2-O-β-D-Glucopyranosyl-L-ascorbic acid,an ascorbic acid derivative isolated from the fruits of Lycium barbarum L.,ameliorates high fructose-induced neuroinflammation in mice: involvement of gut microbiota and leaky gut
Wei Dong,Yuji Peng,Guijie Chen,Zhiyong Xie,Weiqi Xu,Wngting Zhou,Ji Mi,Lu Lu,Yi Sun,Xioxiong Zeng,*,Youlong Co,*,Ymei Yn,*
a College of Food Science and Technology,Nanjing Agricultural University,Nanjing 210095,China
b Institute of Wolfberry Engineering Technology,National Wolfberry Engineering Research Center,Ningxia Academy of Agriculture and Forestry Sciences,Yinchuan 750002,China
Keywords:Neuroinflammation Gut microbiota Leaky gut Lipopolysaccharide Fecal microbiome transplantation 2-O-β-D-Glucopyranosyl-L-ascorbic acid
ABSTRACT Western diet (rich in highly refined sugar and fat) can induce a range of metabolic dysfunctions in animals and humans,including neuroinflammation and cognitive function decline.Neuroinflammation and cognitive impairment,two critical pathological characteristics of Alzheimer’s disease,have been closely associated with microbial alteration via the gut-brain axis.Thus,the present study aimed to investigate the influence of 2-O-β-D-glucopyranosyl-L-ascorbic acid (AA-2βG) isolated from the fruits of Lycium barbarum on preventing the high-fructose diet (HFrD) induced neuroinflammation in mice.It was found that AA-2βG prevented HFrD-induced cognitive deficits.AA-2βG also predominantly enhanced the gut barrier integrity,decreased lipopolysaccharide entry into the circulation,which subsequently countered the activation of glial cells and neuroinflammatory response.These beneficial effects were transmissible by horizontal fecal microbiome transplantation,transferring from AA-2βG fed mice to HFrD fed mice.Additionally,AA-2βG exerted neuroprotective effects involving the enrichment of Lactobacillus and Akkermansia,potentially beneficial intestinal bacteria.The present study provided the evidence that AA-2βG could improve indices of cognition and neuroinflammmation via modulating gut dybiosis and preventing leaky gut.As a potential functional food ingredient,AA-2βG may be applied to attenuate neuroinflammation associated with Western-style diets.
1. Introduction
Total fructose consumption has drastically increased due to the extensive commercial use of high-fructose corn syrup (HFCS) as sweeteners for beverages,snacks and baked goods[1].Accumulating evidence demonstrated that fructose could induce inflammatory responses in peripheral tissues,and attention has also been paid on the ability of this sugar to in duce neuroinflammation[2].Neuroinflammation is a pathological hallmark of neurodegenerative disease,including Alzheimer’s disease (AD)[3].AD,an increasingly common neurodegenerative disease,afflicts more than 50 million people worldwide[4].It also drastically impairs individual’s quality of life and increases health care cost,which has become social problem[5].However,there is no specific drug to cure effectively the disease or reverse the process of disease.Therefore,it needs to consider valuable approaches to prevent AD based on various pathological pathw ays.
In recent years,accumulating evidence indicates that gut microbiota can influence the central nervous system (CNS) through the gut-brain axis,subsequently modulating the brain function and host behavior[6].For instant,recent work indicated a causative relationship between the gut microbiota and the development of cognitive deficits with transferability of phenotypes via fecal microbiota transplantation (FMT)[7].For another,the diet,as one of the important mediators of gut microbiota,has an impact on neurodegenerative disease[6].Of note,ample evidence indicates that the cognitive declines and neuroinflammation induced by Western diet seem to be mediated,at least partly,by gut microbiota[8].Specifically,other studies have shown that high-fructose diet(HFrD) could alter the microbial composition and increase gut permeability,which exacerbated the leakage of lipopolysaccharide(LPS) into the blood circulation[9,10].Increased access of LPS into the circulation could further trigger the neuroinflammation process,subsequently contributing to cognitive impairment[9].In line with this,the administration of peripheral LPS could aggravate the activation of astrocytes and microglia,elevating the levels of pro-inflammatory factors in the brain[11].Hence,gut dysbiosis may be an underlying risk factor in neuroinflammation,cognitive deficiency and related diseases.Consequently,developing a safe,effective and easily implemental dietmediated intervention strategy that can alter gut microbiota will be very important to prevent or delay the progression of AD.
Lycium barbarumL.,as a tonic food and traditional Chinese medicine,has been demonstrated to have various biological functions including anti-tumor,anti-inflammatory,immunomodulatory and neuroprotective activities[12].Specifically,it has been proved by both cell and animal models thatL.barbarumcan exert direct neuroprotective effects on neuronal diseases,including AD,Parkinson’s disease and other typical neurodegenerative diseases[13].As a stable ascorbic acid (AA) derivative firstly separated from the fruits ofL.barbarum,the content of 2-O-β-D-glucopyranosyl-L-ascorbic acid (AA-2βG) is about 0.5% in the dried fruits ofL.barbarum[14].It has been reported that AA-2βG extracted fromL.barbarumexhibited antioxidant,anti-tumor and immunomodulatory effects[15-17].In addition,we previously reported that AA-2βG could reduce the inflammation in dextran sodium sulfate-induced colitis mice by decreasing endotoxin,enhancing the intestinal permeability and modulating the gut microbiota[18].However,the possibility that whether AA-2βG can attenuate neuroinflammation and cognitive deficits has not been reported.Hence,we hypothesized that AA-2βG protected against neuroinflammation by remodeling of the gut microbiota and preventing leaky gut.Our results indicated that AA-2βG ameliorated intestinal barrier defect,reduced serum LPS,suppressed glial cells activation and neuroinflammation,and ultimately alleviated cognitive impairment.These neuroprotection effects were transferable by horizontal FMT,manifesting that the gut microbiome mediated the neuroprotection effects produced by AA-2βG.All these findings elucidated that AA-2βG possibly contributed to the inhibition of hippocampal neuroinflammation in HFrD-fed mice.
2. Materials and methods
2.1 Preparation of AA-2βG
AA-2βG (purity >90%) was prepared from the fruits ofL.barbarum(Ningnonggouqi No.7) provided by the National Wolfberry Engineering Research Center (Yinchuan,China).The method of preparation (see Supplementary Materials and Methods)was described in detail in our previous study[18].The structure of the obtained AA-2βG was confirmed by high-performance liquid chromatography (HPLC),matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS),and nuclear magnetic resonance (NMR) spectrometry (Fig.S1).
2.2 Animal experiments
2.2.1 Experimental animals
C57BL/6 (7 weeks old) male mice ((20 ± 1) g,average body weight (bw)) were purchased from Shanghai SLAC Laboratory Animal Co.,Ltd.(Shanghai,China).All the mice were housed in a specific pathogen-free (SPF) condition (SYXK -(Jiangsu)-2011-0037,Nanjing,China) under 12/12 h dark-light cycles.The humidity was(50 ± 5)% and ambient temperature was (25 ± 2) °C.
2.2.2 Ethics statement
The experiments were approved by the Guidelines of the Ethical Committee (SYXK -(Jiangsu) -2011-0037,Nanjing,China) of Nanjing Agricultural University (Approval No.PZ2020089).
2.2.3 Animal protocol 1: experimental scheme for HFrD-induced neuroinflammation and cognitive deficits in mice
Mice that acclimated for 1 week were allocated to 3 groups(n=13 per group) and treated as follows (Fig.1A): The control mice(NC group) were fed with a normal diet (starch (34.5%) and sucrose(35.5%)) for 8 weeks.The model mice (HFrD group) were fed with HFrD (starch (8.9%),sucrose (1%) and fructose (60.1%),Jiangsu Synergy Pharmaceutical Biological Engineering Co.,Ltd.,Nanjing,China) for 8 weeks.Based on the results of our previous research[18],150 mg/(kg·day) of AA-2βG dosage was chosen in rodent models from a preventive perspective.Thus,the mice were fed with HFrD and treated with AA-2βG (AA group) at 150 mg/(kg·day) by intragastric gavage for 8 weeks (200 µL/mice).
Fig.1 Scheme of animal experiment.
2.2.4 Animal protocol 2: FMT
The recipient mice (n=13 per group) were fed HFrD and treated daily with transplant material from either HFrD-fed mice or AA-2βG-fed mice (animal protocol 1) for 8 weeks (Fig.1B).FMT was performed and slightly modified based on the previous report[19].Fresh fecal pellet samples were collected from individual donor mice and subsequently mixed in sterile saline containing 0.5 g/L cysteine hydrochloride (400 mg stools dissolved in 4 mL sterile saline).FMT groups of fecal slurry were centrifuged at 4 °C (1 000 r/min) for 20 s and transferred fecal supernatant to another tube.To prevent changes in intestinal flora,the collection of fecal supernatant and oral gavage(0.2 mL for each mouse) were completed within 15 min.
2.2.5 Animal protocol 3: intestinal permeability experiment
In animal protocol 1 and animal protocol 2,3 mice from each group were used for experiment of intestinal permeabilityin vivo.Fluorescein isothiocyanate (FITC)-dextran permeability assay was used to assess intestinal permeability as described previously[10].Briefly,all animals fasted for 4 h were administered FITC-dextran(4 kDa;Sigma Chemical Co.,St.Louis,MO,USA) by intragastric gavage (0.6 mg/g bw,25 mg/mL solution),3 h before sacrifice.Plasma was collected by centrifuging the blood at 3 000 r/min for 15 min at 4 °C.The assay of fluorescence intensity of FITC-dextran was performed by fluorescence spectrophotometer.The concentrations of plasma FITC-dextran were calculated from a standard curve.
2.3 Morris water maze (MWM) test
To evaluate cognitive function,all mice were tested on the MWM after experimental diets (n=8 per group).The experimental procedure was performed by following the comprehensive description by Liu et al.[20]with minor modifications.In brief,the water maze was a round pool (4 quadrants,120 cm diameter and 60 cm height),which was filled with water ((24 ± 1) °C) to a deep of 30 cm.A hidden platform which is about 1 cm deep from the surface of the water was maintained in one of the quadrants.The test included a 4-day training trail and a spatial probe trial on day 5.Consecutive 4-day training for each mouse was performed,in which the latency to escape from the water and the mice’s swimming trajectory was calculated.After removing the platform,the spatial probe test was performed.Each mouse was placed in the pool for a maximum 90 s (probe test) on day 5.Parameters,including latency,number of platform crossings,etc.,were recorded.
2.4 mRNA analysis
Total RNA of colon,cerebral cortex and hippocampus tissues were extracted by using total RNA extraction kit (Yi Fei Xue Biotechnology,Nanjing,China).The purity and concentration of extracted RNA were tested and quantified on a NanoDrop 2000(Thermo Fisher Scientific,USA).The complementary DNA (cDNA)was immediately synthesized by reverse transcription operated with a PrimeScript RT master mix (TaKaRa Co.,Ltd.,Japan).Primer sequences for PCR are presented in Table S1.Primers were provided by Sangon Biotech (Shanghai) Co.,Ltd.(Shanghai,China).Real time quantitative PCR (RT-qPCR) reaction was performed with 384-well plates and SYBR Green Master Mix (ABI,USA) in the Quant Studio 6 Flex System (ABI,USA).The relative amount of mRNA was normalized to level of glyceraldehyde-3-phosphate dehydrogenase(GAPDH) as housekeeping gene,and the data were calculated using the 2−ΔΔCtmethod.
2.5 Biochemical analysis
The cytokine concentrations were determined by ELISA,using kits for interleukin (IL)-6,tumor necrosis factor (TNF)-α,and IL-10 (Neobioscience,Shenzhen,China) based on the instructions,and the LPS concentration was assessed by a commercial kit (Nanjing Jiancheng Bioengineering Institute,Nanjing,China).
2.6 Hematoxylin and Eosin (H&E) histopathological analysis
The fresh colon and brain tissues were fixed with 4%paraformaldehyde for more than 24 h.Subsequently,the specimens were embedded in paraffin.Finally,the fresh colon and brain tissue samples stained with H&E (Sigma,USA).Slices were detected by a Nikon microscope (Tokyo,Japan) and images were captured.
2.7 Immunofluorescence analysis
Immunofluorescence staining was carried out as described before[18].In brief,the colon and brain tissues were exposed to primary antibodies (diluted with phosphate buffered saline (PBS,pH 7.4)appropriately) at 4 °C overnight,incubated with FITC-labeled secondary antibody for 50 min in the dark and washed 3 times with PBS for 5 min each.Finally,the nucleus was counterstained with 4’,6-diamidino-2-phenylindole (DAPI) solution.The slides were detected and captured images using an inverted fluorescence microscope (Nikon,Tokyo,Japan).For image quantification,the mean fluorescence intensity of images was calculated by Image J.
2.8 Microbial community analysis
The fecal pellets of mice were collected and transported to Center for Genetic &Genomic Analysis,DeepBiome Co.,Ltd.(Jinan,China).Stool DNA samples were extracted,and the 16S rDNA gene comprising V3-V4 regions were subjected to amplification.Purified amplicons were sequenced on an Illumina MiSeq platform.High-quality sequences were selected,and Zero-radius operational taxonomic units (ZOTUs) were clustered with 97% similarity cutoff using USEARCH.Principal component analysis (PCA) was conducted by R software (Version 3.4.3).
2.9 Statistical analysis
The results are expressed as mean ± standard error of mean (SEM).The differences between three groups and more than three groups of data were measured by one-way analysis of variance (ANOVA) procedure followed by Duncan’s test with SPSS software.A two-tailed Student’sttest was applied in the two group comparisons.Gut microbial data were also evaluated using Tukey’s honest significant difference (HSD) test with false discovery rate (FDR-P<0.05) correction.
3. Results
3.1 AA-2βG alleviates cognitive impairment and neuroinflammation
To investigate whether AA-2βG could display a neuro-protective effect,we used HFrD-fed mice for 8 weeks as a model (see animal protocol 1 in Fig.1A).Next,MWM tests were conducted to evaluate recognition memory of mice.As shown in Fig.2B,the mean escape latency decreased progressively for all groups during the training period.Furthermore,in the last day,the mice in the AA group showed shorter latency than the mice in the HFrD group (P<0.05),revealing that AA-2βG could enhance the spatial learning and memory.After the training trial on day 5,the platform was removed and the probe test was performed.The mice in the AA group searched for the platform in the best search strategy,whereas the mice in the HFrD group found the platform without direction (Fig.2A).The mice in the HFrD group displayed the diminution in the number of crossings,the percentage of distance and the percentage of time spent in the target quadrant,in contrast to the mice in the NC group (Figs.2C-E).Conversely,AA-2βG supplementation significantly showed an increase in parameters related to cognitive function,indicating that AA-2βG could ameliorate HFrD-induced memory impairment in mice.We further examined the effects of AA-2βG on systemic inflammation,neuron loss or damage and neuroinflammation.Compared with the HFrD group,serum levels of LPS,TNF-α and IL-6 were diminished markedly by AA-2βG supplementation (Fig.2F).Also,histology of the hippocampus and cortex revealed that HFrD increased nuclei pyknosis and damaged neurons compared with the NC and AA groups(Fig.2G).Furthermore,we examined glial fibrillary acidic protein(GFAP) and ionized calcium-binding adaptor molecule 1 (Iba-1)via immunofluorescence staining.AA-2βG supplementation significantly reduced the mean immunofluorescence intensity of Iba-1 and GFAP in hippocampus regions of the mice brain (Fig.2H).In addition,the mRNA expression levels of neuroinflammation related parameters in the HFrD group,such asTNF-α,IL-1β,monocyte chemoattractant protein 1 (MCP-1),IL-6,inducible nitric oxide synthase (iNOS),toll-like receptor 4 (TLR4),nod-like receptor protein 3 (NLRP3)and myeloid differentiation factor 88 (MyD88),were significantly elevated.The aberrant mRNA expression in the hippocampus caused by HFrD was significantly inhibited by AA-2βG (Fig.2I).In addition,AA-2βG remarkably suppressed the up-regulation ofIL-1β,TNF-α,IL-6,iNOS,TLR4andMyD88mRNA expression in the cortex (Fig.2J).All these data suggested that AA-2βG could alleviate the HFrD-induced systemic inflammation,activation of glial cells,neuroinflammation and cognitive impairment.

Fig.2 AA-2βG alleviates cognitive impairment and neuroinflammation in HFrD-fed mice.(A) Representative swimming paths of mice.(B) Mean latency to reach platform during training session of MWM (**P <0.01).(C) The number of crossings during the probe test of MWM.(D) The percentage of distance in the target quadrant (where the platform was located during training session) during the probe test of MWM.(E) The percentage of time spent in the target quadrant.(F) LPS,TNF-α,IL-6,and IL-10 levels (n=5−8).(G) Representative photomicrographs of H&E staining of hippocampal DG,CA1,and CA3 region,and cortex(scale bar,50 μm),bottom left corner of each image representative higher magnification image.(H) The immunofluorescent staining of Iba-1 and GFAP in DG and CA of the hippocampus (n=3,200× magnification).The mean density of the Iba-1 and GFAP was analyzed by Image J software.TNF-α, IL-1β,IL-6,iNOS,MCP-1,TLR4,NLRP3 and MyD88 mRNA levels in the hippocampus (I) and cortex (J) (n=6).Significantly differences: different letters,P <0.05.Statistical significance was calculated using one-way ANOVA.
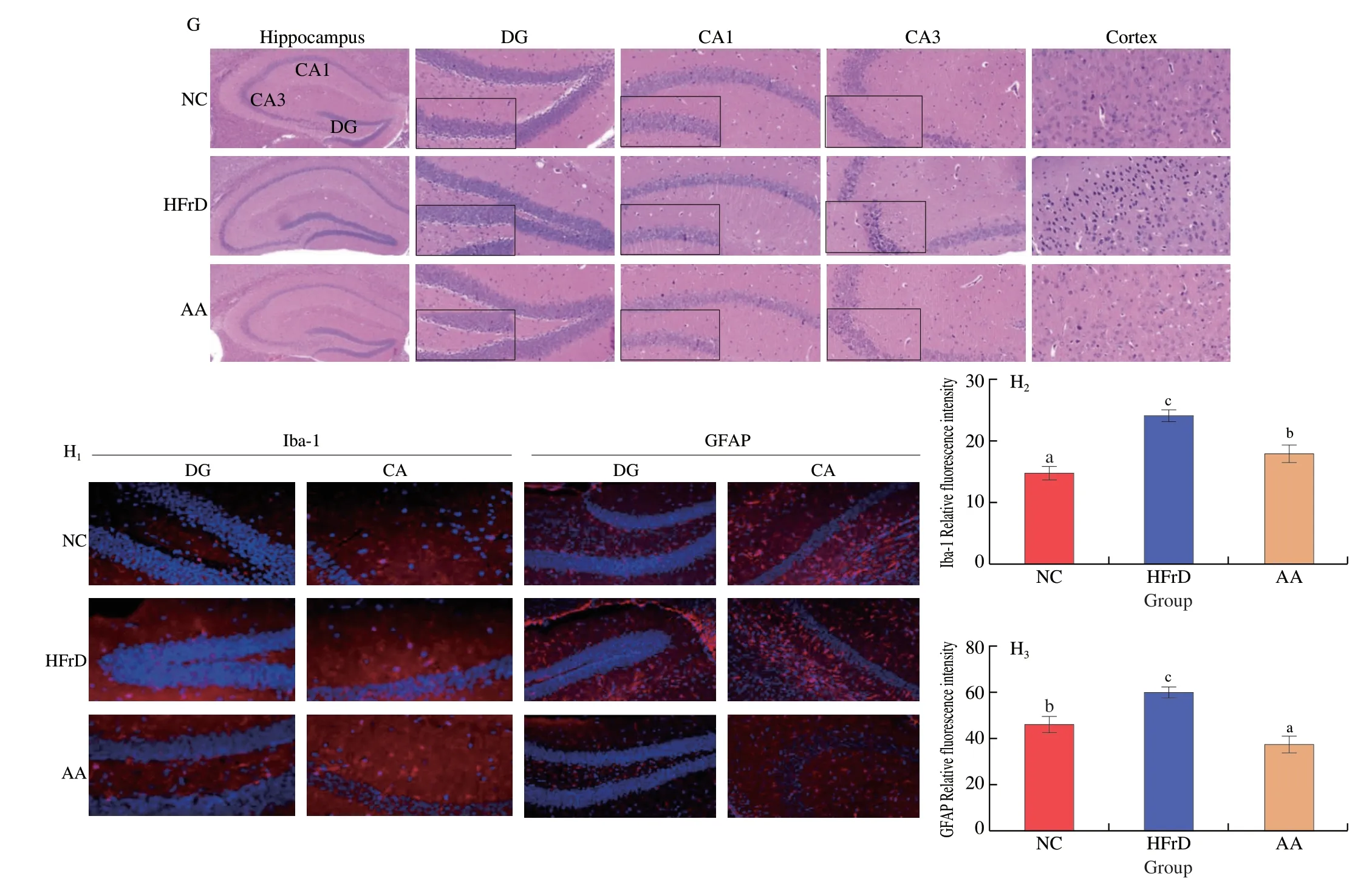
Fig.2 (Continued)
3.2 AA-2βG reduces intestinal epithelial barrier impairment
Next,we examined whether oral administration of AA-2βG prevented intestinal epithelial barrier impairment.According to H&E staining,the histological changes characterized by discrete infiltration of inflammatory cells in the HFrD mice were reversed by AA-2βG(Fig.3A).Furthermore,the tight junction (TJ) proteins and mucin 1(MUC1) were analyzed by immunofluorescence assay (Fig.3B).As illustrated in Fig.3,HFrD induced considerable losses of MUC1 and TJ proteins.Notably,AA-2βG supplementation showed significantly(P<0.05) increased MUC1 level relative to that in the HFrD group (Fig.3C).Occludin and ZO-1 were also increased (P>0.05)after treatment with AA-2βG (Figs.3E-F).Consistently,AA-2βG supplementation remarkably elevated the colonic mRNA expression levels ofMUC1andOccludin(Figs.3G,I).Subsequently,AA-2βG alleviated the plasma level of FITC-dextran,which was elevated by HFrD (Fig.3K),suggesting that AA-2βG could maintain the intestinal epithelial barrier integrity and attenuated gut permeability.These findings suggested that AA-2βG prevented intestinal epithelial barrier impairment,which subsequently might suppress LPS entry into the circulation.
3.3 AA-2βG reverses HFrD-induced gut dysbiosis
Emerging evidences have highlighted that gut-brain bidirectional communication mediated by gut microbiota[6],we therefore assessed the influence of AA-2βG on the gut microbiota composition by performing 16S rDNA amplicon sequencing analysis of bacteria in feces.The results of PCA observed a distance clustering of microbiota composition for three groups (Fig.4A).The structure of gut microbiota was changed significantly.In particular,the relative abundance of Verrucomicrobia was notably increased (P=0.032)by AA-2βG (Fig.S2A).Among the altered family identified,in comparison to the NC group,the HFrD group displayed a remarkably elevation in Rikenellaceaeand reduction in Lactobacillaceae,while AA-2βG protected against the effects in a large part (Fig.S2B).To further assess the composition of the bacterial microbiota community in details,we analyzed the extent of the bacterial taxonomic similarity at the genus level (Fig.4B).Notably,the levels of two genera,includingLactobacillusandAkkermansiawere diminished in the HFrD group,while AA-2βG supplementation protected against the effect (Figs.4C-D).Meanwhile,Alistipeswas dramatically enriched by HFrD,which was decreased by AA-2βG supplementation (Fig.4E).We further used linear discriminant analysis effect size (LEfSe) to confirm the bacterial phylotypes that were significantly altered by AA-2βG supplementation.In HFrD-fed mice,supplementation with AA-2βG significantly increased 10 ZOTUs (LDA score >3.5).It was found thatLactobacillusgenus andAkkermansia_muciniphilaspecies were differentially enriched in gut microbiota community in the AA group (Fig.4F).Meanwhile,we found a consistent increase in the concentrations of propionic acid,i-butyric acid,and total acids in feces and cecal contents (Figs.S3A-M).The relative expression of G-protein couple receptor 41 (GPR41) and GPR43 in colonic tissue was both upregulated in the AA group (Fig.S3N).The results indicated that AA-2βG had significant effect in modulating the gut microbiota composition in HFrD-fed mice.

Fig.4 AA-2βG alleviated HFrD-induced gut dysbiosis.Gut microbial community structures in mice (n=10).(A) PCA analysis of microbiota composition for the NC,HFrD and AA groups.(B) Microbial community bar plot by genus (relative abundance >1%).Relative abundance of Lactobacillus (C),Akkermansia (D),Alistipes (E). (F) (a) Distribution histogram based on linear discriminant analysis (LDA);(b) Heatmap shows the relative abundances of 18 ZOTUs in two groups based on LDA;(c) In the left panel,the dots (●) and circles (○) represent the more and less abundant ZOTUs in two groups,respectively.In the right panel,ZOTUs represent bacterial taxa information.Tukey’s HSD test was conducted using R package (FDR-P <0.05).
3.4 The neuro-protection of AA-2βG is transferable by FMT
To further demonstrate the protection of AA-2βG mediated by gut microbiota and subsequently effects on the host cognitive impairment,we did FMT (see animal protocol 2 in Fig.1B).After 8-week of colonization,horizontal fecal microbiota transfer from AA-2βG mice(AA→HFrD) demonstrated similar cognitive protective effects as observed in the AA group mice (Figs.5A-E).In contrast with the horizontal fecal microbiota transfer from HFrD mice (HFrD→HFrD)group,the content of IL-10 was remarkably elevated in the horizontal fecal microbiota transfer from AA-2bG mice (AA→HFrD) group,while reducing level of LPS was observed (Fig.5F).To identify whether FMT affected the neuroinflammation in the HFrD mice,we assessed neuron loss or damage and neuroinflammation.It was found that there was a much more expression of Iba-1 and GFAP in hippocampus in the HFrD→HFrD group mice.Whereas,the AA receivers reversed the tendency,which suggested that FMT could inhibit the glial activation in HFrD mice (Figs.5G-I).Additionally,the expression of genes involved in neuroinflammation includingTNF-α,IL-1β,iNOS,TLR4andMyD88in the hippocampus were significantly decreased,whileTNF-α,iNOS,andTLR4mRNA levels in the cortex were dramatically down-regulated in the AA→HFrD group (Figs.5J).These findings indicated that the neuro-protection of AA-2βG in HFrD-fed mice might be due to modulation of the gut microbiota and metabolites.

Fig.5 Fecal microbiota transplantation of AA-2βG reversed HFrD-induced memory impairment and suppressed neuroinflammatory response.Horizontal fecal transferred from HFrD-treated mice to HFrD mice are referred as HFrD receivers (HFrD→HFrD).Horizontal fecal transferred from AA-treated mice are referred as AA-2βG receivers (AA→HFrD).(A) Representative swimming paths of mice from each group.(B) Mean latency to reach platform during training session of MWM (* P <0.05).(C) The number of crossings during the probe test of MWM.(D) The percentage of distance in the target quadrant.(E) The percentage of time spent in the target quadrant.n=6−8 animals per group.(F) LPS and IL-10 levels in the serum (n=6).(G) Histopathology observation of hippocampal DG,CA1,and CA3 region,and cortex (scale bar,50 μm),bottom left corner of each image representative higher magnification image.(H) The immunofluorescent staining of Iba-1 in DG and CA of the hippocampus (n=3,200× magnification).The fluorescence intensity of Iba-1 was analyzed by Image J.(I) The immunofluorescent staining of GFAP in DG and CA of the hippocampus (n=3,200× magnification).The fluorescence intensity of GFAP was analyzed by Image J.TNF-α,IL-1β,IL-6,iNOS,MCP-1,TLR4,NLRP3 and MyD88 mRNA levels in the hippocampus (J1) and cortex (J2) (n=6).All values are presented as mean ± SEM.Statistical significance was calculated using two-tailed Student’s t test,* P <0.05;** P <0.01;ns,no significant.
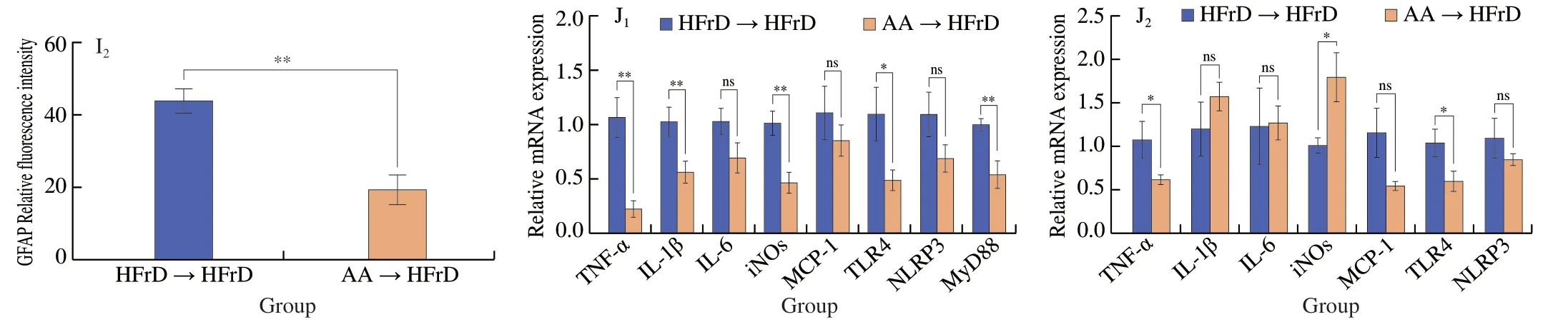
Fig.5(Continued)
Given that gut microbiota alteration in these recipient mice might affect gut permeability,we examined whether AA-2βG could modulate gut integrity in order to test this hypothesis.Large area of inflammatory cells infiltration was observed in the HFrD→HFrD group,reversely,it was not observed in the AA→HFrD group (Fig.6A).Expression ofZO-1,OccludinandMUC1genes were also elevated in the colon of HFrD mice after transfer of fecal microbiota derived from AA-2βG-treated mice and the immunofluorescence staining results were consistent with these observations (Figs.6B-J).In parallel,AA-2βG receivers (AA→HFrD) showed a significant decrease in plasma FITC-dextran level compared with HFrD receivers(HFrD→HFrD) after 8-week of colonization (Fig.6K).These results showed that fecal microbiota transfer from mice fed with AA-2βG(AA→HFrD) enhanced the expression of genes associated with gut integrity.Collectively,these findings supported the hypothesis that AA-2βG remodeled the gut microbiota and prevented the leaky gut in HFrD mice.
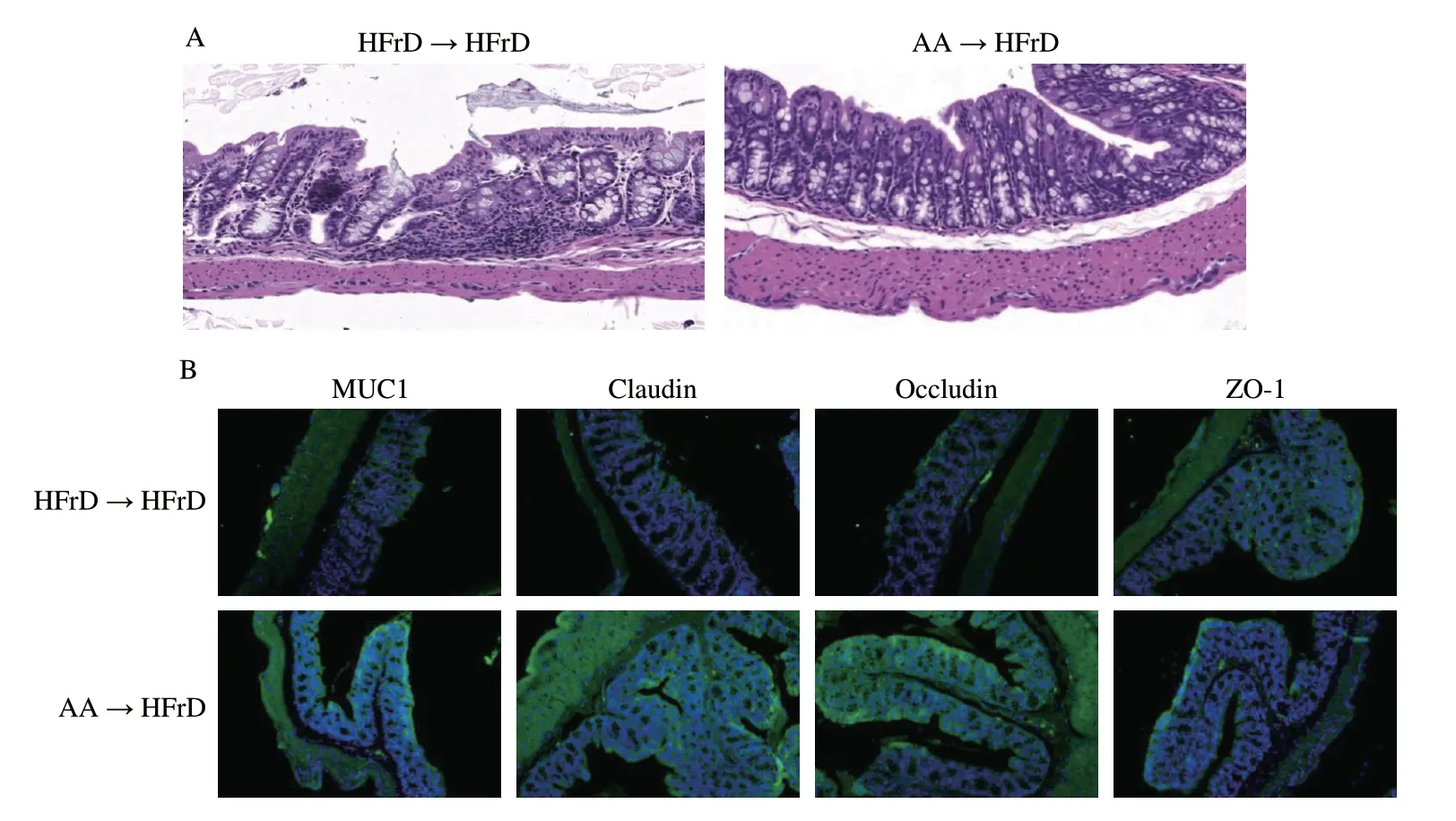
Fig.6 Fecal microbiota transplantation of AA-2βG ameliorated intestinal tight junctions and plasma FITC.(A) Colonic tissue H&E sections in two groups.(B) The immunofluorescent staining of MUC1,Claudin-1,Occludin and ZO-1 for each group (200× magnification).The fluorescence intensity was analyzed by ImageJ software,(C) MUC1,(D) Claudin-1,(E) Occludin,(F) ZO-1 (n=3).MUC1 (G),Claudin-1 (H),Occludin (I) and ZO-1 (J) mRNA levels in the colon (n=6).(K) Plasma FITC-dextran concentration (n=3).All values are represented as mean ± SEM.Statistical significance was obtained using two-tailed Student’s t test,* P <0.05;** P <0.01;ns,no significant.

Fig.6 (Continued)
To further verify the cognitive protective effects of AA-2βG mediated through the gut microbiota,we did FMT and conducted 16S rDNA sequencing based on amplicon sequence variants.The results of PCA showed significant separation of the gut microbiota of mice between the AA→HFrD and the HFrD→HFrD groups(Fig.7A).Family level analysis revealed that Lactobacillaceae,Verrucomicrobiaceae and Rikenellaceae were altered by FMT of AA-2βG (Fig.S4).These bacteria included two genus whose the relative abundances ofLactobacillusandAkkermansiawere remarkably enriched,while the relative abundance ofAlistipessignificantly decreased in the intestine of the AA→HFrD group,indicating an efficient transfer of specificbacterialduring FMT (Figs.7B-E).According to LEfSe,18 ZOTUs were identified,which made a contribution to microbial community between the HFrD→HFrD and AA→HFrD groups (Fig.7F).In line with the AA group,as shown in Fig.7,fecal transfer from the AA group (AA→HFrD) also displayed increases in relative abundances ofLactobacillusgenus andAkkermansia_muciniphilaspecies,indicating that FMT from the AA group recapitulated the microbia as observed in the donor mice.Therefore,AA-2βG alleviated the neuroinflammation by the intervention of gut microbiota,which might have the same effects via FMT.
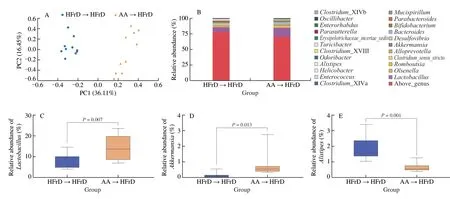
Fig.7 AA-2βG fecal microbiota transplants modulated gut microbiota composition.Gut microbial community structures in mice (n=10).(A) PCA analysis of microbiota composition for the HFrD→HFrD and AA→HFrD groups.(B) Gut microbial composition at genus level.Relative abundance of Lactobacillus (C),Akkermansia (D),Alistipes (E).(F) (a) Distribution histogram based on (LDA);(b) Heatmap shows the relative abundances of 18 ZOTUs in two groups based on LDA;(c) In the left panel,the dots (●) and circles (○) represent the more and less abundant ZOTUs in the AA→HFrD group in comparison to the HFrD→HFrD group,respectively.In the right panel,the ZOTUs represent bacterial taxa information.Relative abundance of ZOTU3 (G) and ZOTU21 (H).Tukey’s HSD test was conducted using R package (FDR-P <0.05).
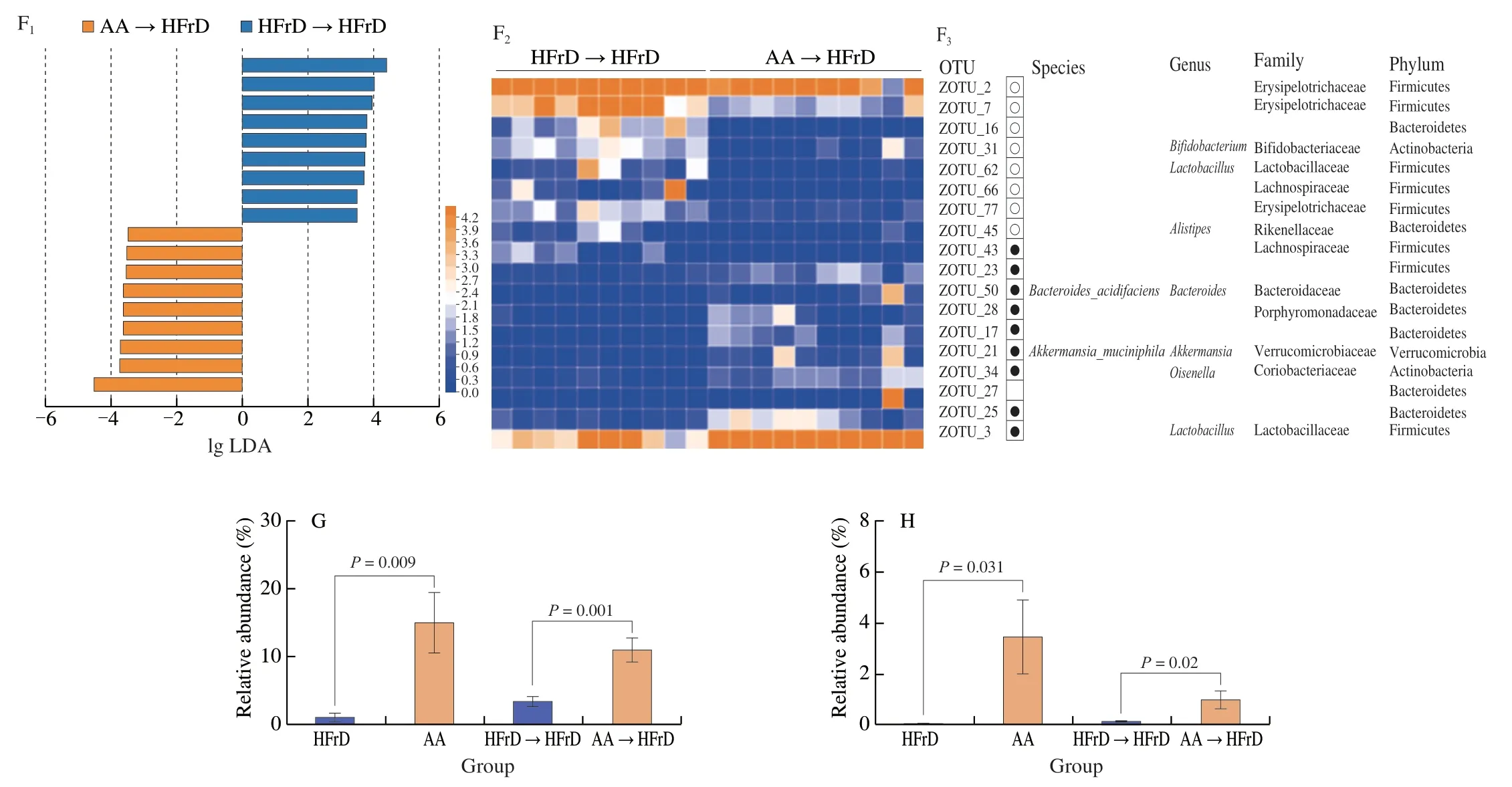
Fig.7 (Continued)
4. Discussion
The high prevalence of AD has become a growing global public health problem.Moreover,the exact etiology of AD is still obscure,and there is no effective therapeutic regimen in clinical at present[5].Meanwhile,a growing number of studies have focused attention on the prevention or delay of a potentially modifiable risk factor in dementia before the occurrence of the irreversible symptoms of dementia.Diet,as a way of non-pharmacological intervention,may be a main chance to modulate neurocognitive function in AD[5].These present results revealed that AA-2βG reduced the HFrDinduced neuroinflammation and cognitive disorder by altering gut microbiota and enhancing intestinal integrity.The concept is proved by transferable neuro-protection by FMT.The observation of Kim et al.[7]that FMT from healthy wild-type mice could alleviate AD support the notion that AD has a strong causal relationship with gut microbiota.Previous studies have shown that AA-2βG exhibited a satisfied effect on healthy host gut micro-ecology via altering the gut microbiome structure[21],which also firmly supports this idea.Our present finding that AA-2βG could exert neurocognitive protection by gut microbiota,therefore,represents a novel potential prebiotic for the therapy of dementia and provides a novel understanding for the therapeutic evaluation of phytochemicals.
It is well-known that diets rich in fructose produce epithelial barrier dysfunction by increasing gut permeability[10,22].Gut permeability is closely controlled by colonic barrier including a mucus layer and TJ proteins[23].Transmembrane mucins are another barrier against invading enteric bacteria,such as MUC1[24].Indeed,we observed that AA-2βG could improve,at least in part,the damage of gut barrier function caused by dysregulation of TJ proteins and epithelial damage.Moreover,MUC1 was significantly increased by AA-2βG supplementation.
Next,as gut permeability increased,more LPS can enter the blood circulation generating systemic inflammation[9].In our study,it was found that the HFrD group had higher gut permeability and levels of LPS and cytokines than the NC group.Based on these findings,we speculated that dietary high-fructose impaired the integrity of intestinal cells,which allowed increased LPS entry into the blood culminating in neuroinflammation.Previous studies have shown that the LPS levels in AD patients were elevated three-fold in plasma and two-fold or three-fold in brains[25].Excessive exposure of LPS stimulated the activation of microglia and astrocytes,thereby produced inflammatory cytokines in the hippocampus of mice[9].The structure of blood-brain barrier (BBB) defects in mice was previously observed in HFrD-induced hippocampal neuroinflammation,in which cognitive dysfunction was exacerbated by inflammatory mediators including TNF-α,IL-6 and LPS[26].In our study,dietary AA-2βG constrained glial activation (microglia and astrocytes) and reduced neuroinflammation in the cortex and hippocampus.These findings are consistent with previous research that administration of AA in colchicineinduced AD rat model was effective in reducing inflammatory markers(TNF-α and IL-1β) through suppressing glia-mediated inflammation,thereby preventing memory impairment[27].Moreover,studies indicated that the ascorbate uptake and the overexpression of sodium-vitamin C cotransporter 2 (SVCT2) blocked LPS induced microglia activation,which was essential for the homeostasis of microglia[28].Hence,it is speculative that the beneficial effects of AA-2βG on both antiinflammation and cognitive function in the brains of mice may be attributed to the increment of intestinal permeability and the diminishment of endotoxins translocation.
Gut microbiota is known as key regulator of the function of the intestine and CNS including brain function and behavior[6].Therefore,the beneficial effect of AA-2βG may occur through remodeling of gut microflora.In the present study,we demonstrated that AA-2βG consumption alleviated a shift of gut microbiota induced by HFrD.Based on 16S rDNA sequencing analysis,elevation of relative abundances of genusLactobacillusandAkkermansiawere found,and the relative abundance of genusAlitipeswas remarkably lower due to AA-2βG intervention.Furthermore,the LEfSe analysis showed that AA-2βG intervention elevated not only theAkkermansiagenus,but also its lower taxa,includingA.muciniphilaatspecies.Similar changes in the decreased levels ofLactobacillusandAkkermansiawere observed in animals with AD[20].Lactobacillus,gram-positive,one of the most common probiotics,has been shown to exert health-promoting influence on neuroinflammation-related disease[29].At species,the recent research found that the probiotic (includingLactobacillus acidophilus,L.casei,L.fermentumandBifidobacterium bifidum) intervention for 12 weeks positively affected some metabolic parameters and cognitive functions in AD patients[30].Studies have demonstrated thatA.muciniphila(a potential probiotics)was considerably reduced in AD mice[31].The decreased level ofAkkermansiawas reported to be related to metabolic related diseases,such as obesity,type 2 diabetes and dysfunction of the gut barrier[32-34],most of which are crucial risk factors for AD.Previous studies have demonstrated that administration ofA.muciniphilacould restore gut barrier integrity,promote neuronal function,and attenuate neuroinflammation,thereby effectively preventing cognition dysfunction in high-fat diet (HFD)-fed mice[35].Additionally,Alistipeswas shown to be correlated with inflammation as pathobiont or opportunistic pathogens in rodent animals.Further,it is proved that an increase inAlistipeswas associated with depressionviadisrupting the gut-brain axis[36].Overall,these findings suggest that AA-2βG supplementation can reverse HFrD-induced gut microbiota shift.
For another,LactobacillusandAkkermansiahave also positive impacts on their host intestinal barrier function and intestinal mucosa[37].As an example,oral administration ofL.caseiLC122 could enhance gut barrier and cause an increase of mucus secretion related gene expression in aged mice[38].Akkermansiaeffectively improved intestine barrier dysfunction,dyslipidemia and cognitive function in APP/PS1 mice[39].In our research,along with elevated abundance ofLactobacillusandAkkermansia,AA-2βG prevented HFrD-induced gut leakage and microbiota encroachment.We further found that the gut barrier function was enhanced through AA-2βG consumption along with reduced systemic LPS,revealing increased intestinal permeability and reduction of endotoxins enter the circulation.These effects transferable through FMT strongly support the concept that AA-2βG may serve as platform factor,utilized byLactobacillusandAkkermansia,to improves gut barrier function.In addition,Spearman’s correlations analysis indicated that the levels ofLactobacillus,AkkermansiaandAlistipeswere remarkably associated with serum LPS and cognitive behavior index.
5. Conclusion
In conclusion,we found that AA-2βG isolated from the fruits ofL.barbarumcould attenuate neuroinflammation and cognitive deficits in HFrD-fed mice,modulating gut microbiota and preventing leaky gut could constitute the underlying mechanisms responsible for the beneficial neuroprotective effects of AA-2βG.AA-2βG improved cognitive impairment involving the enrichment ofLactobacillusandAkkermansia,potentially beneficial intestinal bacteria.Further studies are needed to investigate the present evidence.Our results highlighted that prolonged application of AA-2βG should be of value in reversing detrimental features of gut dysregulation associated with Western diets.
Data availability
Raw reads reported in this paper were deposited in the National Center for Biotechnology Information’s Sequence Read Archive(SRA) database under the BioProject ID number PRJNA758221.
Declaration of competing interest
All the authors declare that they have no competing interests.
Acknowledgments
We greatly appreciate the financial support from the Key Research and Development Program of Ningxia Hui Autonomous Region of China (2021BEF02008),the National Natural Science Foundation of China (32272330),and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Appendix A.Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://doi.org/10.26599/FSHW.2022.9250020.
- 食品科学与人类健康(英文)的其它文章
- GUIDE FOR AUTHORS
- Targeting gut microbiota in osteoporosis: impact of the microbial based functional food ingredients
- Weizmannia coagulans: an ideal probiotic for gut health
- Natural sources,refined extraction,biosynthesis,metabolism,and bioactivities of dietary polymethoxyflavones (PMFs)
- A review of salivary composition changes induced by fasting and its impact on health
- Minerals in edible insects: a review of content and potential for sustainable sourcing

