ERK1/2 and promotes proliferation of keratinocytes[37,38].Our results indicated that expression of p-ERK1/2 was elevated by IMQ treatment.In contrast,API treatment reversed IMQ-activated p-ERK1/2 levels (Fig.6B).
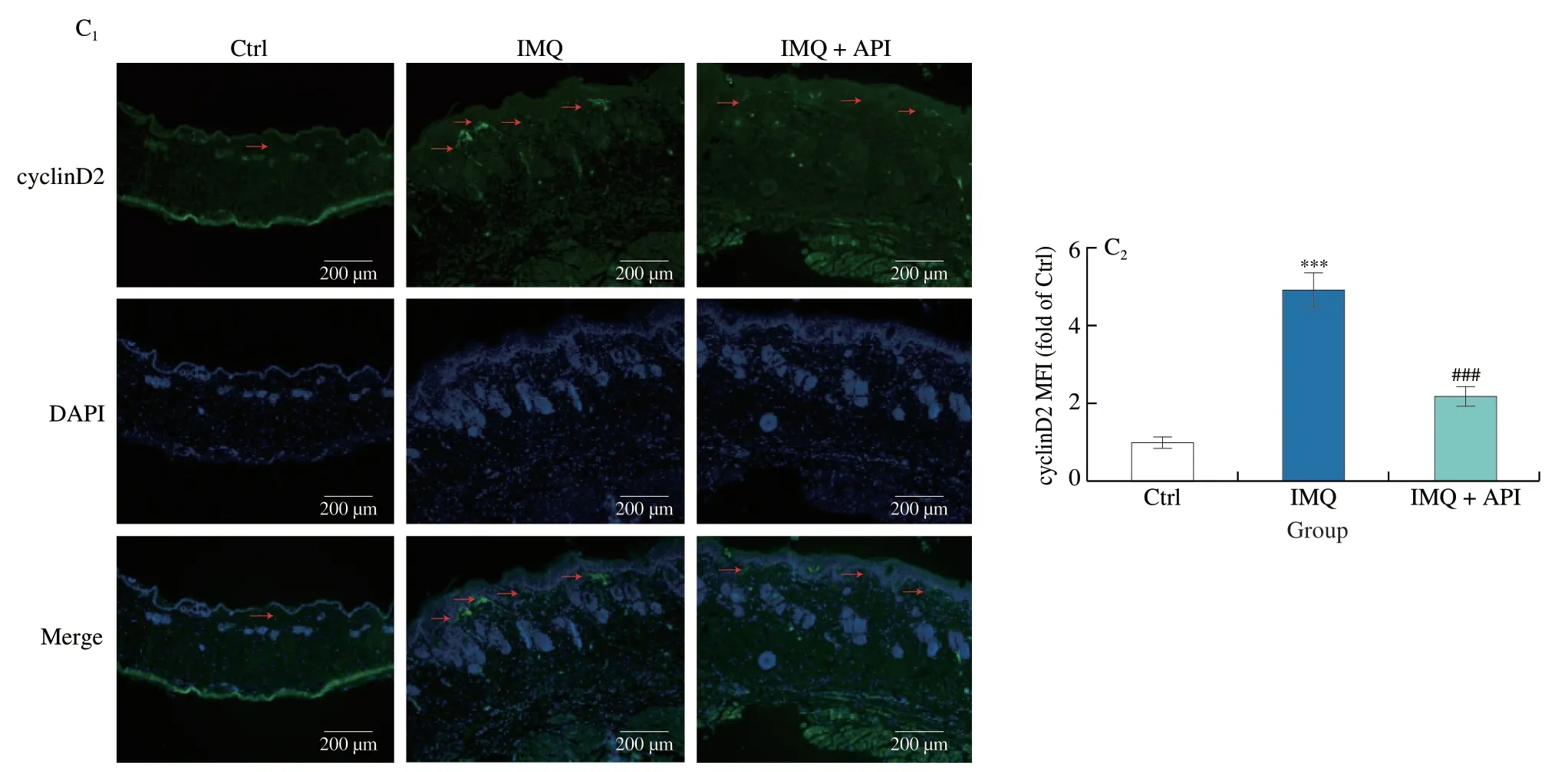
Fig.6 (Continued)
Furthermore,we verified the inhibitory effects of API on cell proliferationin vitro.As shown in Fig.7A,API treatment decreased IMQ-induced cyclinD2 protein expression and ERK1/2 activation in HaCaT cells,which was consistent with thein vivoresults (Fig.6).It has been reported that K16 and K17 levels are associated with hyperproliferation of keratinocytes.K16 increases VEGFA expression through activation of ERK1/2 pathway,while K17 is positively regulated by activated STAT3[39].Our results showed that API treatment decreased mRNA expression ofK16,K17,VEGFAandKi-67in HaCaT cells (Fig.7B).Consistent with thein vivoresults(Fig.3C),the results of immunofluorescence staining demonstrated that API treatment significantly inhibited p-STAT3 levels in HaCaT cells (Fig.7C).Both NF-κB and ERK1/2 can stimulate nuclear translocation of STAT3[40,41].As shown in Figs.7D and E,NF-κB and ERK1/2 were activated after IMQ treatment.Consequently,expression of p-STAT3 was significantly upregulated by IMQ.In contrast,treatment with Bay11-7082 (a NF-κB inhibitor) and PD98059 (an ERK1/2 inhibitor) decreased p-STAT3 levels.Moreover,we found co-treatment of PD98059 or Bay11-7082 with API showed additional inhibitory effects on p-STAT3.Taken together,API inhibits IMQ-induced cell proliferation by suppressing STAT3 activation,which is partly contributed by inhibition of NF-κB and ERK1/2.
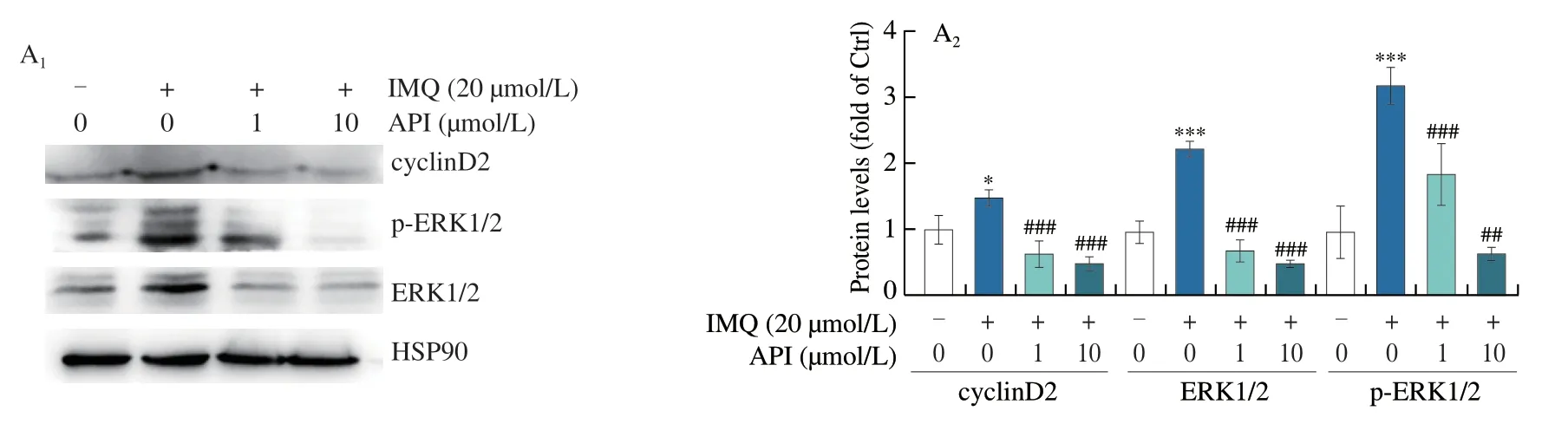
Fig.7 API inhibits IMQ-induced cell proliferation by inactivating STAT3 in HaCaT cells.A-C,HaCaT cells in 6-or 48-well plates were pre-treated with API for 12 h,then treated with IMQ (20 μmol/L) for 6 h.Protein expression of cyclinD2,ERK1/2 and p-ERK1/2 was determined by Western blot with quantitative analysis of band density (A).mRNA expression of Ki-67,K16,K17 and VEGFA was determined by qRT-PCR (B).Protein expression of p-STAT3 in HaCaT cells was determined by immunofluorescent staining with quantitation of MFI (C).D,HaCaT cells in 6-well plates were pre-treated with API or Bay or API+Bay for 12 h,then treated with IMQ (20 μmol/L) for 6 h.Protein expression of p-NF-κB,NF-κB,p-STAT3 and STAT3 was determined by Western blot with quantitative analysis of band density.E,HaCaT cells in 6-well plates were pre-treated with API or PD or API+PD for 12 h,then treated with IMQ (20 μmol/L) for 6 h.Protein expression of p-ERK1/2,ERK1/2,p-STAT3 and STAT3 was determined by Western blot with quantitative analysis of band density.*P <0.05,** P <0.01,*** P <0.001 vs.Ctrl group;# P <0.05,## P <0.01,### P <0.001 vs.IMQ treated group (n=3).PD: PD98059;Bay: Bay11-7082.
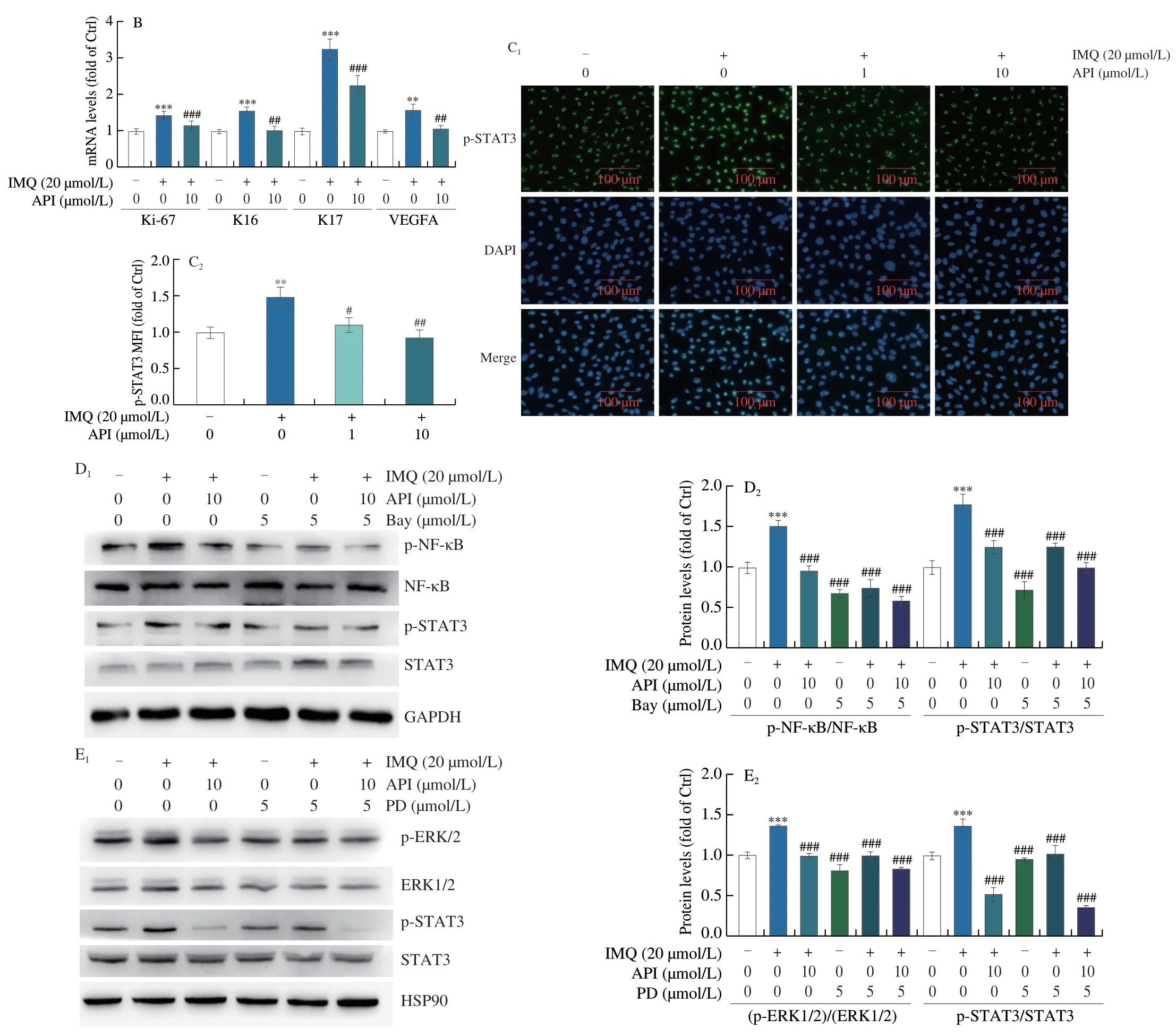
Fig.7 (Continued)
4. Discussion
Psoriasis is a chronic inflammatory skin disease caused by the interaction of immune cells and keratinocytes[31].The exact mechanisms of psoriasis remain unclear,and the therapeutic drugs are also limited.The IL-23/STAT3/IL-17A axis is the most important mechanism mediating the chronic inflammation in psoriasis.Meanwhile,an increasing number of new targets have been shown to be involved in the pathogenesis of psoriasis-like inflammation.Previous studies have proven that inhibition of GPR43,bromodomain and extraterminal domain and interleukin-2-inducible T-cell kinase by inhibitors can largely attenuate psoriasis-like inflammation,which provide new therapeutic targets or therapy strategies against psoriatic inflammation[31,42,43].In this study,we explored the pharmacological effects of API on IMQ-induced psoriasis-like symptoms in C57BL/6J mice.Our results showed that API has protective effect on IMQinduced psoriasis,which is evidenced by relieved erythema and scaling on mouse back,and decreased PASI score.Mechanistically,API regulates immune function through inhibiting IL-23/STAT3/IL-17A signaling axis and inhibits the secretion of inflammatory cytokines by reducing NF-κB pathway.In addition,API reduces cell hyperproliferation via inhibiting p-STAT3 levels bothin vivoandin vitro,at least through inactivation of NF-κB and ERK1/2.
Both innate and adaptive immune systems mediate the process of psoriasis[44].The lymphatic system serves as the most important immune system of the body,and DCs are regarded as the starting point for activating immune response.Targeting DCs is a potential strategy for psoriasis treatment.Bruton’s tyrosine kinase (BTK)plays important roles in regulation DCs and T cells.Recent study has demonstrated that PCI-32765 (a BTK inhibitor) suppresses IMQinduced psoriasis-like inflammation through IL-23/IL-17A pathway[5].Ibrutinib,another effective inhibitor of BTK,has been proven to attenuate psoriatic inflammation by enhancing antioxidants enzymes and inhibiting inflammatory cytokines in neutrophils and DCs[45].More and more studies have demonstrated that spleen tyrosine kinase (SYK) is critical in inflammatory signaling cascade of DCs.Inhibition of SYK by R406 can attenuates IMQ-induced inflammation by reducing Th17 cells and enhancing Treg cells,suggesting that SYK inhibition could be a prospective therapeutic approach for psoriasis treatment[34].Previous studies have shown that API reduces the severity of experimental autoimmune encephalomyelitis by modulating function of DCs and other immune cells[46].Our results revealed that API treatment significantly reduced spleen size and weight,as well as the percentage of DCs in spleen and lymph nodes (Figs.1D,2).In addition,we also found API reversed IMQ-induced the percentage of Th lymphocytes and neutrophils(Fig.2A),indicating API can regulate immune hyperreaction in psoriasis.Studies have proven that DCs in skin plays critical role in psoriasis development[5,45].However,we only detected DCs in spleen and lymph node in this study.It suggests that if API can affect the function DCs in skin need further exploration.
A mount of studies have proven that immune suppressive agents are effective for psoriasis treatment.Previous studies have demonstrated that API markedly inhibits activation of Th1 and Th17 cells in lupus erythematosus[47].Interestingly,we also noticed a significant decrease in protein expression of IL-17A and IFN-γ in the skin of API-treated mice (Figs.3A-D).STAT3,a central transcription factor for regulating inflammation and immune response,plays a key role in the pathogenesis of psoriasis[48].IL-23 is secreted by DCs and can promote nuclear translocation of STAT3.Phosphorylated STAT3 promotes the differentiation of Th17 cells[49].One study has shown that API inhibits STAT3/CD36 signaling axis to reduce visceral obesity[27].Another study has demonstrated that API induces apoptosis in primary effusion lymphoma by inhibiting STAT3[17].Consistent with previous findings,we found API significantly reduced IL-23 and p-STAT3 levels (Figs.3C and D),suggesting API regulates immune system through IL-23/STAT3/IL-17A axis.Therefore,we indicate that API improves psoriasis may through inhibiting DCs,then reduces Th cells activation.Psoriatic inflammation is associated with other diseases,such as asthma and depression.Ahmed Nadeem et al.,have demonstrated that IL17A led to an induction in IMQ-induced depression-like symptoms,which is mediated by NF-κB/p38MAPK signaling pathway[50].In addition,they also showed that psoriatic inflammation in cockroach extract-induced allergic mice is associated with enhanced airway inflammation,as well as increasement of Th2/Th17 cells/signature cytokines/transcription factors,which is mainly regulated by IL-23/STAT3 signaling pathway[51].Above researches provide new insight in the treatment of asthmatics with psoriasis and psoriasis associated depression,and also suggest that API may have other functions.
NF-κB plays a crucial role in the inflammatory process of psoriasis.NF-κB can directly regulate expression of pro-inflammatory factors,TNF-α,IL-6 and IL-1β.In turn,TNF-α stimulates NFκB activation,which regulates inflammatory response in a positive feedback loop.Previous evidence suggests that API showed a significant inhibitory effect on TNF-α-activated NF-κB in PC-3 cells by inhibiting DNA binding of NF-κB,IκBα phosphorylation and IKK activation[52].In this study,we found API inhibited p-NF-κB levels,nuclear translocation of NF-κB and TNF-α,IL-6 and IL-1β expression bothin vivoandin vitro(Figs.4 and 5).Our results indicate that Bay11-7082 and API showed similarly inhibitory effects on p-NF-κB levels (Fig.7D),indicating the directory role of API on NF-κB.
Inflammatory factors not only mediate activation of immune cells,but also lead to hyperproliferation of keratinocytes[53].We found API significantly inhibited IMQ-induced Ki-67 protein expressionin vivoand mRNA levelsin vitro(Figs.6A and 7B),and reduced cyclinD2 protein levels (Figs.6A and 7A).K16 and K17 are biomarkers of hyperproliferation of keratinocytes.K16 enhances excessive VEGFA secretion through activation of ERK1/2 pathway,resulting in increased skin vascularization[54].In cutaneous cancer research,API suppressed proliferation and migration of melanoma cells by inhibiting p-ERK1/2 levels[55].Consistent with previous studies,our results also showed that API inhibited p-ERK1/2 levels bothin vivoandin vitro,reduced mRNA levels of K16,K17 and VEGFA in HaCaT cells (Figs.6 and 7),indicating API reduces keratinocytes hyperproliferation.ERK1/2 activation plays key roles in promoting cell survival and proliferation,which may be the reason of that IMQ enhanced cell viability of HaCat at low concentrations.In psoriasis,STAT3 not only regulates the immune response but also correlates with cell proliferation.It has been shown that STAT3 is involved in regulating K17 expression[39].Our results demonstrated that API reduced p-STAT3 levels (Figs.3C and 7C).Previous studies have shown that both NF-κB and ERK1/2 can activate STAT3[40,41].Reciprocally,in this study,we observed that inhibition of NF-κB and ERK1/2 by API leads to reduction of p-STAT3 expression,which may make contributions to its inhibition on cell proliferation.
In conclusion,in this study,we demonstrated that API can improve IMQ-induced psoriasis.Mechanistically,we indicated that API modulates immune response by inactivation of DCs,then inhibits Th cells differentiation and keratinocytes hyperproliferation,which is mainly mediated by STAT3 and NF-κB pathways.Our study suggests that API may be a potential drug for treatment of psoriasis or a lead compound for development of anti-psoriasis drugs.
Declaration of competing interest
The authors declare that they have no known competing economic interests or personal relationships that may affect the work described in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (81973316,82173807);the China Postdoctoral Science Foundation (2020M681914);the Fund from Tianjin Municipal Health Commission (ZC200093),and the Open Fund of Tianjin Central Hospital of Obstetrics and Gynecology/Tianjin Key Laboratory of human development and reproductive regulation (2021XHY01).
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250018.
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing

